In recent years, many studies have investigated metformin and its effects on lung cancer. However, since previous studies have shown that the relationship between metformin and lung cancer is complicated, we performed a meta-analysis to analyze this relationship.
Material and methodsAn electronic database search was conducted using PubMed, Embase, and Cochrane library. Outcomes were quantified with hazard ratios and 95% confidence intervals to compare lung cancer survival in patients treated with or without metformin.
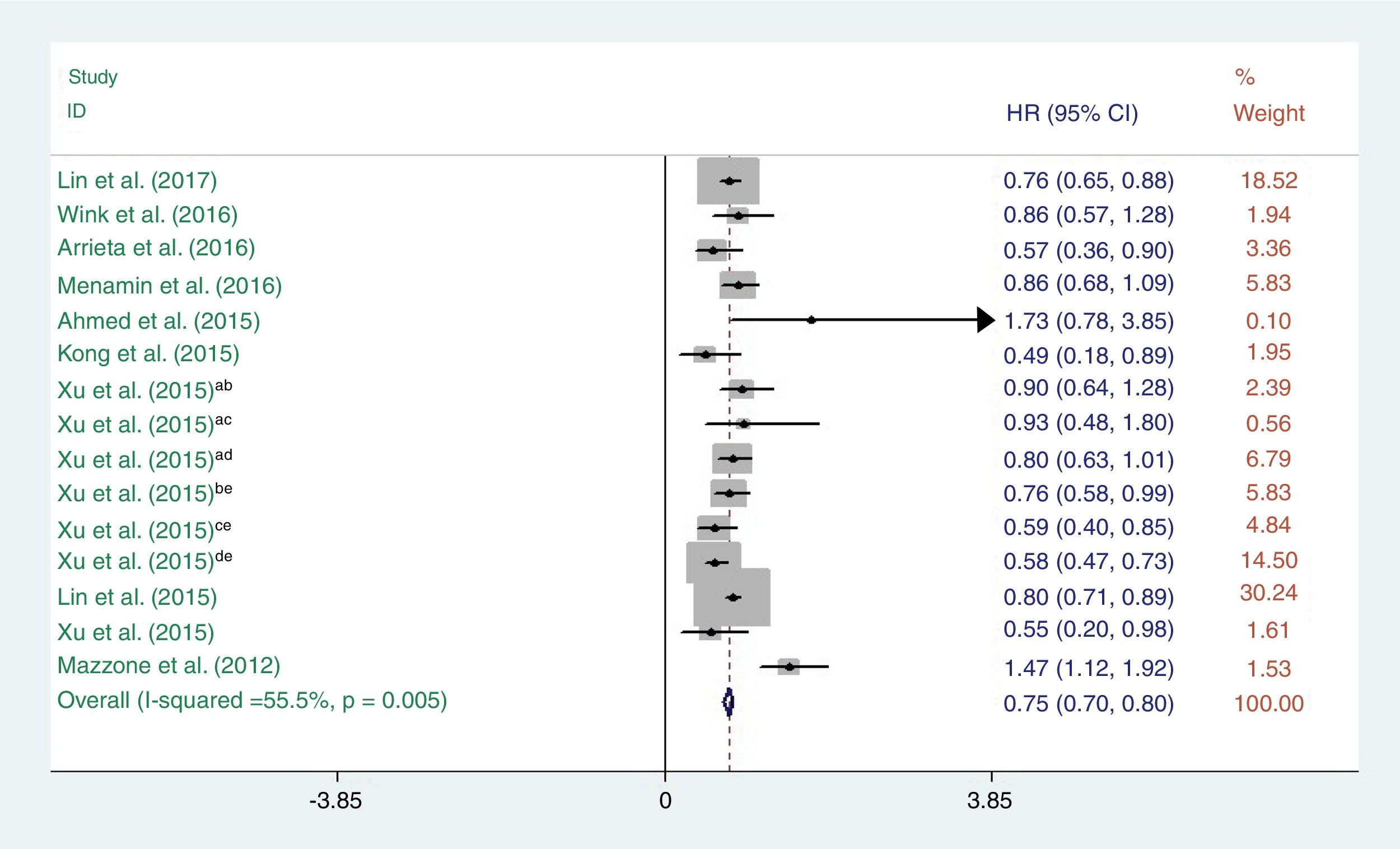
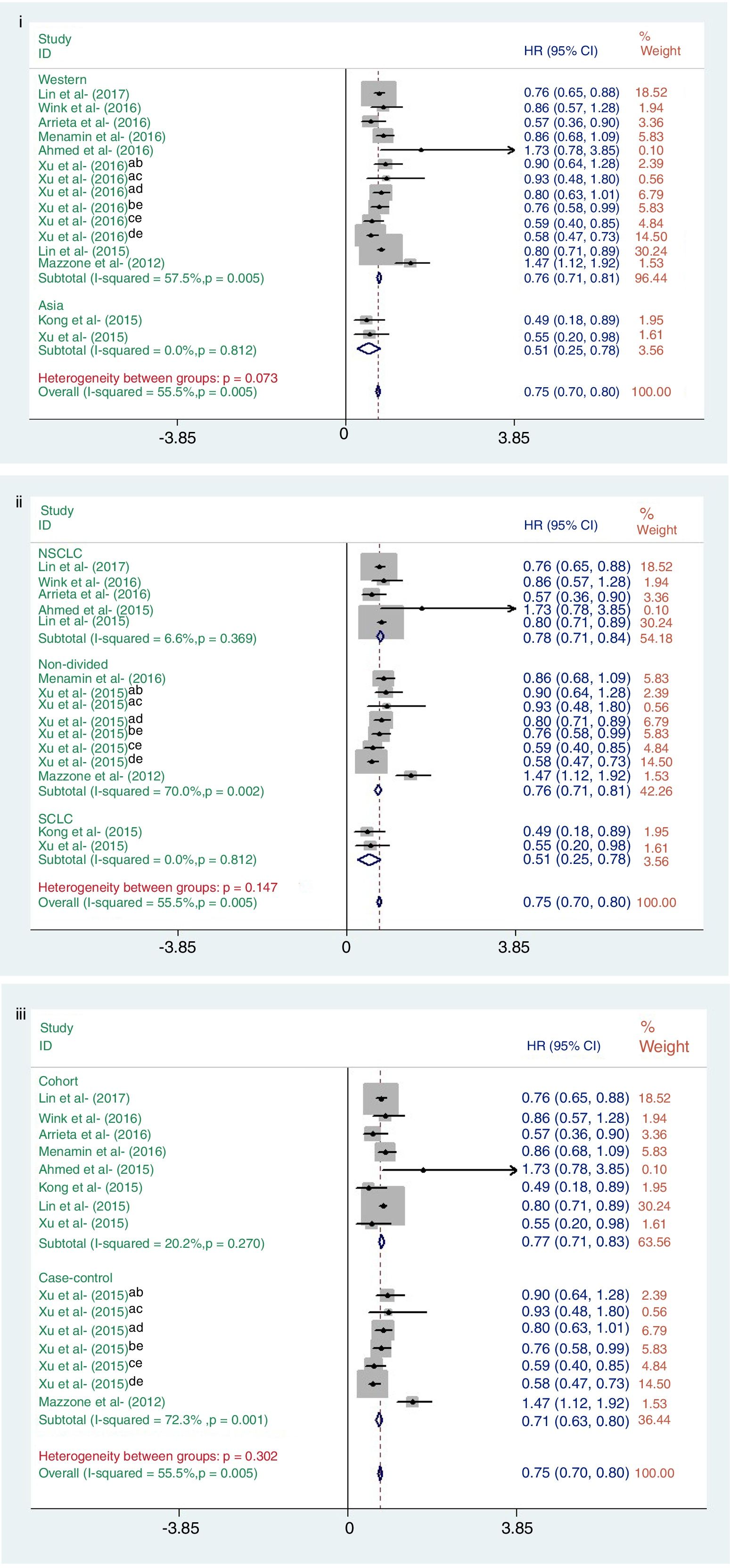
ResultsTen studies, involving 4397 participants, were included. In the pooled analysis, we found that metformin treatment significantly improved the survival of lung cancer patients (hazard ratio=0.75, 95% confidence interval: 0.70–0.80; P<0.001). Subgroup analysis showed that when stratified by geographic region, the hazard ratios for overall survival were 0.76 (95% confidence interval: 0.71–0.81, P<0.001) and 0.51 (95% confidence interval: 0.25–0.78, P<0.001) for Western and Asian countries, respectively. When stratified by lung cancer subtype, the hazard ratios for overall survival were 0.78 (95% confidence interval: 0.71–0.84, P<0.001), 0.73 (95% confidence interval: 0.66–0.81, P<0.001), and 0.51 (95% confidence interval: 0.25–0.78, P<0.001) for non-divided, non-small cell, and small-cell lung cancer subtypes, respectively. When stratified by study design, the hazard ratios for overall survival were 0.77 (95% confidence interval: 0.71–0.83, P<0.001) and 0.71 (95% confidence interval: 0.63–0.80, P<0.001) for cohort-based and case–controlled studies, respectively.
En los últimos años, muchos estudios han investigado los efectos de la metformina sobre el cáncer de pulmón. Sin embargo, dado que estudios previos habían demostrado que la relación entre la metformina y el cáncer de pulmón era complicada, realizamos un metaanálisis para estudiar esta relación.
Materiales y métodosSe realizó una búsqueda en las bases de datos electrónicas PubMed, Embase y Cochrane Library. Los resultados se cuantificaron con cociente de riesgos instantáneos e intervalos de confianza del 95% para comparar la supervivencia del cáncer de pulmón en pacientes tratados con o sin metformina.
