The role of in-hospital dipeptidyl peptidase-4 inhibitors in very old patients has not been widely described. This work analyzes the simplification of in-hospital antihyperglycemic management (less insulin use) and reductions in hypoglycemia events using linagliptin in patients aged≥80 years with type 2 diabetes.
Patients and methodsThis real-world observational study included hospitalized patients≥80 years with type 2 diabetes treated with an antihyperglycemic protocol of either basal-bolus insulin or linagliptin between January 2016 and December 2023. A 1:1 propensity score matching analysis was performed.
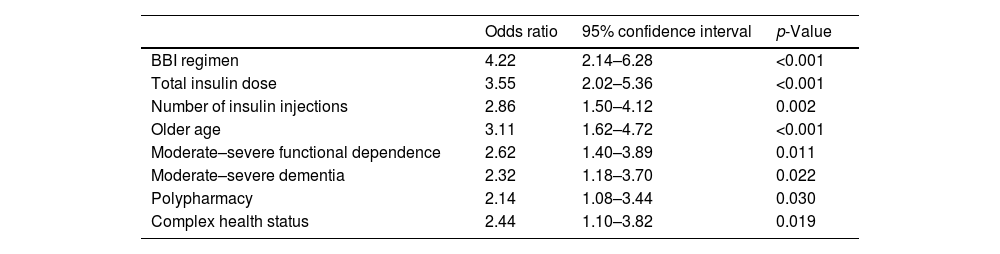
ResultsPost-matching, 944 patients were included in each group. The total and basal insulin doses and number of daily injections were significantly lower in the linagliptin group than the basal-bolus insulin group with no differences in glycemic efficacy. Regarding safety, patients on the basal-bolus insulin regimen had more hypoglycemic events. The use of basal-bolus insulin regimen (odds ratio: 4.22; 95% confidence interval: 2.14–6.28; p<0.001), a higher total insulin dose (odds ratio: 3.55; 95% confidence interval: 2.02–5.36; p<0.001) and the number of insulin injections (odds ratio: 2.86; 95% confidence interval: 1.50–4.12; p=0.002) were associated with a greater risk of hypoglycemia. Other hypoglycemia risk factors were older age, moderate–severe functional dependence, moderate–severe dementia, polypharmacy, and complex health status.
ConclusionsThe linagliptin regimen simplified in-hospital antihyperglycemic management and reduced hypoglycemia events compared to basal-bolus insulin regimen in patients with type 2 diabetes aged≥80 years. Basal-bolus insulin use and clinical factors were associated with hypoglycemia. The linagliptin regimen could be considered as standard of care for older adult type 2 diabetes patients in the hospital setting.
El papel de los inhibidores de la dipeptidil-peptidasa-4 intrahospitalario en pacientes de edad avanzada no ha sido ampliamente descrito. Este estudio analiza la simplificación del manejo antihiperglucémico intrahospitalario (uso de menos insulina) y la reducción de hipoglucemia usando linagliptina en pacientes de ≥80 años con diabetes mellitus tipo 2.
Materiales y métodosEstudio observacional en pacientes hospitalizados con ≥80 años con diabetes tipo 2 tratados con el protocolo antihiperglucémico que incluye el regimen insulina en bolo-basal o linagliptina, entre enero 2016-diciembre 2023. Se realizó un análisis de puntuaciones de propensión 1:1.
ResultadosTras la propensión, 944 pacientes fueron incluidos por grupo. Las dosis de insulina total y basal y el número de inyecciones fueron significativamente menores en el grupo linagliptina sin diferencias en la eficacia glucémica. Respecto a la seguridad, los pacientes con insulina bolo-basal tuvieron más hipoglucemias. El uso de insulina bolo-basal (Odds Ratio: 4.22; Intervalo de confianza 95%: 2.14-6.28; p<0.001), mayor dosis de insulina total (Odds Ratio: 3.55; Intervalo de confianza 95%: 2.02-5.36; p<0.001) y número de inyecciones (Odds Ratio: 2.86; Intervalo de confianza 95%: 1.50-4.12; p=0.002) fueron asociados con mayor riesgo de hipoglucemia. Otros factores fueron la edad avanzada, dependencia funcional moderada-severa, demencia moderada-severa, polifarmacia y estado de salud complejo.
ConclusionesLinagliptina simplificó el manejo antihiperglucémico y redujo hipoglucemias respecto al regimen de insulina en bolo-basal en pacientes de ≥80 años con diabetes tipo 2. El uso del régimen bolo-basal y factores clínicos fueron asociados con la hipoglucemia. Linagliptina intrahospitalaria podría ser considerada como el tratamiento estándar para pacientes de edad avanzada con diabetes tipo 2.
Type 2 diabetes (T2D) is expected to progressively increase worldwide in the aging population and has been associated with higher mortality and a greater risk of hospitalizations.1,2
A basal-bolus insulin (BBI) regimen is the preferred treatment for most noncritically ill hospitalized patients with T2D.3 The BBI regimen has been shown to improve glycemic control and reduce complications when compared to sliding scale insulin regimen.4,5 However, a BBI regimen is time- and labor-intensive, requiring several daily injections, and has been associated with frequent, clinically significant hypoglycemic events.3,6
In recent years, multiple randomized trials7–11 and real-world observational studies12–15 have reported that some dipeptidyl peptidase-4 inhibitors (DPP-4i) were safe and effective for treating patients with T2D during hospitalization. The use of other noninsulin antihyperglycemic drugs in hospitalized patients has been limited due to their potential side effects or contraindications.3
In light of the high prevalence of T2D among older adult patients in the hospital setting, the risk of drug-induced hypoglycemia associated with the intensive antidiabetic therapy, and the limited evidence on the role of DPP-4i in very old hospitalized patients with T2D, this work aimed to analyze the simplification of antihyperglycemic management, in terms of reduction of daily insulin doses and injections, and safety, in terms of a reduction in hypoglycemic events, associated with the use of linagliptin 5mg/day in patients aged≥80 years with T2D during the hospitalization.
