In this retrospective study, with prolonged follow-up, we analyze the outcomes of allogeneic hematopoietic stem cell transplantation (allo-HSCT) in adult acute lymphoblastic leukemia (ALL) and the impact of pre-transplantation measurable residual disease (pre-HSCT MRD).
MethodsDetection of MRD was performed by multiparametric flow cytometry (MFC) for Philadelphia chromosome-negative ALL (Ph-neg ALL) and by classic genetic tests for Ph-pos ALL.
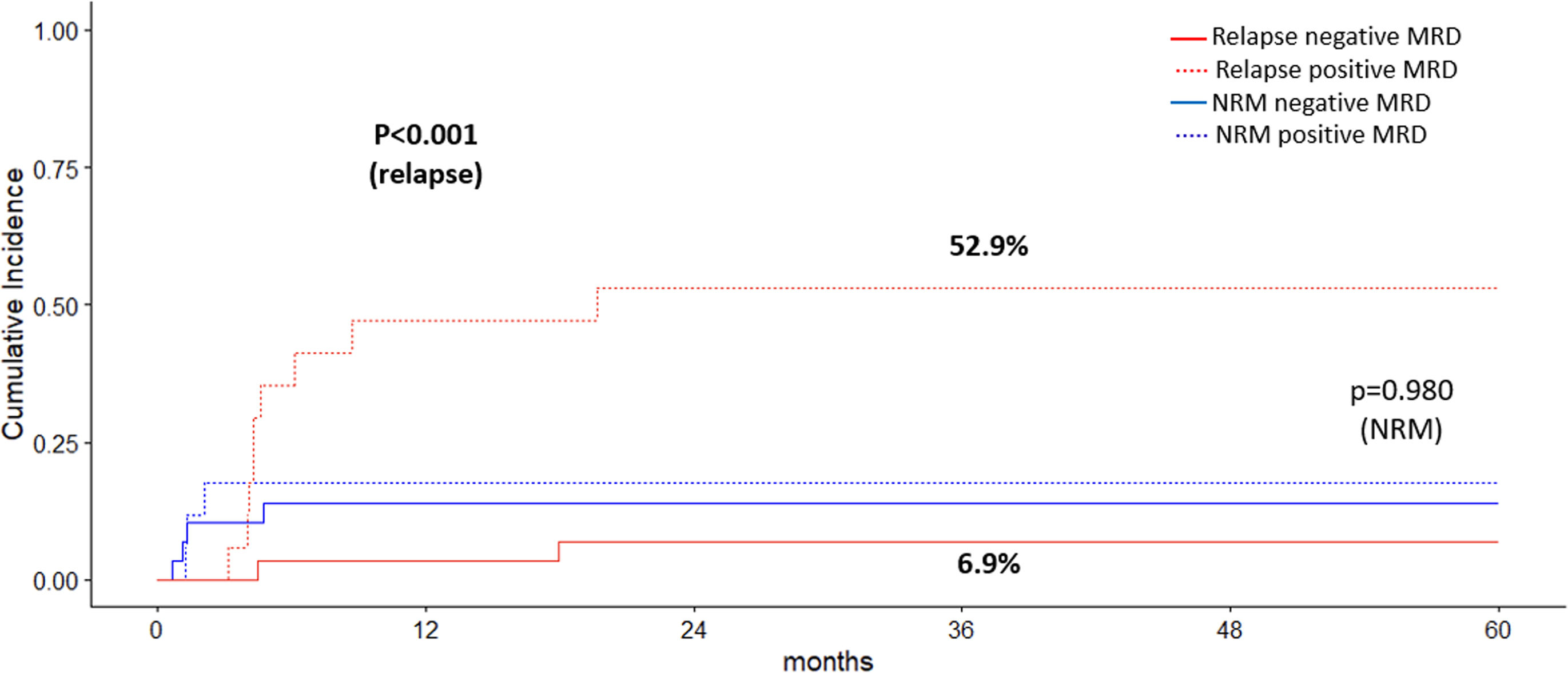
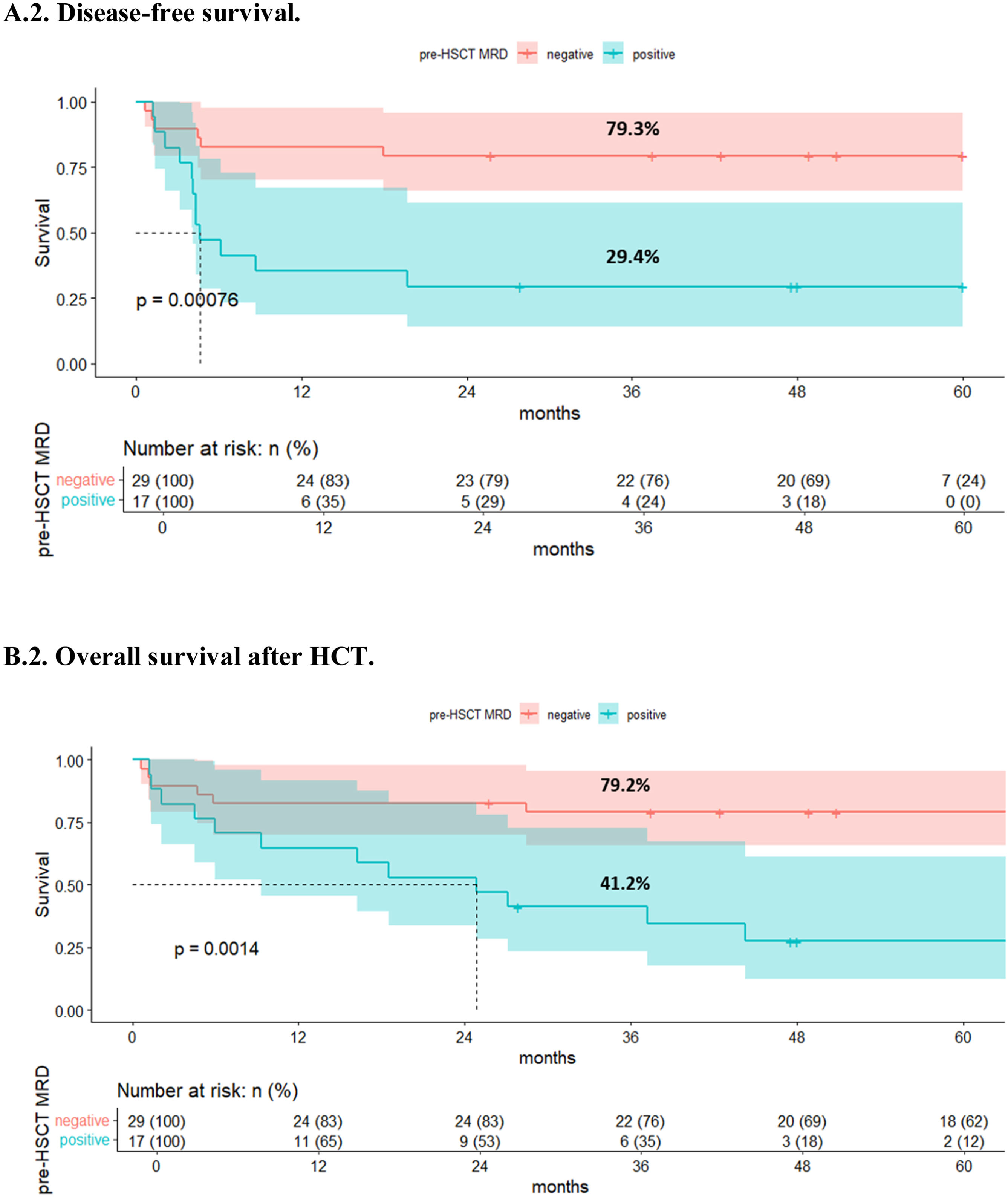
ResultsAmong 46 patients in first complete remission (CR1) who had available MRD data, 1- and 3-year cumulative incidences of relapse (CIR) for patients with positive and negative MRD were 47.1% and 52.9% vs. 3.4% and 6.9%, respectively (p<0.001). Disease free survival (DFS) at 1 and 3 years was 82.8% (95% CI 70.1–97.7) and 79.3% (95% CI 65.9–95.5) in the negative MRD group and 35.3% (95% CI 18.5–67.2) and 29.4% (95% CI 14.1–61.4) in the positive MRD group (p<0.001). With a median follow up of 29 months in the entire cohort and 177.6 months (14.8 years) in survivors, 1- and 3-year overall survival (OS) for the pre-HSCT negative MRD group was 82.8% (95% CI 70.1–97.7) and 79.2% (95% CI 65.6–95.5), respectively, compared to 64.7% (95% CI 45.5–91.9) and 41.2% (95% CI 23.3–72.7) in the positive MRD group (p=0.001). In a multivariate model, positive pre-HSCT MRD is associated with increased CIR and poorer DFS and OS.
ConclusionThese results support that pre-HSCT MRD should be eradicated to improve survival of adult ALL patients who undergo allo-HSCT.
En este estudio retrospectivo con seguimiento prolongado analizamos los resultados del trasplante alogénico de células progenitoras hematopoyéticas (alo-TPH) en los adultos con leucemia aguda linfoblástica (LAL) y el impacto de la enfermedad medible residual (EMR) antes del trasplante (pre-TPH).
MétodosLa detección de la EMR se realizó mediante citometría de flujo multiparamétrica (CFM) para la LAL cromosoma Filadelfia negativa (LAL Ph-neg) y mediante técnicas moleculares para la LAL Filadelfia positiva (LAL Ph-pos).
