Following the publication of the RECOVERY collaborative group,1 glucocorticoids (GCs) have become the first-line treatment for hospitalized patients with the 2019 coronavirus disease (COVID-19). The Journal of the American Medical Association (JAMA) subsequently published three other articles about critically ill patients that partially confirmed this result (CoDEX, CAPE-COVID, and REMAP-CAP COVID-19 trials).2 In contrast, the METCOVID study,3 which included patients with varying severity levels, did not confirm said finding. Explanations have been proposed for the differences between the results obtained in these studies. We believe that the most important aspect of this apparent contradiction is that it reminds us that there are still unanswered questions about the use of GCs in COVID-19.
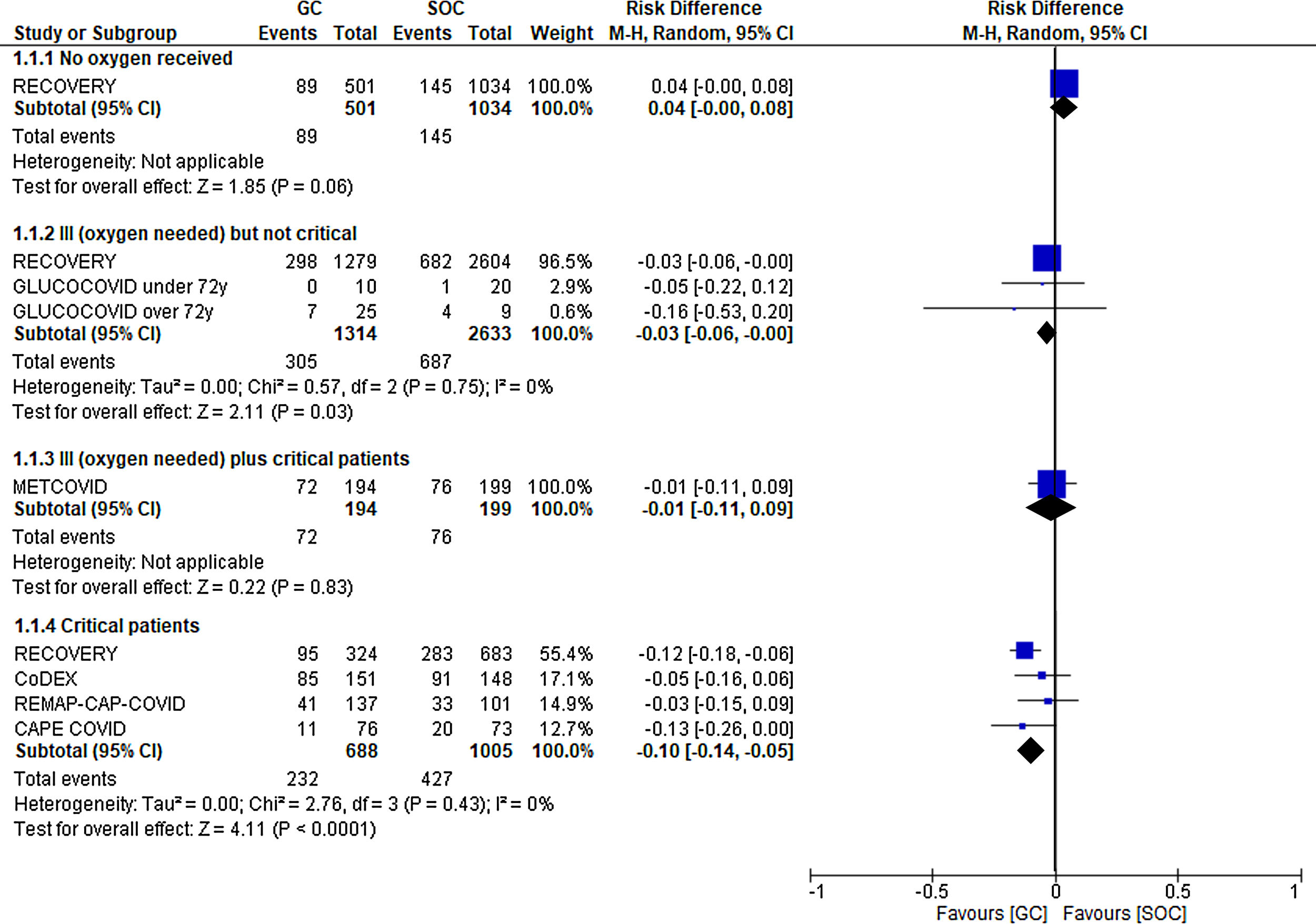
First, which patients benefit from treatment with steroids? In other words, can we establish a severity level starting from which patients will benefit from the use of GCs? Of all questions, this seems to be the one with the most certain answer (Fig. 1). Glucocorticoids have been associated with benefits in more severe patients. Thus, the RECOVERY, CoDEX, CAPE-COVID, and REMAP-CAP COVID-19 trials demonstrated a clear reduction in mortality among critically ill patients (absolute risk reduction [ARR] in mortality at 21–28 days of 10%, with a 95% confidence interval [CI] of −5% to −14%). This improvement was not as significant among critically ill patients requiring oxygen, but not invasive mechanical ventilation (ARR of 3%, with a 95% CI of −3% to 0%, according to data from the RECOVERY and GLUCOCOVID trials). Finally, no benefit was observed when the treatment was used in hospitalized patients without the need for supplementary oxygen (RECOVERY trial), and there was even a trend toward a worse clinical evolution in the treated group.
Second, which is the right GC and dose? Up to three GCs (dexamethasone, hydrocortisone, and methylprednisolone) have been evaluated, and it would be reasonable to believe that there are no significant differences between them at equivalent doses. Intermediate to high doses have been used in the clinical trials performed thus far (from 30 mg of prednisone equivalent per day in the RECOVERY trial to 125 mg in the CoDEX trial). Several observational studies performed in Spain have shown a potentially better response when GCs are administered at high doses in short cycles (equivalent to 300−600 mg of prednisone per day).4,5 The additional benefit of these doses could be explained by the activation of the non-genomic pathway of these corticosteroids, which would result in a maximum anti-inflammatory effect.