MethodsStudy design and patientsA real-world, observational, multicentre study was carried out on patients with T2D aged≥80 years who were hospitalized at the Hospital Regional Universitario de Málaga, Hospital Universitario Virgen de la Victoria de Málaga, Hospital Helicópteros Sanitarios de Marbella, and Hospital Cenyt de Estepona, from January 2016 to December 2023.
The antihyperglycemic protocol includes the possibility of using two regimens: BBI or linagliptin. The BBI regimen is recommended for all patients as standard of care but is specifically recommended in patients who meet the following criteria: an admission blood glucose (BG) level>250mg/dL, at-home treatment with ≥30units of insulin, or linagliptin contraindication (expected admission to an intensive care unit, history of acute diabetic complications, hyperglycaemia without a known history of diabetes, gastrointestinal obstruction, pregnancy, those expected to be without oral intake or with artificial nutrition [enteral or parenteral], and history of active pancreas–gallbladder disease). As an alternative, the linagliptin regimen is recommended for patients who do not meet the criteria for using the BBI regimen. Linagliptin is initially used as monotherapy when an admission BG level is <180mg/dL, and in combination with once-daily basal insulin when an admission BG level is between 180 and 250mg/dL. The choice of which to use is made by healthcare providers according to their own medical judgment. The insulin dose is calculated according to admission BG levels, age, and serum creatinine, and is modified during hospitalization when required. Patients on the BBI regimen start on a total daily dose of 0.3units of insulin per kg when the following criteria are met: admission BG concentrations of <180mg/dL, patients ≥70 years old, serum creatinine ≥2mg/dL and/or body mass index≤20kg/m2. A total daily dose of 0.4units per kg is used for patients who meet the criterion of admission BG concentrations between 180 and 250mg/dL and 0.5 units per kg is used for patients who meet the criterion of admission BG concentrations of >250mg/dL. Fifty percent of total daily dose is ordered as basal insulin at the same time each day (04:00p.m.) and fifty percent is ordered as rapid-acting insulin divided into doses of 30%, 40% and 30% before breakfast, lunch and dinner, respectively. Patients on the linagliptin regimen who need to be treated in combination with basal insulin start on a daily dose of 0.2units of insulin per kg. Linagliptin 5mg/day is administered at 09:00a.m. Fasting, pre-prandial, and bedtime capillary BG levels are measured using a point-of-care glucose meter. Additionally, BG levels are measured any time a patient experiences symptoms of hypoglycemia or when requested by the medical provider. Level 1 hypoglycemia is defined as a measurable BG level<70mg/dL and ≥54mg/dL, level 2 hypoglycemia as a measurable BG level<54mg/dL, and level 3 hypoglycemia as a severe event characterized by altered mental and/or physical status requiring assistance, accordingly to American Diabetes Association criteria.16
When patients on the linagliptin arm experience treatment failure, defined as either two consecutive measurements or a mean daily BG level>180mg/dL, basal insulin are added or adjusted until reaching, if necessary, the BBI regimen. The target of therapy is to maintain fasting and pre-prandial BG levels between 140 and 180mg/dL. More in-depth details about our antihyperglycemic protocol are reported in the official Hospital Regional Universitario de Málaga website.17
Data on sociodemographic, anthropometric, diabetes, previous medical history variables were collected during the hospitalization. Dementia was determined using the Global Deterioration Scale and the Functional Assessment Staging; functional dependence was determined using the Barthel index; polypharmacy was defined as patients treated simultaneously with ≥5 drugs; and complex health status was defined as ≥1 of the following criteria: moderate–severe dementia, moderate–severe functional dependence, cardiovascular disease and/or advanced renal failure.
Measured outcomesThe primary endpoint was antihyperglycemic management simplification, measured via a reduction in daily insulin doses and number of daily injections, and safety, measured via a reduction in hypoglycemic events (total number, the number of patients with 1 or ≥2 events, incidence rates, and the number of patients with level 1, 2 and 3 hypoglycemia). Glycemic effectiveness (differences in mean daily values, at mealtimes and bedtime, BG levels 140–180mg/dL and >180mg/dL, and treatment failures between regimens), length of hospital stay, hospital complications, and mortality were also analyzed.
Statistical analysesIn order to match each patient who started BBI with a patient who started linagliptin in a 1:1 manner, propensity score matching (PSM) with a caliper of 0.2 and a greedy matching algorithm were used. The probability of starting BBI was estimated using a logistic regression model that included clinical variables that could have affected the treatment assignment or outcomes as independent variables (age, gender, anthropometric characteristics, diabetes characteristics, previous medical history, and admission characteristics). PSM adequacy was assessed using the standardized difference, with a difference>10% between baseline variables considered a significant imbalance.
Baseline characteristics were analyzed using descriptive statistics. Continuous and categorical variables were shown as means±standard deviation (SD) and as absolute value and percentage, respectively. Daily total units of insulin and number of insulin injections following our antihyperglycemic protocol were taken into account in order to calculate the mean of insulin units and insulin injections per patient. The hypoglycemia incidence rate per 100 patients-year was calculated. Differences between groups were determined using the two-sample Student's t-test or the Mann–Whitney–Wilcoxon rank-sum test for continuous variables and Pearson's Chi-squared test for categorical variables. In our analysis, we assessed multicollinearity among the predictor variables using the Variance Inflation Factor (VIF). For our model, we calculated the VIF for each predictor variable and removed those variables with VIF values exceeding 10. After this adjustment, logistic regression was used for the multivariate analysis of factors independently associated with the presence of hypoglycemia. The regression analysis values were expressed as odds ratio (OD) and 95% confidence interval (95% CI). Values were considered to be statistically significant when p<0.05. Multiple comparisons across different days of therapy were adjusted conservatively using Tukey's adjustment. Statistical analyses were performed using 22.0 SPSS Statistics, and 9.3 SAS for Windows.