ResultadosSe incluyeron 10 estudios con un total de 4.397 participantes. En el análisis combinado, el tratamiento con metformina mejoró significativamente la supervivencia de los pacientes con cáncer de pulmón (cociente de riesgos instantáneos=0,75; intervalo de confianza del 95%: 0,70-0,80; p<0,001). El análisis de subgrupos mostró que cuando se estratificó por región geográfica, los cocientes de riesgos para la supervivencia global fueron de 0,76 (intervalo de confianza del 95%: 0,71-0,81; p<0,001) y 0,51 (intervalo de confianza del 95%: 0,25-0,78; p<0,001) para los países occidentales y asiáticos, respectivamente. Cuando se estratificó por subtipo de cáncer de pulmón, los cocientes de riesgo para la supervivencia global fueron de 0,78 (intervalo de confianza del 95%: 0,71-0,84; p<0,001), de 0,73 (intervalo de confianza del 95%: 0,66-0,81; p<0,001) y de 0,51 (intervalo de confianza del 95%: 0,25-0,78; p<0,001) para subtipos de cáncer de pulmón no microcítico, de células no pequeñas y de células pequeñas, respectivamente. Cuando se estratificó según el diseño del estudio, los cocientes de riesgo para la supervivencia global fueron de 0,77 (intervalo de confianza del 95%: 0,71-0,83; p<0,001) y de 0,71 (intervalo de confianza del 95%: 0,63-0,80; p<0,001) para estudios de cohortes y de casos-controles, respectivamente.
Lung cancer is the number one cause of cancer deaths worldwide.1 Many clinical epidemiologic factors, such as cigarette smoking, air pollution, pulmonary tuberculosis, exposure to carcinogens, and familial history of lung cancer, have been identified2–3 Despite the rapid development of diagnosis and treatment modalities, the prognosis of lung cancer remains poor, with a 5-year survival of only about 15%.4 Therefore, there is an urgent need to find treatments that have a therapeutic effect on lung cancer.
Diabetes is a common and devastating chronic disease, with a projected global population of 366 million having this disease by 2030.5 With an aging population, the number of cancer patients with diabetes is on the rise. Increasing epidemiological evidence suggests that there is an association between diabetes mellitus (DM) and various cancers. Early epidemiological studies suggest that diabetes is not associated with the development of lung cancer; however, several recent studies suggest that diabetes is significantly associated with the development of lung cancer.6,7
Metformin, an oral anti-diabetic drug that is less expensive than any anti-cancer agent that is currently in routine use, first attracted attention in 2005 for its potential to suppress not only serum glucose levels, but also the incidence of various cancers in an observational study.8 Metformin has subsequently been investigated as an anti-cancer medicine for malignancies, including lung cancer, in diabetic and non-diabetic subjects. In recent years, an increasing number of studies have investigated metformin and lung cancer, with the results showing that metformin may improve chemotherapy outcomes and survival for patients who have NSCLC with diabetes.9,10 However, Medairos et al. reported that Metformin exposure in diabetic patients with early-stage non-small cell lung cancer may be associated with improved progression-free survival, but no effect was seen on overall survival.11 Based on these studies, the relationship between metformin and lung cancer is complicated.
In order to elucidate the relationship between metformin and lung cancer, we conducted a meta-analysis of the existing relevant observational studies in the literature and systematically re-evaluated the impact of metformin on the survival outcomes of diabetic patients.
Materials and methodsLiterature searchAn electronic search of the Medical literature was conducted for articles with dates of publication up to 27 July 2017, in the following databases: PubMed, Embase, and Cochrane library. The search was without language restrictions and independently checked by two of the authors. We developed a search strategy using the following terms: “Metformin” or “Dimethylbiguanidine” or “Dimethylguanylguanidine” or “Glucophage” or “Metformin Hydrochloride” or “Hydrochloride, Metformin” or “Metformin HCl” or “HCl, Metformin” and “lung neoplasms” or “Pulmonary Neoplasms” or “Neoplasms, Lung” or “Lung Neoplasm” or “Neoplasm, Lung” or “Neoplasms, Pulmonary” or “Neoplasm, Pulmonary” or “Pulmonary Neoplasm” or “Lung Cancer” or “Cancer, Lung” or “Cancers, Lung” or “Lung Cancers” or “Pulmonary Cancer” or “Cancer, Pulmonary” or “Cancers, Pulmonary” or “Pulmonary Cancers” or “Cancer of the Lung” or “Cancer of Lung”. In addition, we screened the bibliographies of selected original studies and review articles to identify any other relevant studies that were not retrieved through the initial database search.
Selection criteriaStudies, or subsets of studies, were included if they satisfied all the following criteria: (a) randomized controlled clinical trials, data-based, or cohort studies; (b) studies evaluating the effect of metformin on survival of lung cancer in patients with diabetes; and, (c) studies reporting the hazard ratios (HRs) with corresponding 95% confidence intervals (CIs), or providing sufficient data to calculate these values. If more than one paper was based on the same study, we included the one which provided the most abundant information or the one containing the largest number of cases. Studies were excluded if they were published as letters, editorials, reviews, cell-line studies, animal studies, and studies without controls.
Exclusion criteriaTwo authors performed the data extraction independently. For the eligible studies, collected data included the following: (1) basic information of each study, such as author names, year of publication, country or area of origin, and study design; (2) characteristics of patients, such as median or mean age, gender composition, and tumor type; (3) information of study designation, such as the number of enrolled subjects, group sample size, and follow-up time; and, (4) results of treatment, such as overall survival (OS), median disease-free survival (DFS), or progression-free survival (PFS) and adjusted HRs with the associated 95% CIs.
Data extraction quality assessmentTo assess the quality of the included studies, the Newcastle-Ottawa Scale (NOS) was applied to this meta-analysis.12 We evaluated the included studies based on selection of participants, comparability of participants, and ascertainment of outcomes, with the methodological quality then being scored. Quality assessment was independently conducted by two authors, with third party assessment (Liu) being performed when necessary.