ResultadosEntre los 46 pacientes que disponían del dato de EMR en primera remisión completa (RC1), la incidencia acumulada de recaída (IAR) al primer y tercer año para los pacientes con EMR pre-alo-TPH positiva y negativa fue del 47,1% y del 52,9% vs. el 3,4% y el 6,9%, respectivamente (p<0,001). La supervivencia libre de enfermedad (SLE) al primer y tercer año fue del 82,8% (IC95%: 70,1-97,7) y del 79,3% (IC95%: 65,9-95,5) vs. el 35,3% (IC95%: 18,5-67,2) y el 29,4% (IC95%: 14,1-61,4) en el grupo de EMR negativa y positiva, respectivamente (p<0,001). Con una mediana de seguimiento de 29meses de la cohorte entera y de 177,6meses en los supervivientes, la supervivencia global (SG) al primer y tercer año en el grupo de EMR negativa y positiva fue del 82,8% (IC95%: 70,1-97,7) y del 79,2% (IC95%: 65,6-95,5) vs. el 64,7% (IC95%: 45,5-91,9) y el 41,2% (IC95%: 23,3-72,7), respectivamente (p=0,001). En el modelo multivariable, la EMR positiva pre-alo-TPH se asoció con aumento de recaída y peor supervivencia.
ConclusiónEstos resultados apoyan la necesidad de erradicar la EMR pre-TPH para mejorar la supervivencia de los pacientes con LAL que se someten a un alo-TPH.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is widely indicated for the treatment of adult patients with high-risk acute lymphoblastic leukemia (ALL). This procedure is the gold standard in Philadelphia chromosome-positive ALL (Ph-pos ALL) in first complete remission (CR1), early T cell-precursor ALL (ETP-ALL) and ALL with high-risk chromosomal abnormalities [(t(4;11)/KMT2A alterations)].1 In Ph-neg ALL, inadequate response with persistence of minimal residual disease (MRD) after chemotherapy is nowadays the most accepted indication for allo-HSCT.2 Allo-HSCT is considered an effective treatment for preventing relapse based on the combination of myeloablative conditioning and graft-versus-leukemia (GVL) reaction. It is also associated with significant incidence of non-relapse mortality (NRM) that should be considered in each case.3 While improved supportive care has reduced the toxicity/NRM,4,5 the high incidence of relapse associated to ALL remains an important challenge.