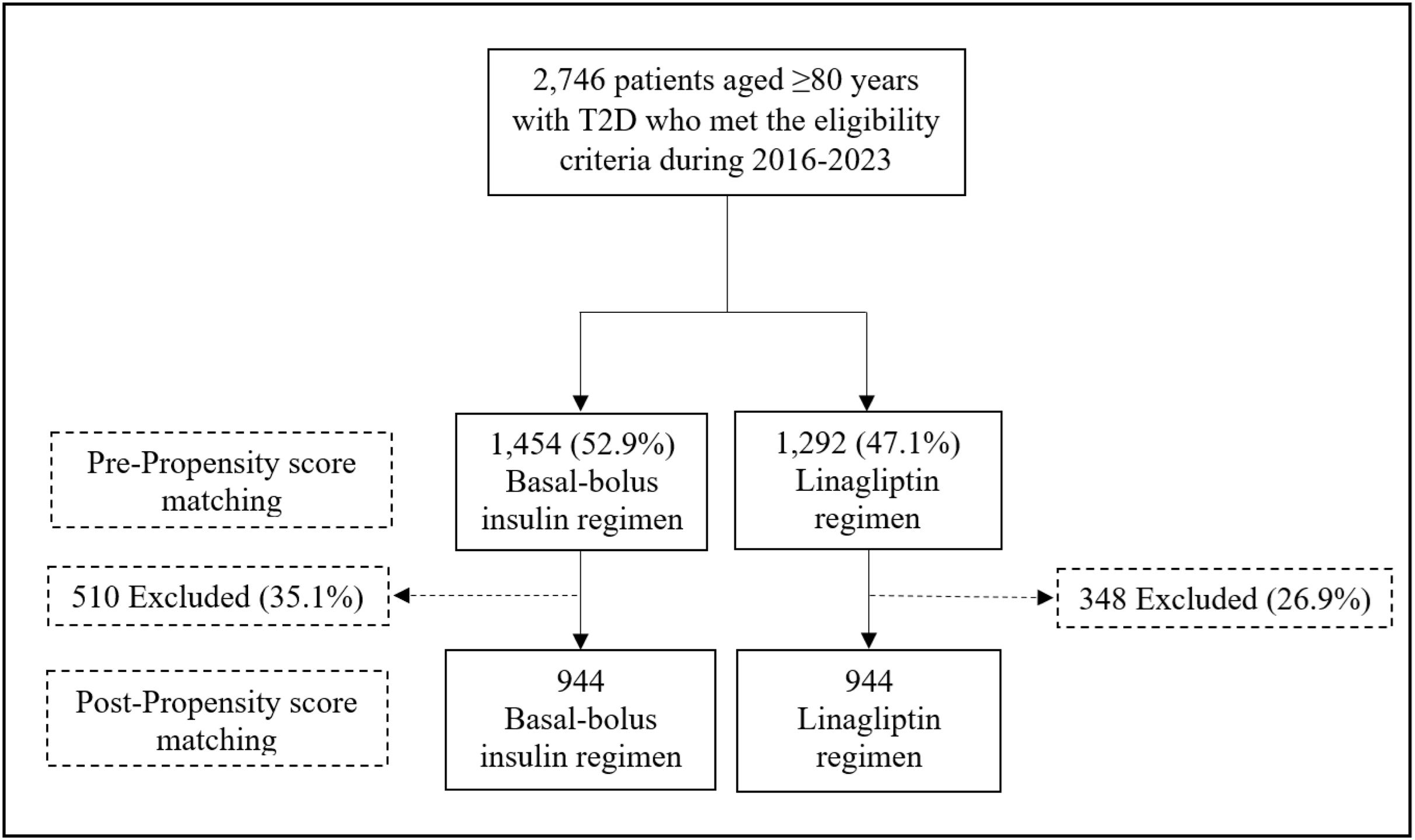
ResultsA total of 2746 patients aged≥80 years with T2D who did not meet the criteria for using the BBI regimen were identified. Of them, 1454 patients (52.9%) were managed with the BBI regimen and 1292 (47.1%) with the linagliptin regimen. Finally, after PSM, 944 patients were included in each treatment group. A flow chart for patient inclusion for both regimens is shown in Fig. 1.
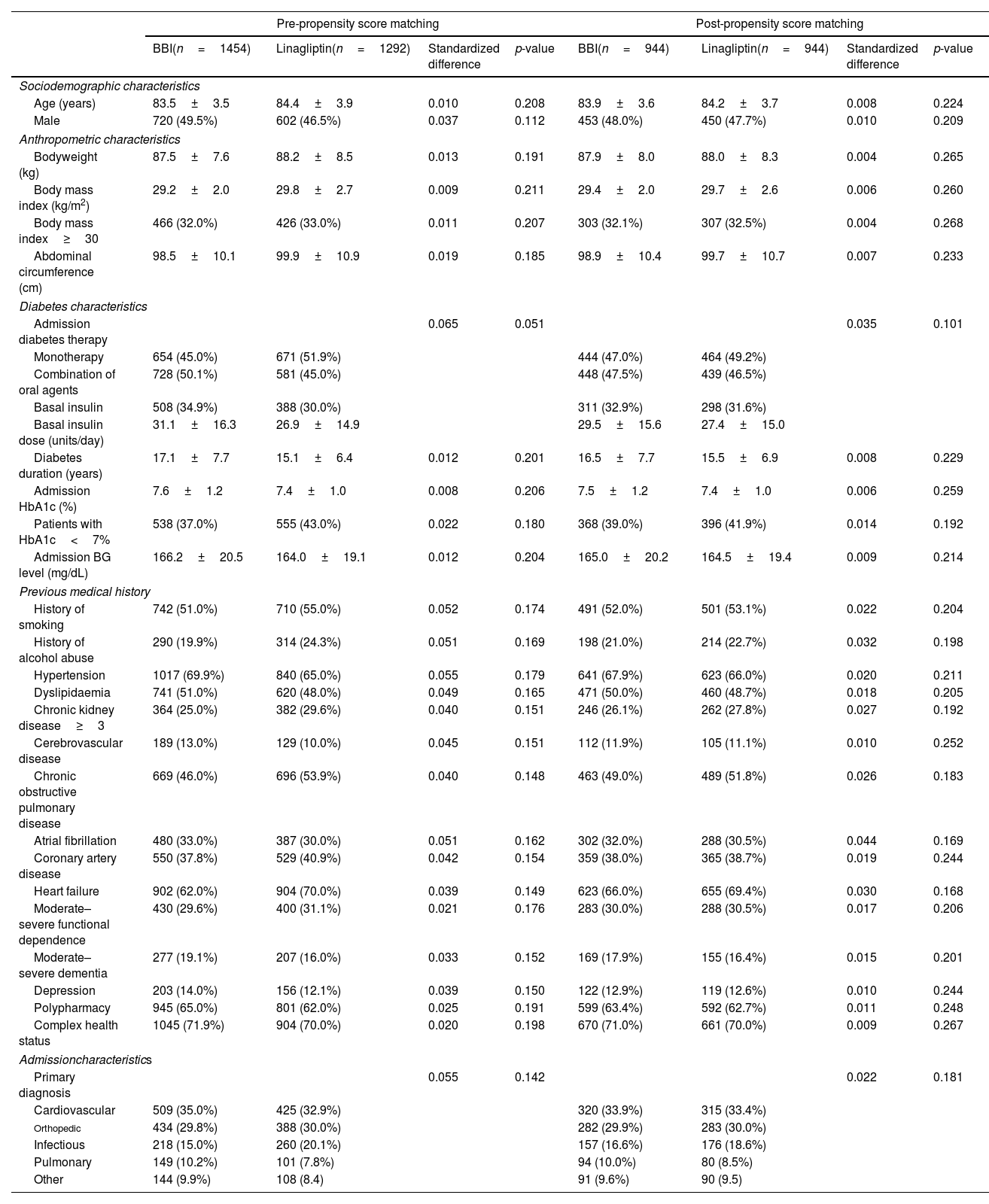
Baseline clinical characteristics between groups showed non-significant differences. The pre- and post-PSM sociodemographic, anthropometric, diabetes, previous medical history, and admission characteristics of very old patients according to antihyperglycemic regimen are shown in Table 1.