Statistical analysesTo assess the association between metformin treatment and the survival of patients with lung neoplasms, summary HRs with their corresponding 95% CIs were calculated through meta-analysis. Heterogeneity analysis was performed using the Cochran Q statistic and the I2 statistic. Statistical significance for heterogeneity was considered if P<0.05 or I2>50%. A fixed-effects model was applied when P>0.05 and I2<50%, while a random-effects model was chosen when P<0.05 or I2>50%. To identify the sources of heterogeneity, we performed subgroup analyses. In addition, we conducted a sensitivity analysis by removing one study at a time and re-calculating the pooled effects. Potential publications bias (considered present if P≤0.1) was assessed by conducting statistical tests for funnel plot asymmetry, as well as Egger's test and Begg's test. All statistical analyses were conducted using Stata version 12.0 software (Stata Corp., College Station, TX, USA).
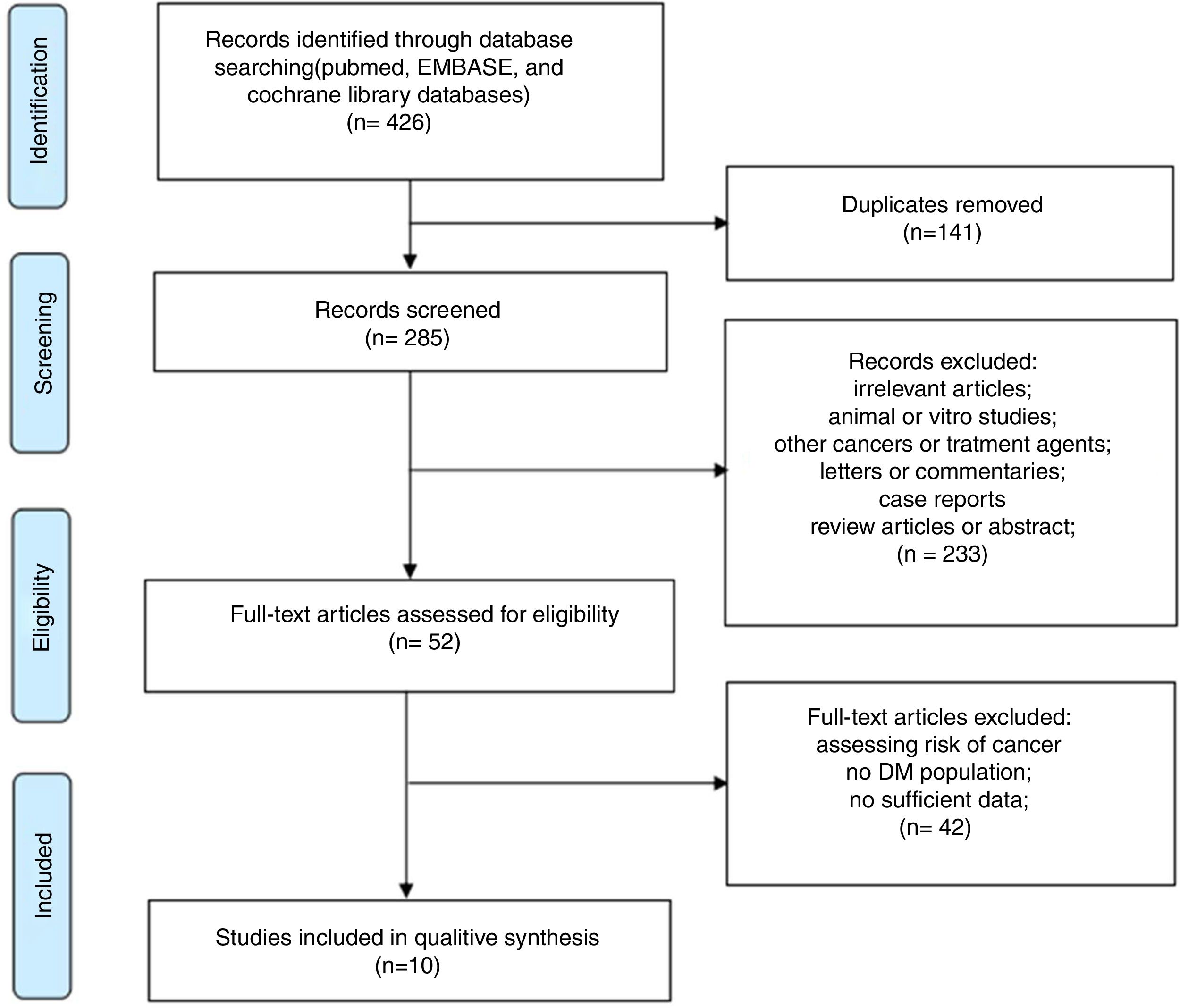
ResultsLiterature searchOur detailed study selection process is described in Fig. 1. The literature search identified 426 potentially relevant articles, with 141 papers being excluded because of duplicates. After screening titles and