Despite CR rates up to 90% are achieved with high-intensity chemotherapy-protocols, at CR1 30–50% of adult patients still have positive MRD, defined as the presence of leukemic cells in patients with cytomorphologic CR. Several methods allow a detection sensitivity of up to 0.01% cells (10−4).6 The most commonly available is multiparametric flow cytometry (MFC) which discriminates leukemia-associated immunophenotype in more than 90% of patients with any subtype of ALL.7 Up to 40% of B-cell precursor ALL (B-ALL) and 20% of T-cell ALL (T-ALL) patients have specific chromosome aberrations detectable by traditional genetic methods. Less frequently available specific molecular techniques, such as assessment of rearrangements of immunoglobulin (Ig) and T cell receptor (TCR) genes, can also be used to detect residual leukemic cells.8
MRD persistence indicates resistance to chemotherapy and has proven to be the most important risk factor for hematologic relapse in adult and pediatric ALL.9 Current treatment protocols for Ph-neg ALL are based on MRD detection for transplant indication. Indeed, a persistent positive MRD just before the transplant indicates a higher risk of post-transplant relapse in Ph-neg ALL.10 Conversely, although Ph-pos ALL is still an indication for allo-HSCT in CR1, the achievement of a deep molecular remission with improved combinations of new generation tyrosine kinase inhibitors (TKIs) and chemotherapy, has led to question the role of transplant in Ph-pos ALL therapy.11
The aim of this study was to explore the long-term outcome of allo-HSCT in 141 ALL adult patients, older than 15 years, including the impact of pre-HSCT MRD on transplantation results.
Patients and methodsStudy cohortThis retrospective study included patients with ALL from January 1984 to January 2020 who underwent allo-HSCT at La Princesa University Hospital (Madrid, Spain). The Hematology department has performed more than 2000 hematopoietic transplants along more than 35 years, the first one in 1982. Inclusion criteria were age ≥15 years, diagnosis of B-ALL, T-ALL or Ph-pos ALL and at least one allo-HSCT. Patients who underwent subsequent allo-HSCT were not excluded and their follow-up time for overall survival (OS) assessment was based on the addition of time from first HSCT to second HSCT and time from second HSCT to end of follow up.
Data were extracted from the transplantation database and from individual chart review. Collected data included patients and ALL characteristics, as well as first allo-HSCT characteristics such as stem cell source (SCS), donor type, conditioning regimens and graft versus host disease (GVHD) prophylaxis, and analyses of outcome variables, including OS, disease free survival (DFS), NRM and cumulative incidence of relapse (CIR).
This study was conducted in accordance with the local legislation and institutional requirements and was approved by Research Ethics Committee of University Hospital La Princesa, Madrid (3751-EO) to gather data from patient records and databases. The study data base was locked as of March 2022.
Transplantation protocolsDecisions concerning transplantation were discussed in the HSCT committee. Bone marrow (BM) was employed until 2001; from this date on peripheral blood (PB) was set as preferred stem cell source (SCS). HLA matched sibling or related donor (RD) was the preferred donor choice, followed in preference order by unrelated donor (URD) (including one mismatched HLA allele), related haploidentical donor (haploD) and cord blood donor (CBD). Myeloablative conditioning regimen based on cyclophosphamide (Cy) 60mg/kg for two days (−6 and −5) followed by fractioned total body irradiation (fTBI) of 12Gy divided in 4 days (−4 to −1) was mostly employed. Other regimens consisted of Cy plus busulfan (CyBu), fludarabine plus busulfan (FluBu) and specific protocols for haplo-HSCT12 and CBD-HSCT.13 For second allo-HSCT, an individualized conditioning regimen was applied depending on disease status and previous treatments received.
For GVHD prophylaxis, cyclosporine (CsP) 3–5mg/kg/day (from day −1) plus short methotrexate (sMTX) 15mg/m2 (day +1) and 10mg/m2 (days +3, +6, +11) was most employed. For some patients with high risk of relapse sMTX was omitted. Mycofenolate mofetil (MMF) 10–15mg/kg/8h instead of sMTX was employed in CBD and haploD allo-HSCT, with de addition of posttransplant Cy 50mg/kg (days +3, +4) in the latter. In most HLA mismatched donor (MMD) and CBD allo-HSCT, antithymocyte globulin (ATG) 2mg/kg (days-4 – only in CBD-, -3,-2) was added to GVHD prophylaxis. Antimicrobial prophylaxis and surveillance as well as other supportive measures were implemented according to updated local transplantation protocols.
Detection of MRDPatients were categorized as positive or negative pre-HSCT MRD based on MFC assessment for Ph-neg ALL and on genetic test result for Ph-pos ALL from BM samples obtained right before the transplant. MFC for ALL was available in our institution from 2001 and BCR-ABL rearrangements by polymerase chain reaction (PCR) from 1996.
MRD by MFC was considered positive for values above 10−4 (i.e. 0.01%). The detection sensitivity was 10−4 until 2017 and 10−5 from there on. A BD FACSCalibur flow cytometer with BD CellQuestPro software was employed from 2001 to 2010. From 2010 on, BD FACSCanto II with BD FACSDiva software was used for acquisition, and the analysis was performed with the Infinicyt software (Cytognos SL, Salamanca, Spain). The panels of antibodies used for B- or T-lineage ALL are detailed in Supp. Material. All antibodies were obtained from Becton Dickinson Biosciences (BD Biosciences, San Jose, CA, USA) unless otherwise specified.
Regarding the molecular assay of BCR/ABL rearrangement, qualitative nested PCR (sensitivity 10−6) was performed from 1996 to 2004. After this date, the method was changed to real-time quantitative PCR (sensitivity 10−5) performed using the Light cycler (LC-480) (Roche Diagnostics, Basel, Switzerland).
Definitions and statistical analysesNeutrophil recovery was defined as an absolute neutrophil count of 500/mm3 on 3 consecutive days and platelet recovery as a platelet count of 20,000/mm3 without transfusions for 7 consecutive days. For acute and chronic GVHD gradation Glucksberg classification and NIH system were employed, respectively.
OS was defined as the duration from HSCT to death from any cause or cut-off date. DFS was defined as survival in CR after HSCT. NRM was defined as death from any cause without previous relapse. Cumulative incidence of relapse (CIR) was determined from the date of HSCT to the date of relapse or the end of follow-up. For patients without an event, observation was censored at the cutoff date of 1st March 2022.