Pre- and post-propensity score matching sociodemographic, anthropometric, diabetes, previous medical history and admission characteristics of ≥80 year-old patients according to antihyperglycemic regimen.
| Pre-propensity score matching | Post-propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| BBI(n=1454) | Linagliptin(n=1292) | Standardized difference | p-value | BBI(n=944) | Linagliptin(n=944) | Standardized difference | p-value | |
| Sociodemographic characteristics | ||||||||
| Age (years) | 83.5±3.5 | 84.4±3.9 | 0.010 | 0.208 | 83.9±3.6 | 84.2±3.7 | 0.008 | 0.224 |
| Male | 720 (49.5%) | 602 (46.5%) | 0.037 | 0.112 | 453 (48.0%) | 450 (47.7%) | 0.010 | 0.209 |
| Anthropometric characteristics | ||||||||
| Bodyweight (kg) | 87.5±7.6 | 88.2±8.5 | 0.013 | 0.191 | 87.9±8.0 | 88.0±8.3 | 0.004 | 0.265 |
| Body mass index (kg/m2) | 29.2±2.0 | 29.8±2.7 | 0.009 | 0.211 | 29.4±2.0 | 29.7±2.6 | 0.006 | 0.260 |
| Body mass index≥30 | 466 (32.0%) | 426 (33.0%) | 0.011 | 0.207 | 303 (32.1%) | 307 (32.5%) | 0.004 | 0.268 |
| Abdominal circumference (cm) | 98.5±10.1 | 99.9±10.9 | 0.019 | 0.185 | 98.9±10.4 | 99.7±10.7 | 0.007 | 0.233 |
| Diabetes characteristics | ||||||||
| Admission diabetes therapy | 0.065 | 0.051 | 0.035 | 0.101 | ||||
| Monotherapy | 654 (45.0%) | 671 (51.9%) | 444 (47.0%) | 464 (49.2%) | ||||
| Combination of oral agents | 728 (50.1%) | 581 (45.0%) | 448 (47.5%) | 439 (46.5%) | ||||
| Basal insulin | 508 (34.9%) | 388 (30.0%) | 311 (32.9%) | 298 (31.6%) | ||||
| Basal insulin dose (units/day) | 31.1±16.3 | 26.9±14.9 | 29.5±15.6 | 27.4±15.0 | ||||
| Diabetes duration (years) | 17.1±7.7 | 15.1±6.4 | 0.012 | 0.201 | 16.5±7.7 | 15.5±6.9 | 0.008 | 0.229 |
| Admission HbA1c (%) | 7.6±1.2 | 7.4±1.0 | 0.008 | 0.206 | 7.5±1.2 | 7.4±1.0 | 0.006 | 0.259 |
| Patients with HbA1c<7% | 538 (37.0%) | 555 (43.0%) | 0.022 | 0.180 | 368 (39.0%) | 396 (41.9%) | 0.014 | 0.192 |
| Admission BG level (mg/dL) | 166.2±20.5 | 164.0±19.1 | 0.012 | 0.204 | 165.0±20.2 | 164.5±19.4 | 0.009 | 0.214 |
| Previous medical history | ||||||||
| History of smoking | 742 (51.0%) | 710 (55.0%) | 0.052 | 0.174 | 491 (52.0%) | 501 (53.1%) | 0.022 | 0.204 |
| History of alcohol abuse | 290 (19.9%) | 314 (24.3%) | 0.051 | 0.169 | 198 (21.0%) | 214 (22.7%) | 0.032 | 0.198 |
| Hypertension | 1017 (69.9%) | 840 (65.0%) | 0.055 | 0.179 | 641 (67.9%) | 623 (66.0%) | 0.020 | 0.211 |
| Dyslipidaemia | 741 (51.0%) | 620 (48.0%) | 0.049 | 0.165 | 471 (50.0%) | 460 (48.7%) | 0.018 | 0.205 |
| Chronic kidney disease≥3 | 364 (25.0%) | 382 (29.6%) | 0.040 | 0.151 | 246 (26.1%) | 262 (27.8%) | 0.027 | 0.192 |
| Cerebrovascular disease | 189 (13.0%) | 129 (10.0%) | 0.045 | 0.151 | 112 (11.9%) | 105 (11.1%) | 0.010 | 0.252 |