abstracts, 233 articles were excluded because they were animal or in vitro studies, involved other cancers or treatment agents, or were letters, commentaries, case reports, review articles, or Abstracts. After reading the full text of the remaining 52 articles, 42 were excluded due insufficient data. Finally, 10 studies,13–22 including a total of 4397 patients, were included in the current meta-analysis. The current meta-analysis was conducted in accordance with PRISMA guidelines.23
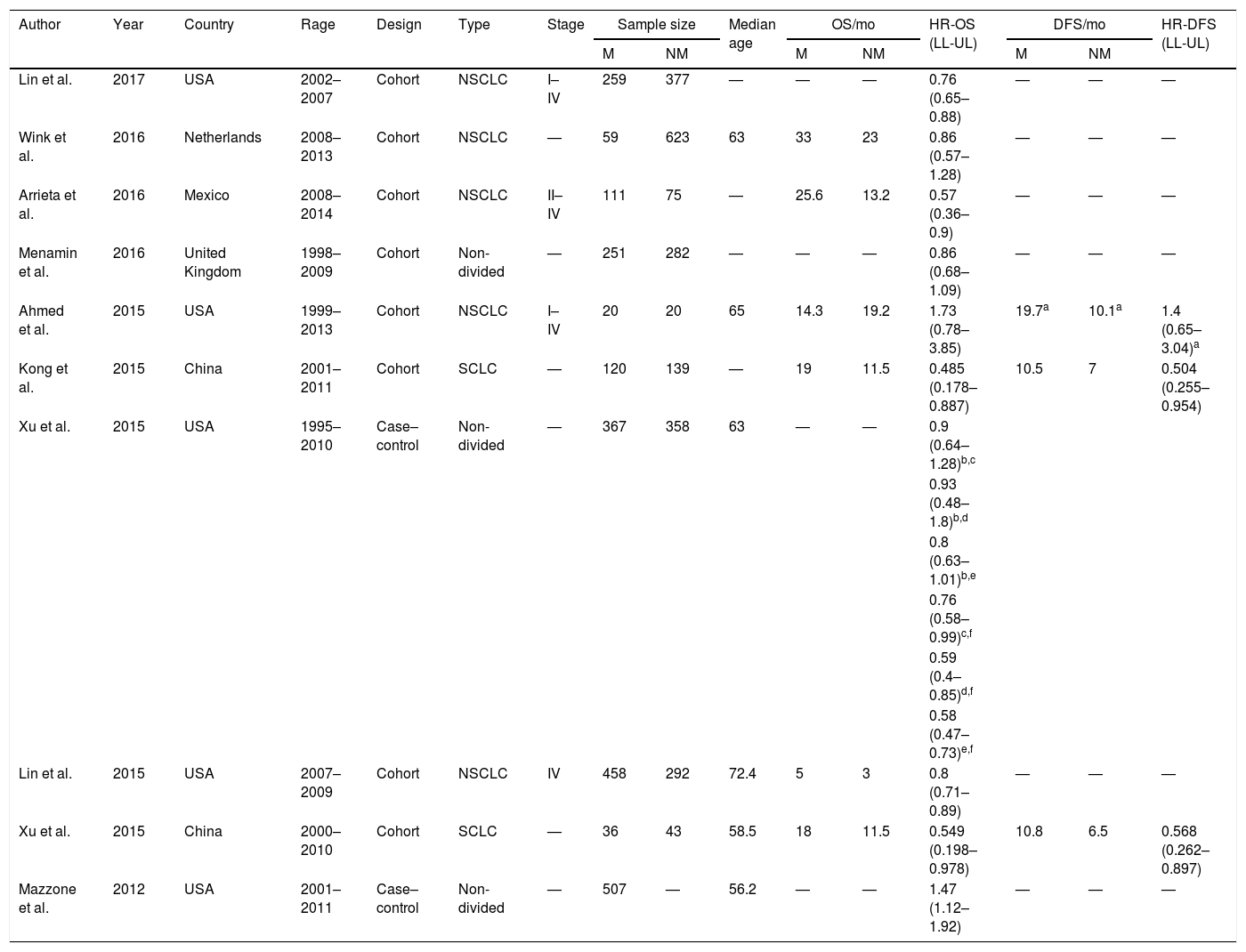
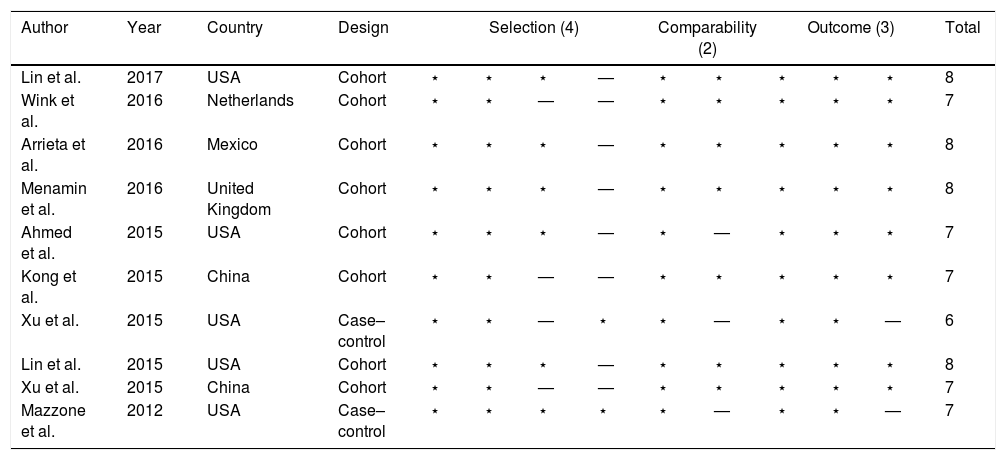
Characteristics of the included studiesThe baseline characteristics of each study are shown in Table 1. These 10 studies were all retrospective, including 8 cohort studies and 2 case–controlled studies. Most of these were published very recently between 2015 and 2017, and originated from China, the USA, Netherlands, Mexico, and the United Kingdom. All subtypes of lung cancer, including small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with stage I–IV disease were involved. The median age of the enrolled patients ranged from 56.2 to 72.5 years. The NOS score of the selected studies ranged from 6–8 stars on the scale, which suggested moderate to high quality studies (Table 2).