Patients age was expressed as median and absolute intequartile range while the remaining patient and transplant demographic and clinical characteristics were expressed as frequencies and percentages. Age group, induction treatment response, disease status, SCS and donor type were compared by study period using Chi-square test.
CI of neutrophil and platelet engraftment, CI of acute and chronic GVHD, CIR and NRM over time were calculated as competing risks in a Fine-Gray competing risk survival model, with death/relapse, death, NRM and relapse being treated as a competing event to hematopoietic engraftment, GVHD, relapse and NRM, respectively. Survival curves were estimated using the Kaplan–Meier method and comparisons between groups were analyzed using the log rank test. Multivariable analysis was performed with the use of a Cox proportional hazard model, adjusted for potential risk factors and effect modifiers. Statistical analyses were performed with SPSS, version 28.0 (IBM Corp, Armonk, NY, USA), and R, version 4.2.1 (R Foundation, Vienna, Austria), softwares.
ResultsPatient and HSCT characteristicsThe characteristics of the study population and allo-HSCT are summarized in Table 1. The study cohort included 141 adult patients with a median age of 30 years (16–66). Forty-four patients (32.1%) had Ph-pos ALL. Twenty-six patients (18.4%) with Ph-neg ALL had unfavorable cytogenetics such as 11q23/MLL gene rearrangement, hypodiploidy (karyotype with <45 chromosomes) or complex karyotype (≥5 chromosomal abnormalities). ALL initial treatment was mainly administered following national protocols (PETHEMA group) available in each period and based on chemotherapy (± ITK in Ph-pos ALL). Twenty-three patients (15.6%) had advanced disease (AD), defined as not being in CR including partial remission (≥5% blasts in BM) or relapsed/refractory (R/R) disease. Fourteen patients (10%) underwent second allo-HSCT at a median of 25.8 months (5.7–208.3) from the first transplant (outcomes in Supp. Material).
Patient, disease and transplant characteristics.
| Patients characteristics | No. of patients (%) |
|---|---|
| Patients | 141 (100%) |
| Median age (years) | 30 (16–66) |
| <40 years | 102 (72.3%) |
| ≥40 years | 39 (27.7%) |
| Sex | |
| Male | 63 (44.7%) |
| Female | 78 (55.3%) |
| CMV receptor serology | |
| Positive | 97 (68.8%) |
| Negative | 40 (28.4%) |
| Unavailable | 4 (2.8%) |
| Clinical presentation | |
| CNS involvement | 16 (11.3%) |
| Extramedular involvement | 45 (31.9%) |
| Hyperleucocitosis | 40 (28.4%) |
| ALL subtype | |
| B-ALL | 78 (55.3%) |
| T-ALL | 19 (13.5%) |
| Ph-pos ALL | 44 (31.2%) |
| Pre-HSCT ALL status | |
| CR1 | 76 (53.9%) |
| CR2+ | 43 (30.5%) |
| AD | 22 (15.6%) |
| MRD prior to HSCT | |
| Negative | 47 (33.3%) |
| Positive | 29 (20.6%) |
| Unavailable/NA | 65 (46.1%) |
| High risk cytogenetic | 70 (49.6%) |
| Ph/t(9;22) | 44 (31.2%) |
| Other unfavorable abnormalitiesa | 26 (18.4%) |
| Allo-HSCT period | |
| Before 2001 | 63 (44.7%) |
| From 2001 | 78 (55.3%) |
| Graft source | |
| BM | 84 (59.6%) |
| PB | 55 (39%) |
| UCB | 2 (1.4%) |
| Donor origin | |
| RD | 100 (70.9%) |
| URD | 36 (25.5%) |
| HaploD/UCB | 3 (2.1%)/2 (1.4%) |
| HLA mismatch | |
| RD | 3 (2.1%) |
| URD | 14 (9.9%) |
| ≥2 HLA mismatch | 5 (3.5%) |
| Conditioning regimens | |
| fTBI+Cy | 115 (81.6%) |
| CyBu | 18 (12.8%) |
| Other | 8 (5.7%) |
| GVHD prophylaxis | |
| CNI+sMTX | 118 (83.7) |
| CsP+MMF | 6 (5.2%) |
| CsP+MMF+Cy post | 3 (2.1%) |
| CsP | 14 (9.9%) |
| ATG administration | 15 (10.6%) |
AD: advanced disease. Allo-HSCT: allogeneic hematopoietic stem cell transplantation. ATG: antithymocyte globulin. BM: bone marrow. Bu: busulphan. CNI: calcineurin inhibitors. Cy: cyclophosphamide. Cy post: posttransplant cyclophosphamide. CsP: cyclosporine. CR1: first complete remission. CR2+: second complete remission or beyond. fTBI: fractionates toral body irradiation. HaploD: haploidentical donor. MMF: mycophenolate mofetil. NA: not applicable. PB: peripheral blood. Ph: Philadelphia chromosome/t(9;22). RD: related donor (sibling). sMTX: short methotrexate. UCB: umbilical cord blood. URD: unrelated donor.
Regarding the characteristics of allo-HSCT, SCS corresponded to BM in 84 patients (59.6%) and donor was RD in 100 cases (70.9%). Twenty-two transplants (15.6%) carried MMD and ATG was added in 15 of them. ABO group mismatch between receptor and donor was detected in 39 transplants (27.7%). Conditioning regimen was mainly myeloablative based on fTBI plus Cy (n=115; 81.6%). GVHD prophylaxis was CsP plus sMTX in 114 transplants (80.9%).
Transplantation periodSixty-three (44.7%) and 78 (55.3%) allo-HSCT were performed until the year 2000 (earlier period) and from 2001 on (later period), respectively. Of the 39 patients who were older than 40 years in the entire cohort, only 12 (30.8%) underwent allo-HSCT until 2000 (p=0.04). Although more patients showed refractory disease to induction chemotherapy and AD prior to allo-HSCT in the earlier period, the differences were not statistically significant. Regarding graft source, PB replaced BM from 2001 on (p<0.001). Alternative donors became more common after 2000 (p<0.001). These data are summarized in Table 2.