| Chronic obstructive pulmonary disease | 669 (46.0%) | 696 (53.9%) | 0.040 | 0.148 | 463 (49.0%) | 489 (51.8%) | 0.026 | 0.183 |
| Atrial fibrillation | 480 (33.0%) | 387 (30.0%) | 0.051 | 0.162 | 302 (32.0%) | 288 (30.5%) | 0.044 | 0.169 |
| Coronary artery disease | 550 (37.8%) | 529 (40.9%) | 0.042 | 0.154 | 359 (38.0%) | 365 (38.7%) | 0.019 | 0.244 |
| Heart failure | 902 (62.0%) | 904 (70.0%) | 0.039 | 0.149 | 623 (66.0%) | 655 (69.4%) | 0.030 | 0.168 |
| Moderate–severe functional dependence | 430 (29.6%) | 400 (31.1%) | 0.021 | 0.176 | 283 (30.0%) | 288 (30.5%) | 0.017 | 0.206 |
| Moderate–severe dementia | 277 (19.1%) | 207 (16.0%) | 0.033 | 0.152 | 169 (17.9%) | 155 (16.4%) | 0.015 | 0.201 |
| Depression | 203 (14.0%) | 156 (12.1%) | 0.039 | 0.150 | 122 (12.9%) | 119 (12.6%) | 0.010 | 0.244 |
| Polypharmacy | 945 (65.0%) | 801 (62.0%) | 0.025 | 0.191 | 599 (63.4%) | 592 (62.7%) | 0.011 | 0.248 |
| Complex health status | 1045 (71.9%) | 904 (70.0%) | 0.020 | 0.198 | 670 (71.0%) | 661 (70.0%) | 0.009 | 0.267 |
| Admissioncharacteristics | ||||||||
| Primary diagnosis | 0.055 | 0.142 | 0.022 | 0.181 | ||||
| Cardiovascular | 509 (35.0%) | 425 (32.9%) | 320 (33.9%) | 315 (33.4%) | ||||
| Orthopedic | 434 (29.8%) | 388 (30.0%) | 282 (29.9%) | 283 (30.0%) | ||||
| Infectious | 218 (15.0%) | 260 (20.1%) | 157 (16.6%) | 176 (18.6%) | ||||
| Pulmonary | 149 (10.2%) | 101 (7.8%) | 94 (10.0%) | 80 (8.5%) | ||||
| Other | 144 (9.9%) | 108 (8.4) | 91 (9.6%) | 90 (9.5) | ||||
Values are shown as mean±standard deviations, absolute values, and percentages. Standardized difference>10% (>0.1) is considered to represent a non-negligible difference. Values were considered to be statistically significant when p<0.05 in the comparison analysis.
BBI: basal-bolus insulin; BG: blood glucose; cm: centimeter; HbA1c: glycated hemoglobin; kg: kilogram; m2: square meter; mg/dL: milligram/decilitre.
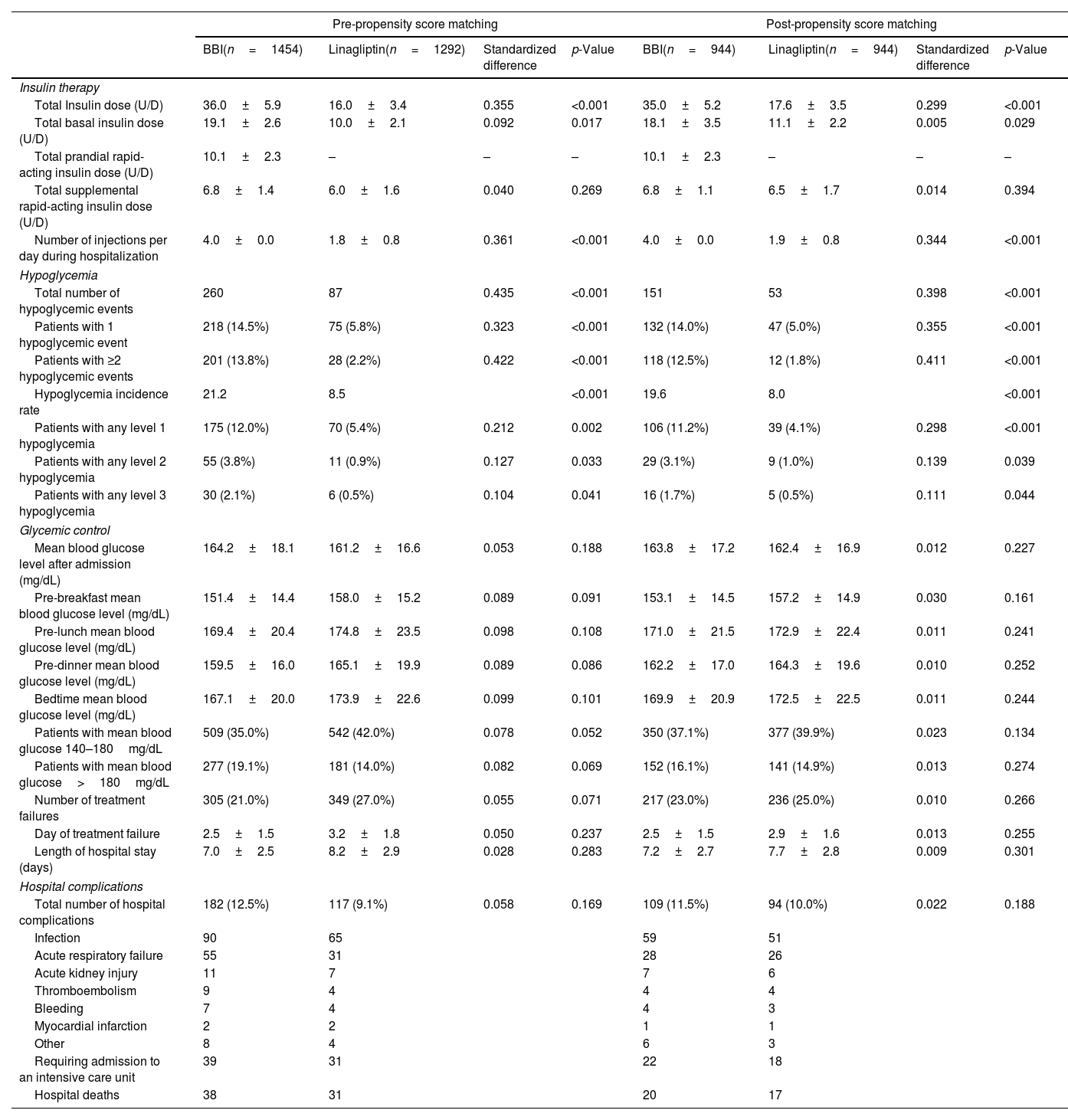
Before and after the PSM, the total and basal insulin doses were significantly lower in the linagliptin group compared with the BBI group. There were no significant differences in the total supplemental rapid-acting insulin doses. In addition, the number of daily injections during the hospital stay were significantly lower for patients in linagliptin group than for patients in BBI group. In regard to hypoglycemic events, patients on the BBI regimen had a higher total number, proportion of patients with 1 or ≥2 events, incidence rate, and proportion of patients who had level 1, level 2, and level 3 hypoglycemia than patients on the linagliptin regimen (Table 2).