The baseline characteristics of each study.
| Author | Year | Country | Rage | Design | Type | Stage | Sample size | Median age | OS/mo | HR-OS (LL-UL) | DFS/mo | HR-DFS (LL-UL) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | NM | M | NM | M | NM | ||||||||||
| Lin et al. | 2017 | USA | 2002–2007 | Cohort | NSCLC | I–IV | 259 | 377 | — | — | — | 0.76 (0.65–0.88) | — | — | — |
| Wink et al. | 2016 | Netherlands | 2008–2013 | Cohort | NSCLC | — | 59 | 623 | 63 | 33 | 23 | 0.86 (0.57–1.28) | — | — | — |
| Arrieta et al. | 2016 | Mexico | 2008–2014 | Cohort | NSCLC | II–IV | 111 | 75 | — | 25.6 | 13.2 | 0.57 (0.36–0.9) | — | — | — |
| Menamin et al. | 2016 | United Kingdom | 1998–2009 | Cohort | Non- divided | — | 251 | 282 | — | — | — | 0.86 (0.68–1.09) | — | — | — |
| Ahmed et al. | 2015 | USA | 1999–2013 | Cohort | NSCLC | I–IV | 20 | 20 | 65 | 14.3 | 19.2 | 1.73 (0.78–3.85) | 19.7a | 10.1a | 1.4 (0.65–3.04)a |
| Kong et al. | 2015 | China | 2001–2011 | Cohort | SCLC | — | 120 | 139 | — | 19 | 11.5 | 0.485 (0.178–0.887) | 10.5 | 7 | 0.504 (0.255–0.954) |
| Xu et al. | 2015 | USA | 1995–2010 | Case– control | Non- divided | — | 367 | 358 | 63 | — | — | 0.9 (0.64–1.28)b,c | |||
| 0.93 (0.48–1.8)b,d | |||||||||||||||
| 0.8 (0.63–1.01)b,e | |||||||||||||||
| 0.76 (0.58–0.99)c,f | |||||||||||||||
| 0.59 (0.4–0.85)d,f | |||||||||||||||
| 0.58 (0.47–0.73)e,f | |||||||||||||||
| Lin et al. | 2015 | USA | 2007–2009 | Cohort | NSCLC | IV | 458 | 292 | 72.4 | 5 | 3 | 0.8 (0.71–0.89) | — | — | — |
| Xu et al. | 2015 | China | 2000–2010 | Cohort | SCLC | — | 36 | 43 | 58.5 | 18 | 11.5 | 0.549 (0.198–0.978) | 10.8 | 6.5 | 0.568 (0.262–0.897) |
| Mazzone et al. | 2012 | USA | 2001–2011 | Case– control | Non- divided | — | 507 | — | 56.2 | — | — | 1.47 (1.12–1.92) | — | — | — |
M, metformin use group; N-M, non-metformin use group; OS, overall survival; HR, hazard ratio; DFS, disease-free survival; y, year; mo, month; LL, lower limit; UL, upper limit; SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
The quality of the 10 included studies appraised in reference to the Newcastle-Ottawa statement.