Third, how long should the treatment cycle last? All these clinical trials have assessed short treatment periods (from 5 days in the METCOVID trial to 14 days in the CAPE-COVID trial). We have no prospective data on the likelihood of exacerbations in patients following discontinuation of the GCs. We are also unaware whether any particular subgroup of patients might benefit from a progressively decreasing cycle duration compared with a pre-fixed cycle duration.
Fourth, when is the best time to begin treatment with GCs? In all of these clinical trials, treatment was started based on the severity of the patients’ condition, without considering the time elapsed since onset of the disease in the inclusion criteria. A recent publication has proposed the administration of this treatment at the second week since onset.
Fifth, could the response to GCs be predicted based on laboratory profiles or imaging data? None of the published clinical trials included the presence of inflammatory markers in their inclusion criteria. Some parameters, such as C-reactive protein levels or the neutrophil-lymphocyte ratio, have been associated with a risk of death. In addition, it could be hypothesized that more inflammatory laboratory profiles might exhibit a greater response. However, the METCOVID trial reported no difference in the response rate regardless of the pre-treatment levels of C-reactive protein, ferritin, or interleukin 6 (IL-6).
Finally, should we administer GCs together with antivirals such as remdesivir? And, should we administer them together with other immunomodulators such as IL-6 or IL-1 antagonists, among others, during the inflammatory phase?
There is evidence that GCs have beneficial effects in critically ill COVID-19 patients. We now need to continue our research to complete the guidelines for the use and implementation of these drugs in cases of infection by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
FundingThe authors of this article have received funding within the context of the call for COVID-19 projects of the regional Health Ministry of Castile and Leon (SACYL, Sanidad Castilla y León), for project GRS COVID 68/A/20. Country: Spain.
Conflicts of interestThe authors declare no conflict of interest.
Please cite this article as: Corral-Gudino L, Abadía-Otero J, Gómez-Barquero J. Cuestiones por resolver en el tratamiento con glucocorticoides de la infección COVID-19. Med Clin (Barc). 2021;156:143–144.