Insulin therapy, hypoglycemic events, glycaemic control, length of hospital stay, and hospital complications according to antihyperglycemic regimen: pre- and post-matching analysis.
| Pre-propensity score matching | Post-propensity score matching | |||||||
|---|---|---|---|---|---|---|---|---|
| BBI(n=1454) | Linagliptin(n=1292) | Standardized difference | p-Value | BBI(n=944) | Linagliptin(n=944) | Standardized difference | p-Value | |
| Insulin therapy | ||||||||
| Total Insulin dose (U/D) | 36.0±5.9 | 16.0±3.4 | 0.355 | <0.001 | 35.0±5.2 | 17.6±3.5 | 0.299 | <0.001 |
| Total basal insulin dose (U/D) | 19.1±2.6 | 10.0±2.1 | 0.092 | 0.017 | 18.1±3.5 | 11.1±2.2 | 0.005 | 0.029 |
| Total prandial rapid-acting insulin dose (U/D) | 10.1±2.3 | – | – | – | 10.1±2.3 | – | – | – |
| Total supplemental rapid-acting insulin dose (U/D) | 6.8±1.4 | 6.0±1.6 | 0.040 | 0.269 | 6.8±1.1 | 6.5±1.7 | 0.014 | 0.394 |
| Number of injections per day during hospitalization | 4.0±0.0 | 1.8±0.8 | 0.361 | <0.001 | 4.0±0.0 | 1.9±0.8 | 0.344 | <0.001 |
| Hypoglycemia | ||||||||
| Total number of hypoglycemic events | 260 | 87 | 0.435 | <0.001 | 151 | 53 | 0.398 | <0.001 |
| Patients with 1 hypoglycemic event | 218 (14.5%) | 75 (5.8%) | 0.323 | <0.001 | 132 (14.0%) | 47 (5.0%) | 0.355 | <0.001 |
| Patients with ≥2 hypoglycemic events | 201 (13.8%) | 28 (2.2%) | 0.422 | <0.001 | 118 (12.5%) | 12 (1.8%) | 0.411 | <0.001 |
| Hypoglycemia incidence rate | 21.2 | 8.5 | <0.001 | 19.6 | 8.0 | <0.001 | ||
| Patients with any level 1 hypoglycemia | 175 (12.0%) | 70 (5.4%) | 0.212 | 0.002 | 106 (11.2%) | 39 (4.1%) | 0.298 | <0.001 |
| Patients with any level 2 hypoglycemia | 55 (3.8%) | 11 (0.9%) | 0.127 | 0.033 | 29 (3.1%) | 9 (1.0%) | 0.139 | 0.039 |
| Patients with any level 3 hypoglycemia | 30 (2.1%) | 6 (0.5%) | 0.104 | 0.041 | 16 (1.7%) | 5 (0.5%) | 0.111 | 0.044 |
| Glycemic control | ||||||||
| Mean blood glucose level after admission (mg/dL) | 164.2±18.1 | 161.2±16.6 | 0.053 | 0.188 | 163.8±17.2 | 162.4±16.9 | 0.012 | 0.227 |
| Pre-breakfast mean blood glucose level (mg/dL) | 151.4±14.4 | 158.0±15.2 | 0.089 | 0.091 | 153.1±14.5 | 157.2±14.9 | 0.030 | 0.161 |
| Pre-lunch mean blood glucose level (mg/dL) | 169.4±20.4 | 174.8±23.5 | 0.098 | 0.108 | 171.0±21.5 | 172.9±22.4 | 0.011 | 0.241 |
| Pre-dinner mean blood glucose level (mg/dL) | 159.5±16.0 | 165.1±19.9 | 0.089 | 0.086 | 162.2±17.0 | 164.3±19.6 | 0.010 | 0.252 |
| Bedtime mean blood glucose level (mg/dL) | 167.1±20.0 | 173.9±22.6 | 0.099 | 0.101 | 169.9±20.9 | 172.5±22.5 | 0.011 | 0.244 |
| Patients with mean blood glucose 140–180mg/dL | 509 (35.0%) | 542 (42.0%) | 0.078 | 0.052 | 350 (37.1%) | 377 (39.9%) | 0.023 | 0.134 |
| Patients with mean blood glucose>180mg/dL | 277 (19.1%) | 181 (14.0%) | 0.082 | 0.069 | 152 (16.1%) | 141 (14.9%) | 0.013 | 0.274 |
| Number of treatment failures | 305 (21.0%) | 349 (27.0%) | 0.055 | 0.071 | 217 (23.0%) | 236 (25.0%) | 0.010 | 0.266 |
| Day of treatment failure | 2.5±1.5 | 3.2±1.8 | 0.050 | 0.237 | 2.5±1.5 | 2.9±1.6 | 0.013 | 0.255 |
| Length of hospital stay (days) | 7.0±2.5 | 8.2±2.9 | 0.028 | 0.283 | 7.2±2.7 | 7.7±2.8 | 0.009 | 0.301 |
| Hospital complications | ||||||||
| Total number of hospital complications | 182 (12.5%) | 117 (9.1%) | 0.058 | 0.169 | 109 (11.5%) | 94 (10.0%) | 0.022 | 0.188 |
| Infection | 90 | 65 | 59 | 51 | ||||
| Acute respiratory failure | 55 | 31 | 28 | 26 | ||||
| Acute kidney injury | 11 | 7 | 7 | 6 | ||||
| Thromboembolism | 9 | 4 | 4 | 4 | ||||
| Bleeding | 7 | 4 | 4 | 3 | ||||
| Myocardial infarction | 2 | 2 | 1 | 1 | ||||
| Other | 8 | 4 | 6 | 3 | ||||
| Requiring admission to an intensive care unit | 39 | 31 | 22 | 18 | ||||
| Hospital deaths | 38 | 31 | 20 | 17 | ||||
Values are shown as mean±standard deviations, absolute values, and percentages. Standardized difference of >10% (>0.1) is considered to represent a non-negligible difference. Values were considered to be statistically significant when p<0.05 on the comparison analysis. mg/dL: milligram/decilitre.