| Author | Year | Country | Design | Selection (4) | Comparability (2) | Outcome (3) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lin et al. | 2017 | USA | Cohort | ⋆ | ⋆ | ⋆ | — | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Wink et al. | 2016 | Netherlands | Cohort | ⋆ | ⋆ | — | — | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Arrieta et al. | 2016 | Mexico | Cohort | ⋆ | ⋆ | ⋆ | — | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Menamin et al. | 2016 | United Kingdom | Cohort | ⋆ | ⋆ | ⋆ | — | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Ahmed et al. | 2015 | USA | Cohort | ⋆ | ⋆ | ⋆ | — | ⋆ | — | ⋆ | ⋆ | ⋆ | 7 |
| Kong et al. | 2015 | China | Cohort | ⋆ | ⋆ | — | — | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Xu et al. | 2015 | USA | Case–control | ⋆ | ⋆ | — | ⋆ | ⋆ | — | ⋆ | ⋆ | — | 6 |
| Lin et al. | 2015 | USA | Cohort | ⋆ | ⋆ | ⋆ | — | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 8 |
| Xu et al. | 2015 | China | Cohort | ⋆ | ⋆ | — | — | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | 7 |
| Mazzone et al. | 2012 | USA | Case–control | ⋆ | ⋆ | ⋆ | ⋆ | ⋆ | — | ⋆ | ⋆ | — | 7 |
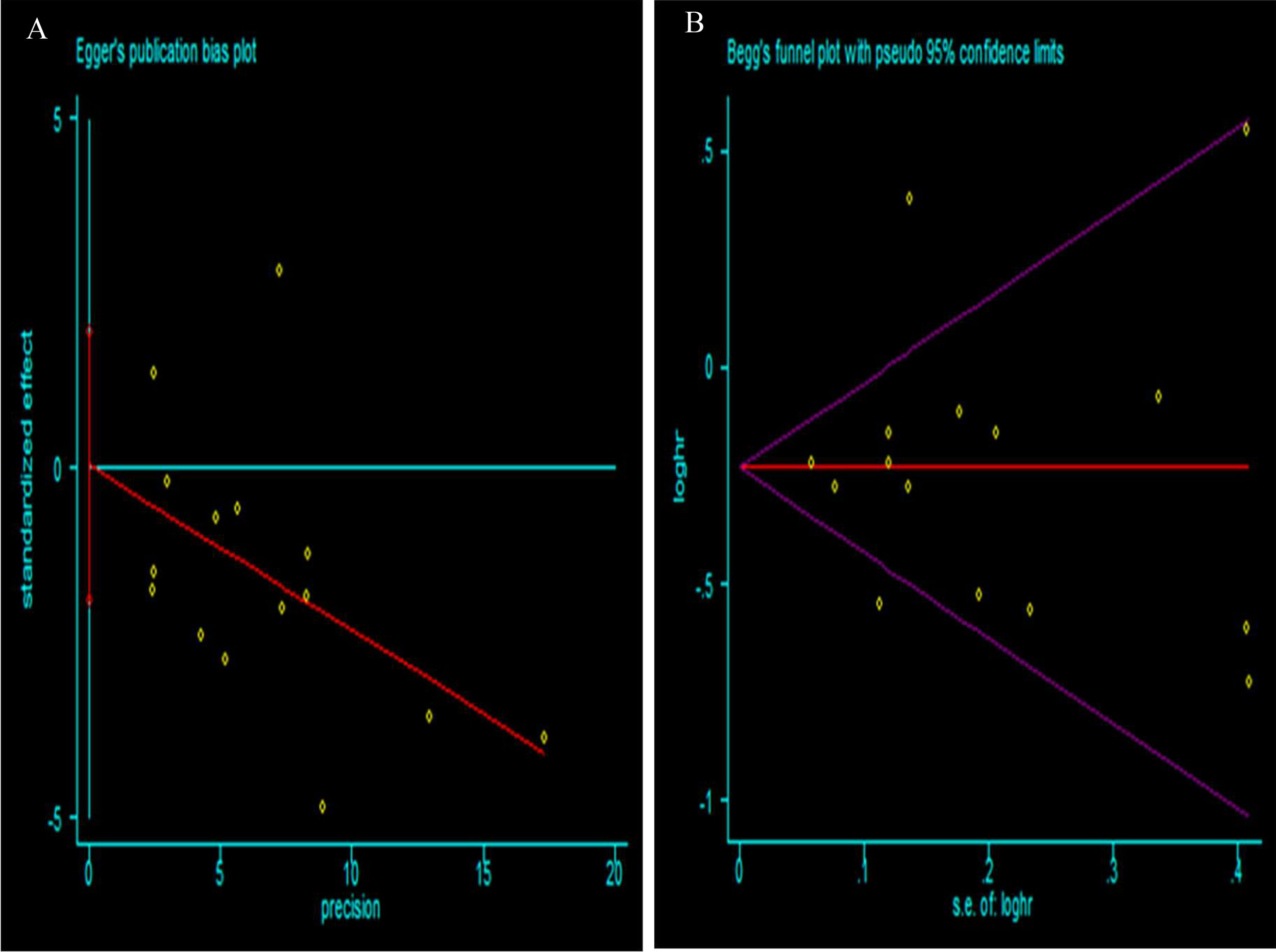
To assess the possibility of publication bias among the studies, funnel plots were generated. No evidence of asymmetry in the funnel plot was detected, indicating that there was no obvious publication bias in our study, which was also supported by the Egger's test and Begg's test (Fig. 2).
Metformin use and survival of lung cancerA total of 10 studies reporting OS were incorporated into this meta-analysis. The heterogeneity test indicated that a fixed-effect model could be selected (I2=55.5%, P=0.005). The pooled results showed that there was a significant difference in OS between lung cancer patients who did and did not receive metformin (HR=0.75, 95% CI: 0.70–0.80; P<0.001) (Fig. 3).
Subgroup analysisSubgroup analysis was adopted to explore the causes of heterogeneity for the analysis of OS (Fig. 4). The analyzed studies were stratified to evaluate OS according to geographic region, study design, and lung cancer subtype.
Subgroup analysis result of OS: (i) geographic region (West or Asian); (ii) lung cancer subtype (NSCLC, non-divided, or SCLC); (iii) study design (cohort or case–control study). Data from: (a) Vanderbilt University Medical Center; (b) metformin vs. other drug; (c) metformin vs. insulin; (d) metformin vs. none; (e) Mayo Clinic medical data.