Patients and transplant characteristics according to allo-HSCT period.
| Characteristics | Number of patients (%) | p-Value | ||
|---|---|---|---|---|
| Total | Period before 2001 | Period from 2001 | ||
| Age (years) | ||||
| <40 | 102 (100%) | 51 (50.0%) | 51 (50.0%) | 0.040 |
| ≥40 | 39 (100%) | 12 (30.8%) | 27 (69.2%) | |
| Response to ICT | ||||
| CR | 113 (100%) | 45 (39.8%) | 68 (60.2%) | 0.059 |
| PR | 8 (100%) | 4 (50.0%) | 4 (50.0%) | |
| REF | 13 (100%) | 8 (61.5%) | 5 (38.5%) | |
| NA | 7 (100%) | 6 (85.7%) | 1 (14.3%) | |
| Pre-HSCT ALL status | ||||
| CR1 | 76 (100%) | 30 (39.5%) | 46 (60.5%) | 0.094 |
| CR2+ | 43 (100%) | 18 (41.9%) | 25 (58.1%) | |
| AD | 22 (100%) | 15 (68.2%) | 7 (31.8%) | |
| Graft source | ||||
| BM | 91 (100%) | 62 (68.1%) | 29 (31.9%) | <0.001 |
| PB | 48 (100%) | 1 (2.1%) | 47 (97.9%) | |
| UCB | 2 (100%) | 0 (0%) | 2 (100%) | |
| Donor origin | ||||
| RD | 100 (100%) | 57 (57%) | 43 (43%) | <0.001 |
| URD | 36 (100%) | 5 (13.9%) | 31 (86.1%) | |
| HaploD | 3 (100%) | 1 (13.9%) | 2 (66.7%) | |
| UCB | 2 (100%) | 0 (80%) | 2 (100%) | |
AD: advanced disease. BM: bone marrow. CR1: first complete remission. CR2+: second complete remission or beyond. HaploD: haploidentical donor. HSCT: hematopoietic stem cell transplantation. ICT: intensive chemotherapy. MMF: mycophenolate mofetil. NA: not applicable. PB: peripheral blood. REF: refractory. RD: related donor (sibling). UCB: umbilical cord blood. URD: unrelated donor.
One hundred and thirty-four (95%) patients achieved neutrophil recovery and 125 (88.7%) achieved platelet recovery. Median time for neutrophil engraftment was 20 days post-transplant (range: 10–38 days) and for platelet engraftment was 19 days (range: 6–38 days). The 30-day cumulative incidence of neutrophil and platelet engraftment was 92.9% [95% confidence interval (CI), 88.6–97.2)] and 79.4% [(95% CI, 72.7–86.1)], respectively. Neutrophil and platelet recovery occurred earlier if PB was employed compared to BM [subHazard ratio (sHR) 1.75 (95% CI, 1.20–2.56), p=0.004] and sHR 1.99 [(95% CI, 1.32–2.99), p<0.001].
Graft versus host diseaseOf the 141 patients, 45 (31.9%) developed grade 2–4 aGVHD of which 19 were grade 3–4 or severe aGVHD. The 100-day cumulative incidences of grade 2–4 and grade 3–4 aGVHD were 29.1% (95% CI, 21.2–37.0) and 10.9% (95% CI, 5.3–16.6), respectively. In a univariate analysis, MMD was associated with higher risk to develop both grade 2–4 aGVHD [sHR=3.04 (95% CI 1.55–5.99), p=0.001] and grade 3–4 aGVHD [sHR=7.52 (95% CI 2.87–19.7), p<0.001], whereas RD was associated with lower risk [sHR=0.45 (95% CI 0.24–0.2), p=0.009 and sHR=0.29 (95% CI 0.12–0.70), p=0.006]. In a multivariable analysis, MMD showed a trend to be associated to a higher risk for grade 2–4 aGVHD [sHR=2.14 (0.92–4.96), p=0.077] and a 6-fold higher risk for grade 3–4 [sHR=6.38 (2.73–14.9), p<0.001]. Later transplantation period (from 2001) and RD resulted in 3-fold and 2.5-fold lower risk, respectively, to develop grade 3–4 aGVHD [sHR=0.35 (95% CI 0.16–0.80), p=0.012 and sHR=0.39 (95% CI 0.18–0.81), p=0.012].
Forty-eight patients (34%) developed cGVHD. It was mild in 20 patients (41.7%), moderate in 21 patients (43.7%) and severe in 7 patients (14.6%). One- and 3-year cumulative incidences of global cGVHD and severe GVHD were 28.2% (95% CI 20.1–36.3) and 41.1% (95% CI 31.9–50.2), and 19.6% (95% CI 12.1–27.2) and 27.3% (95% CI 18.3–36.2), respectively. Of the variables analyzed, none of them was statistically significantly associated with global cGVHD, except from showing a trend to a higher risk in the case of positive pre-HSCT MRD [sHR=1.91 (95% CI 0.91–4.01), p=0.087]. On the other hand, RD was related to 2.6 and 3.6-fold decreased risk, respectively, to develop severe cGVHD both in the univariate [sHR=0.38 (95% CI 0.18–0.78), p=0.008] and multivariable analysis [sHR=0.28 (95% CI 0.09–0.82), p=0.021].