Post-PSM, there were no differences between the BBI and linagliptin groups in regard to: (1) mean daily BG levels after admission; mean pre-breakfast, pre-lunch, pre-dinner and bedtime BG levels; (2) patients with a mean blood glucose level 140–180mg/dL and >180mg/dL; and (3) number and day of treatment failure. Before PSM, patients managed with the linagliptin regimen had higher mean BG level after admission and a greater number had average BG levels of 140–180mg/dL than those managed with the BBI regimen. The length of hospital stay was similar in both treatment groups, with a range between five and 12 days (96.1% of patients were hospitalized between five and nine days). The number of hospital complications and mortality rates were also similar when comparing groups. Pre- and post-PSM insulin therapy, hypoglycemic events, glycemic control, length of hospital stay, and hospital complications according to antihyperglycemic regimen are summarized in Table 2.
On the logistic regression analysis, the use of BBI regimen (p<0.001), a higher total insulin dose (p<0.001) and the number of insulin injections (p=0.002) were associated with a greater likelihood of having hypoglycemia. Older age (p<0.001), moderate–severe functional dependence (p=0.011), moderate–severe dementia (p=0.022), polypharmacy (p=0.030), and complex health status (p=0.019) were also risk factors of having hypoglycemic events. Factors associated with the presence of hypoglycemia are shown in Table 3.
Factors associated with the presence of hypoglycemia.
| Odds ratio | 95% confidence interval | p-Value | |
|---|---|---|---|
| BBI regimen | 4.22 | 2.14–6.28 | <0.001 |
| Total insulin dose | 3.55 | 2.02–5.36 | <0.001 |
| Number of insulin injections | 2.86 | 1.50–4.12 | 0.002 |
| Older age | 3.11 | 1.62–4.72 | <0.001 |
| Moderate–severe functional dependence | 2.62 | 1.40–3.89 | 0.011 |
| Moderate–severe dementia | 2.32 | 1.18–3.70 | 0.022 |
| Polypharmacy | 2.14 | 1.08–3.44 | 0.030 |
| Complex health status | 2.44 | 1.10–3.82 | 0.019 |
Logistic regression was used for the multivariate analysis of factors independently associated with the presence of hypoglycemia. Values were considered to be statistically significant when p<0.05.
This study found that the linagliptin regimen simplified the antihyperglycemic management, in terms of a reduction in daily insulin doses and number of daily injections, and was safer than BBI regimen, in terms of fewer hypoglycemic events during hospitalization, in patients aged ≥80 years with T2D. In addition, the linagliptin regimen was non-inferior to the BBI regimen in regard to glycaemic control and there were no differences in treatment failures, length of hospital stay, mortality, or hospital complications. The use of a BBI regimen with a higher total insulin dose and number of insulin injections, the presence of older age, moderate–severe functional dependence, moderate–severe dementia, polypharmacy, and complex health status were associated with a higher risk of hypoglycemia.
In most instances, the BBI regimen is the preferred treatment for noncritically ill hospitalized patients with T2D because it has been shown to be more effective in terms of improving glycemic control and reducing complications than a sliding scale insulin regimen.3–5 However, the intensive antihyperglycemic therapy is complex, requiring insulin's combinations with several daily injections, and has been associated with drug-induced hypoglycemia, which occur in up to 30% of hospitalized patients3,6 especially in older adult patients.2,18,19
In recent years, different DPP-4i alone or in combination with basal insulin have demonstrated efficacy and safety for the management of mid-to-moderate hyperglycemia in the hospital setting.7–15 However, limited data is available specifically on older adult patients. Recently, greater benefits were reported with the use of a regimen that included linagliptin in combination with basal insulin compared to a BBI regimen in patients aged≥75 years with T2D in terms of reductions in hypoglycemic events and treatment simplification, with no differences in glycemic control during the hospitalization.15 This study provides more data on safety and effectiveness in a larger population followed-up on for a longer period of time and, furthermore, it focuses specifically on very old patients (aged≥80 years), comparing a linagliptin regimen with a BBI regimen in real-world clinical practice. Reductions in total and basal insulin doses and number of daily injections were observed in the linagliptin group compared to the BBI group. In addition, patients on the linagliptin regimen had a lower total number of hypoglycemic events, proportion of patients with 1 or ≥2 events, incidence rate, and proportion of patients with a level 1, level 2, and level 3 hypoglycemia than patients on the BBI regimen. A reduction in hypoglycemic events was also observed in other studies on linagliptin use in hospitalized patients with T2D undergoing non-cardiac surgery10,13 and patients with T2D hospitalized due to heart failure.14 In a report of pooled data from 2 prospective studies using DPP-4i (sitagliptin and linagliptin) in general medicine and surgery patients with T2D, the use of DPP-4i alone or in combination with basal insulin resulted in a lower incidence of hypoglycemia compared to BBI regimen.11 In this study focused on very-old patients, the reduction in hypoglycemic events was observed in all recorded items of hypoglycemia, including the severest forms, and in a greater proportion of patients than in previous studies. These differences between studies may be explained as a result of differences in study design and mean age. Patients in real-world studies and older age were more likely to have hypoglycemic events with BBI.12–15
Hypoglycemia is a known clinically limiting complication in patients with T2D during hospitalization.19,20 Only a few studies have been specifically focused on identification of in-hospital hypoglycemia-associated factors in T2D.21–23 Insulin has been described as the drug with the greatest risk of hypoglycemia, mainly associated with the use of complex insulin combinations that included rapid-acting insulin.3 Furthermore, several patient-related factors have been identified as potential risk factors for hypoglycemia,23 including the advanced age, multiple coexisting chronic illnesses, cognitive impairment, functional dependence, and polypharmacy.24 These findings confirm that the use of BBI regimen with a higher total insulin dose and number of insulin injections, older age, moderate–severe functional dependence, moderate–severe dementia, polypharmacy, and complex health status were associated with a higher risk of in-hospital hypoglycemia.