In this analysis, the studies from Western countries showed that metformin was associated with reduced mortality risk, as well as in Asian countries. For lung cancer subtype, the relative survival benefit associated with metformin remained for all subtypes. When analyzing study design, both cohort-based and case–controlled studies were significant in terms of OS and metformin use). However, the heterogeneity of Western countries, non-divide group and case–control studies were significant (I2=57.7%, P<0.001; I2=70.0%, P<0.001; I2=72.3%, P<0.001; respectively) (Fig. 4).
Sensitivity analysisConsidering the large variations across studies, we performed sensitivity analysis by the sequential omission of individual studies to recalculate the pooled HR, and none of the exclusions of a specific study would substantially alter the trend of our primary pooled results; this showed a significant association of metformin administration and improved OS.
DiscussionIn this study, we analyzed the association of metformin use with the survival of lung cancer using a meta-analysis. To our knowledge, this is the first meta-analysis providing comprehensive insights into the effects of metformin treatment and the survival of lung cancer. This meta-analysis included a pooled analysis of 8 cohort studies and 2 case–control studies, which investigated the effect of metformin use on the survival of lung cancer patients. We found that, metformin treatment significantly improved the survival of lung cancer patients (HR=0.75, 95% CI: 0.70–0.80; P<0.001). In the sub-group analysis, metformin use was significantly associated with good prognosis in patients with lung cancer, independent of tumor type and region.
Diabetes and malignant tumors are common diseases, where the development of urbanization and industrialization has led to a yearly increase in incidence, which seriously affects the health of large populations. There are many common risk factors for diabetes and lung cancer. First, smoking is an important risk factor for lung cancer. Studies have shown that smoking can also increase the risk of diabetes.24 Second, lung cancer and diabetes are age-related diseases. Third, epidemiological studies have shown that vitamin D levels are negatively correlated with the occurrence of lung cancer25 Lucato et al. published a meta-analysis that demonstrated vitamin D supplementation can improve insulin sensitivity and reduce the risk of diabetes.26 The classical pathways shared by diabetes mellitus and cancer are the insulin/IGF-axis. Hyperglycemia is a feature of diabetes mellitus, and Warburg first hypothesized that hyperglycemia itself might have carcinogenic potential.27 Direct carcinogenic effects of hyperglycemia, combined with the Wnt signaling pathway, were recently proposed as promoting carcinogenesis.28
Metformin has been administered in routine clinical use for more than 50 years. It is one of the most widely used oral hypoglycemic agents in the world. In recent years, although a number of new hypoglycemic drugs have been implemented, metformin is still the world's most used classic oral hypoglycemic agent. Evans et al.12 found that metformin was associated with a reduced cancer risk and observed that with increasing use, the protective effect of metformin gradually increased. However, the specific mechanism underlying the anti-tumor effect of metformin remains unknown, with most studies focusing on the AMP activated protein kinase (AMPK) signaling pathway. Many studies have shown that metformin can activate the AMPK pathway, which may not only affect metabolism, but also inhibit the occurrence and development of tumors.29–32 In addition, Metformin reduces insulin levels in the circulatory system, reduces insulin-like growth factor binding protein levels, and reduces the synthesis and secretion of the IGF-1, which plays a role in the metabolic regulation and proliferative inhibition of lung cancer cells.33
The current meta-analysis has several strengths. First, the major strength of this study is that we comprehensively assessed the effects of metformin use on lung cancer, using survival as the primary outcome. Second, the sensitivity analysis did not show that a single study influenced the pooled results and no publication bias was detected. Given these characteristics, this meta-analysis is a comprehensive study of the effect of metformin on lung cancer.
However, several limitations in our study should be acknowledged. First, 2 of the studies included in this meta-analysis did not report adjustments, while the adjustments of the other 8 studies were either inconsistent or incomplete. Many other confounders, such as gender ratio of the subjects, cumulative dose, use of concomitant medications, and time-related bias, were not controlled. These aspects are important in understanding the role of metformin. Second, patients with small-cell lung cancer were all from Asian populations. Therefore, we do not know whether metformin's role in these populations is related to ethnicity. The average age of the subjects in this study was between 56.2 and 74.5. Therefore, we do not know whether metformin is consistent with this study in those who are younger than 54.2 years old or older than 74.5 years old. In other words, we do not know whether age affects the effect of metformin on lung cancer. Finally, there is some heterogeneity between lung cancer survival rates (I2=55.5%, P=0.005). With this in mind, we conducted a subgroup analysis according to region, study design, and cancer subtype. From these results, we observed that heterogeneity may be derived from non-divided lung cancer types and case–control studies.
Compliance with ethical standardsThe manuscript does not contain clinical studies or patient data.
Authors’ contributionsSha Zeng and Hua-Xia Gan contributed equally to this work.
Conflicts of interestThe authors declare that no conflicts of interest exist.
The study was supported by grants from the National Natural Science Funds of China (No. 81260336), Jiangxi Provincial Natural Science Foundation of China Science (No. 20132BAB205017), and Jiangxi Provincial Science and Technology Project of China (No. 20121BBG70045).