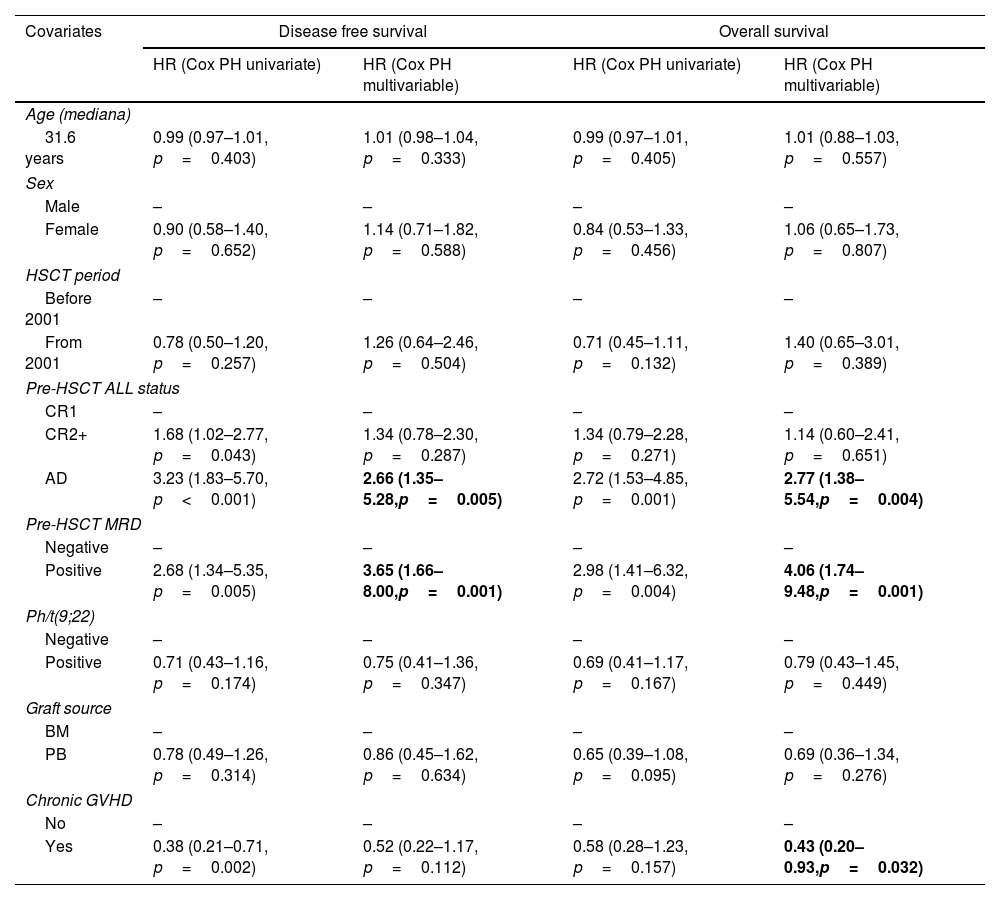
Overall survival and disease-free survivalWith a median follow up of 29 months in the entire cohort and 177.6 months (14.8 years) in survivors, 1-, 3- and 5-year OS values for the entire cohort were 62.4% (95% CI 54.9–70.9), 49.6% (95% CI 42–58.6) and 46.4% (95% CI 38.8–55.5), respectively. AD status of ALL before transplant [HR=2.77 (95% CI 1.38–5.54, p=0.004)] was associated with worse OS, whereas presence of cGVHD [HR=0.43 (95% CI 0.20–0.93; p=0.032)] was associated with better OS in the multivariable model (Table 3).
Univariate and multivariable analyses of factors influencing disease free survival and overall survival.
| Covariates | Disease free survival | Overall survival | ||
|---|---|---|---|---|
| HR (Cox PH univariate) | HR (Cox PH multivariable) | HR (Cox PH univariate) | HR (Cox PH multivariable) | |
| Age (mediana) | ||||
| 31.6 years | 0.99 (0.97–1.01, p=0.403) | 1.01 (0.98–1.04, p=0.333) | 0.99 (0.97–1.01, p=0.405) | 1.01 (0.88–1.03, p=0.557) |
| Sex | ||||
| Male | – | – | – | – |
| Female | 0.90 (0.58–1.40, p=0.652) | 1.14 (0.71–1.82, p=0.588) | 0.84 (0.53–1.33, p=0.456) | 1.06 (0.65–1.73, p=0.807) |
| HSCT period | ||||
| Before 2001 | – | – | – | – |
| From 2001 | 0.78 (0.50–1.20, p=0.257) | 1.26 (0.64–2.46, p=0.504) | 0.71 (0.45–1.11, p=0.132) | 1.40 (0.65–3.01, p=0.389) |
| Pre-HSCT ALL status | ||||
| CR1 | – | – | – | – |
| CR2+ | 1.68 (1.02–2.77, p=0.043) | 1.34 (0.78–2.30, p=0.287) | 1.34 (0.79–2.28, p=0.271) | 1.14 (0.60–2.41, p=0.651) |
| AD | 3.23 (1.83–5.70, p<0.001) | 2.66 (1.35–5.28,p=0.005) | 2.72 (1.53–4.85, p=0.001) | 2.77 (1.38–5.54,p=0.004) |
| Pre-HSCT MRD | ||||
| Negative | – | – | – | – |
| Positive | 2.68 (1.34–5.35, p=0.005) | 3.65 (1.66–8.00,p=0.001) | 2.98 (1.41–6.32, p=0.004) | 4.06 (1.74–9.48,p=0.001) |
| Ph/t(9;22) | ||||
| Negative | – | – | – | – |
| Positive | 0.71 (0.43–1.16, p=0.174) | 0.75 (0.41–1.36, p=0.347) | 0.69 (0.41–1.17, p=0.167) | 0.79 (0.43–1.45, p=0.449) |
| Graft source | ||||
| BM | – | – | – | – |
| PB | 0.78 (0.49–1.26, p=0.314) | 0.86 (0.45–1.62, p=0.634) | 0.65 (0.39–1.08, p=0.095) | 0.69 (0.36–1.34, p=0.276) |
| Chronic GVHD | ||||
| No | – | – | – | – |
| Yes | 0.38 (0.21–0.71, p=0.002) | 0.52 (0.22–1.17, p=0.112) | 0.58 (0.28–1.23, p=0.157) | 0.43 (0.20–0.93,p=0.032) |
AD: advanced disease. BM: bone marrow. CR1: first complete remission. CR2+: second complete remission or beyond. GVHD: graft versus host disease. HR: hazard ratio. HSCT: hematopoietic stem cell transplantation. PB: peripheral blood. Ph: Philadelphia chromosome/t(9;22). SD: standard deviation.
In bold, statistically significant results of multivariable analysis.
DFS values at 1, 3 and 5 years were 51.8% (95% CI 44.1–60.7), 43.2 (95% CI 35.8–52.2) and 42.4% (95% CI 35–51.5), respectively. Disease status different from CR1 before transplant was associated with worse DFS in the univariate analysis [HR=1.68 for CR2+ (95% CI 1.02–2.77, p=0.043), HR=3.23 for AD (95% CI 1.83–5.70, p<0.001)] whereas only AD remained statistically significant in the multivariable analysis (Table 3). Outcome of Ph-pos vs. Ph-neg ALL patients can be seen in Supp. Material.