In order to achieve and maintain glycemic control and prevent both hyper- and hypoglycemia in very old patients during hospitalization, a comprehensive clinical assessment should be performed to determine goals and treatment options.3,25 The evidence found in previous studies7–15,26 has indicated that the use of a DPP-4i alone or in combination with basal insulin may be preferable for managing non-critical T2D patients with mild-to-moderate hyperglycaemia who are not treated at home with high doses of insulin or have no severe complications. According to this study's results, this recommendation should be strongly extended to older adult patients and specially in those with functional dependence, dementia, polypharmacy, or complex health status in order to implement a simpler antihyperglycemic regimen and safeguard patient safety. Although evidence on the role of DDP-4i in older adult patients in the hospital setting is still limited, it has been found to be adequate and safe for treating older adult outpatients with T2D due to their very low risk of hypoglycemia and drug interactions.27–29
The potential beneficial aspects of linagliptin as opposed to insulin therapy for hospitalized very old patients with T2D could include the role of incretin-based therapies on stimulation of insulin secretion and reduction of glucagon secretion, the increase of postprandial incretins as glucagon-like peptide-1 that could potentially affect glucose regulation through multiple effects, such as a delay in gastric emptying and a decrease in caloric intake likely secondary to centrally mediated signaling. These benefits could also be influenced by the patients’ insulin secretion reserve.30 In our real-world study, the C-peptide level was not recorded due to the lack of its routine determination.
Although these findings on the benefits of a linagliptin regimen are important because they support the use of a simpler and safer in-hospital antihyperglycemic regimen in order to prevent hypoglycemic events in very old adult patients, they should be examined within the context of potential limitations. First, despite the large number of patients included and the use of a PSM, the possibility of unmeasured confounding factors cannot be ruled out due to the retrospective nature of the data. Second, an antihyperglycemic protocol was used that is not fully implemented in our area. Indeed, a large proportion of physicians managed hospitalised T2D patients using BBI regardless of the baseline patients’ characteristics. Third, it is not fully known if the benefits of good glycaemic control during hospitalization are due to improvements in BG levels or due to the direct effect of the medication regardless of BG. Fourth, the local protocol only includes the use of linagliptin due to the fact that dose titration according to renal function is not required and the evidence on safety reported on older adults.29,31,32 Thus, these findings cannot be extrapolated to other DPP-4i. Fifth, a group of patients who use basal insulin alone was not included. This option may also be a safer and simpler alternative than BBI in hospitalized patients with high risk of hypoglycemia. Finally, only very old patients (age≥80 years old) were included, although the reduction in hypoglycemic events could be extrapolated to younger age ranges or other subgroups of T2D patients at high risk of hypoglycemia, such as those with functional dependence, dementia, or complex health status.28 Further research with large, prospective randomized studies is required to confirm these results and to provide more evidence.
In conclusion, this study found that a linagliptin regimen simplified the in-hospital antihyperglycemic management, requiring lower daily insulin doses and fewer injections, and was safer than BBI regimen, reducing hypoglycemic events in patients aged≥80 years with T2D. In addition, linagliptin regimen was non-inferior to BBI regimen in regard to glycaemic control. The BBI regimen, older age, moderate–severe functional dependence, moderate–severe dementia, polypharmacy, and complex health status were associated with a higher risk of having hypoglycemia. The linagliptin regimen could be considered as standard of care for older adult T2D patients in the hospital setting.
Authors’ contributionsThe authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Miguel A. Pérez-Velasco: analysis and interpretation of data and preparation of manuscript. Julio Osuna-Sánchez: analysis and interpretation of data and preparation of manuscript. Mercedes Millán-Gómez: acquisition of subjects and data, analysis and interpretation of data and preparation of manuscript. Michele Ricci: analysis and interpretation of data and preparation of manuscript. Almudena López-Sampalo: analysis and interpretation of data and preparation of manuscript. M. Rosa Bernal-López: analysis and interpretation of data and preparation of manuscript. Ricardo Gómez-Huelgas: concept and design, analysis and interpretation of data and preparation of manuscript. Luis M. Pérez-Belmonte: concept and design, acquisition of subjects and data, analysis and interpretation of data and preparation of manuscript. All authors have participated in the writing and/or revision of the manuscript and accept its publication.
Ethics approval and consent to participateThe study was approved by the Institutional Research Ethics Committee of Málaga (Ethics Committee code: REDIME-27-10-2016). Written informed consent for the consultation of medical records was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki. Data confidentiality and patient anonymity were upheld at all times.
Declaration of Generative AI and AI-assisted technologies in the writing processNo AI technology was used in the writing process by the authors.
Sponsor's roleThis research was funded by grants from Internal Medicine Department, cofounded by the Fondo Europeo de Desarrollo Regional-FEDER (“Centros de Investigación En Red” (CIBER, CB06/03/0018)) and the Spanish Society of Cardiology (Proyectos de Investigación en Servicios de Salud de Cardiología de la Sociedad Española de Cardiología). The authors have not received any fees related to the development of the manuscript. Open Access fees has been funded by the Asociación Instituto Malagueño de Investigación Clínica, through an unrestricted grant from Boehringer Ingelheim Spain. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Conflict of interestThe authors have no conflicts.