Non-relapse mortality and relapseFifty-four patients (38.3%) and 26 (18.4%) died due to relapse and NRM, respectively. Death causes were severe infection (12, 46.2%), sinusoidal obstructive syndrome (SOS) (6, 23.1%; half of them after second allo-HSCT), severe GVHD (3, 11.5%), hemorrhagic coagulopathy (2, 7.7%), secondary neoplasm (SN) (1, 3.8%), thrombotic microangiopathy (1, 3.8%) and toxicity-related respiratory failure (1, 3.8%).
One- and 3-year estimates of NRM were 14.2% (95% CI 8.4–20.0) and 16.3% (95% CI 10.2–22.4), respectively. In the univariate analysis, there were no differences in NRM between periods [until 2000 vs. from 2001 on; HR=0.79 (95% CI 0.36–1.74, p=0.560)], SCS [BM vs. PB; HR=0.86 (95% CI 0.35–2.07, p=0.730)] and donor type [URD vs. RD; HR=0.90 (95% CI 0.36–2.28, p=0.830)]. In a multivariable analysis, there was a trend to increased NRM after developing grade 3–4 aGVHD [HR=2.56 (95% CI 0.88–7.45, p=0.085)]. Toxic mortality before day 100 post-transplantation occurred in 18 patients (12.8%).
Relapse occurred in 58 patients (41.1%) within a median of 4.7 months after transplantation (range: 0.72–204.6). One- and 3-year CIR for the entire cohort were 34.0% (95% CI 26.2–41.9) and 40.5% (95% CI 32.3–48.6), respectively. Significant increase of CIR was observed associated with the disease status, while decrease of CIR with the presence of cGVHD in the univariate analysis: HR were 2.79 (95% CI 1.54–5.04, p=0.001) and 4.43 (95% CI 2.14–9.19, p<0.001) for CR2+ and AD, respectively, while HR was 0.45 (95% CI 0.24–0.84, p=0.012) for the presence of cGVHD. In a multivariable model, ALL status different from CR1 was associated with increased risk of relapse [HR=2.71 for CR2+ (95% CI 1.40–5.22, p=0.003) and HR=4.38 for AD (95% CI 1.90–10.10, p=0.001)], whereas the presence of cGVHD was a protective factor (HR=0.38, 95% CI 0.20–0.71, p=0.003).
Apart from 14 patients who underwent a second allo-HSCT (outcome in Supp. Material), 12 of the 58 relapsed patients (20.7%) received rescue treatment based on immunotherapy [10 donor lymphocyte infusion (DLI), 1 blinatumomab and 1 inotuzumab ozogamicin]. Only 4 of 58 relapsed patients remained alive: three who underwent a second allo-HSCT after being rescued with chemotherapy (2) and blinatumomab (1) and one with Ph-pos ALL who was treated with DLI plus dasatinib.
MRD impact on relapse and survivalOf the 76 patients in CR1, 46 patients (60.5%) had available MRD data. Twenty-two patients with Ph-neg ALL had MFC-MRD data being positive 10 of them. Twenty-four patients with Ph-pos ALL had available BCR-ABL rearrangement data for MRD, being positive 7 of them. Relapse occurred in 9 of 17 patients and 2 of 29 with positive and negative pre-HSCT MRD, respectively, indicating significantly higher frequency of relapse in the positive MRD group (52.9% vs. 6.9%; p=0.001).
One and 3-year CIR values were higher for patients with positive and negative pre-HSCT MRD (47.1% and 52.9% vs. 3.4% and 6.9%, p<0.001), respectively (Fig. 1). Patients with positive pre-HSCT MRD showed a 4-fold-increased risk of relapse compared with those with negative MRD, both in a univariate (HR=3.62; 95% CI 1.59–8.23, p=0.002) and a multivariable model (HR=4.25, 95% CI 1.87–9.66, p=0.001).
DFS values at 1 and 3 years were higher in the negative MRD group compared with the positive group [82.8% (95% CI 70.1–97.7) and 79.3% (95% CI 65.9–95.5), respectively, and 35.3% (95% CI 18.5–67.2) and 29.4% (95% CI 14.1–61.4), respectively (p<0.001)] (Fig. 2a). Positive pre-HSCT MRD was associated with worse DFS in univariate analysis, (HR=2.68, 95% CI 1.34–5.35, p=0.005), showing more than 4-fold-decrease in DFS compared to negative MRD in a multivariable model (HR=4.48, 95% CI 2.02–9.94, p<0.001).
With a median follow-up of 29 months in the entire cohort and 177.6 months (14.8 years) in survivors, 1- and 3-year OS values were higher for the negative pre-HSCT MRD group compared with the positive group [82.8% (95% CI 70.1–97.7) and 79.2% (95% CI 65.6–95.5), respectively, compared to 64.7% (95% CI 45.5–91.9) and 41.2% (95% CI 23.3–72.7), respectively (p=0.001)] (Fig. 2b). Positive pre-HSCT MRD was associated with 3- and 5-fold-decrease in OS compared to negative MRD both in univariate (HR=2.98, 95% CI 1.41–6.32, p=0.004) and multivariable analyses (HR=5.39, 95% CI 2.25–12.94, p<0.001), respectively (Table 3).
Outcome of patients with AD and survivors’ late effectsOnly 3 of the 22 patients (13.6%) who had AD prior to HSCT remained alive. Relapse was the main cause of death (13 patients, 68.4%), followed by infection (4 patients, 21.1%). Among the remaining patients, one passed away 34 years after HSCT because of advanced colorectal neoplasia and the other died because of SOS early after second allo-HSCT.
Sixty-two of 88 patients (70.5%) who survived≥1 year after allo-HSCT developed≥1 late effect with a median time of 48.5 months (range: 12.4–336). Cardiovascular risk factors (hypertension, diabetes mellitus and dyslipidemia) (n=25; 40.3%) were the most frequent ones followed by sexual disfunction (n=21; 33.9%), hypothyroidism (n=18; 29%) and cataracts (n=13; 21%). Other common late effects (>10%) were osteopenia/osteoporosis (n=12; 19.4%), SN (n=9; 14.5%), autoimmune diseases (n=7; 11.3%) and ischemic complications (n=7; 11.3%). Non-melanoma skin cancer (n=5) was the most frequent SN. Other miscellaneous complications (kidney diseases, hyperferritinemia, acute pericarditis, C hepatitis virus, etc.) were detected in 23 patients (37.5%).
DiscussionIn this study, we retrospectively analyzed the long-term outcome of a cohort of 141 patients older than 15 years with ALL who underwent allo-HSCT recruited along more than three decades in a single transplant center. Adult ALL is a heterogeneous disease with worse prognosis than pediatric ALL which can be cured with high intensity chemotherapy-protocols difficult to complete in adults.14,15 Hence, historically many adult ALL patients have received allo-HSCT at some point during their disease.16 Nonetheless, relapse after transplant remains the main problem, as well as the identification of patients who would most likely benefit from alloHSCT. There is increasing evidence about the strong relevance of positive pre-HSCT MRD for subsequent ALL relapse.3 Most of the studies published were carried out in pediatric population where MRD was evaluated by Ig/TCR gene rearrangement instead of MFC.17,18
The present study has several noteworthy results. The outcome of our allo-HSCTs reflects the high curative potential of this procedure in ALL, which provides excellent OS and DFS, as well as low NRM rate, both in Ph-neg and pos-ALL, even beyond CR1. Our study suggests that positive pre-HSCT MRD, detected by traditional and widely available methods such as MFC for Ph-neg ALL and qPCR for Ph-pos ALL, is the strongest risk factor for relapse (Fig. 1). Nevertheless, not all patients with positive MRD relapsed after allo-HSCT, probably influenced by development of cGVHD, which was associated with lower CIR and better OS in our study (Table 3). In any case, general OS beyond 3 years was close to 50% in a cohort in which almost half of the patients had many high-risk features such as ALL status different from CR1 and adverse cytogenetic profile (Table 1). Furthermore, patients with negative pre-HSCT MRD showed excellent sustained OS near 80% (Fig. 2b), thus emphasizing the importance of MRD control prior to transplantation as relapse seems extremely unlikely in this group.
In the current study, persistence of MRD before transplant was strongly associated with higher risk of relapse and worse outcome in adult ALL patients who underwent alloHSCT. Similarly, in a retrospective study in which the impact of MFC-based pre-alloHSCT MRD screening for ALL was analyzed, DFS values at 1 and 3 years were 69% and 61% in the negative MRD group compared to 41% and 34% in the positive MRD group, respectively.19 Our findings are also in line with other studies showing that cGVHD has a significant antileukemic effect as its presence results in lower risk of relapse and longer DFS.20,21 On the other hand, neither period nor donor type significantly affected outcome in the present study. Although URD and MMD was associated with increased risk of acute and chronic GVHD in line with other reports,4,22,23 this association did not translate into higher NRM possibly due to lower number of URD in our study.
Some of our results were in line with already published data, such as those related with transplant period, hematopoietic recovery and GVHD.4,22 However, there are some other points to be highlighted. Firstly, positive pre-HSCT MRD showed a nearly significant effect to present global cGVHD, probably due to the faster immunosuppression withdrawal applied to induce GVL effect.20,21 Secondly, the higher rate of SN24 possibly related with the long follow-up of the patients underwent allo-HSCT in the earliest period of the cohort. Finally, the dismal prognosis of relapsing after transplant with <10% survival rate. In recent years, the development of more potent TKIs and highly effective monoclonal and bispecific antibodies have provided more options for treating patients with R/R ALL. In Ph-pos ALL, increasingly emerging data have supported the addition of a TKI either as preemptive or maintenance therapy after allo-HSCT.25 For R/R ALL, inotuzumab ozogamicin and blinatumomab have clearly demonstrated superiority versus chemotherapy.26,27 More recently, in a study where adults with positive MRD received blinatumomab, complete MRD responders had longer DFS and OS.28 In our cohort, two of the four patients who remained alive after post-transplant relapse were rescued with targeted therapies.
This study has several limitations. The data were collected retrospectively, the patient population is heterogeneous and both ALL treatment protocols and allo-HSCT supporting therapy have evolved and improved throughout the study time. However, it is coherent with real-world clinical practice in ALL along the years, including most of the conditioning regimens based on myeloablative fTBI plus Cy combination and 10% of the study patients who underwent a second allo-HSCT. Moreover, we believe this long-term observation provides strong evidence that MRD prior to allo-HSCT should be eradicated, whenever possible, as it was the main prognostic factor associated with increased relapse and decreased survival after transplant. The observed long-term OS in the positive MRD group (around 30%) may be clearly improved as the number of options for treating patients with both R/R ALL and positive MRD has increased. When MRD cannot be eradicated, classical adjustments of the transplantation procedures such as conditioning intensification to eradicate residual disease and selection of URD and weaker GVHD prophylaxis to favor development of cGVHD should be applied. In the post-transplant setting, frequent MRD monitoring should be mandatory to guide early implementation of measures to avoid relapse such as faster immunosuppression withdrawal followed by preemptive DLI and/or treatment with targeted therapies including CAR T therapy (tisagenlecleucel)29 and the above-mentioned antibodies and new generation TKIs.30 Future and ongoing studies will probably elucidate the optimal sequencing of allo-HSCT and these novel targeted agents in R/R and MRD-positive ALL.
In conclusion, our findings suggest that allo-HSCT still plays an important role to achieve prolonged survival in adult high risk ALL. Detection of MRD before transplant could identify a population of ALL patients at extremely high risk of relapse. This fact supports the utilization of already available novel therapies for MRD eradication before allo-HSCT, apart from classic transplant adjustments, in order to maximize its curative potential and improve survival of adult ALL patients who undergo this therapy.
Ethics approvalThe study was formally approved by Research Ethics Committee of Hospital Universitario La Princesa, Madrid, with the following registration number: 3751-EO.
FundingOA publishing charges were covered by Universidad Autónoma de Madrid.
Conflicts of interestThe authors have no conflicts of interest to disclose.