The present systematic review analyses the role of soluble fms-like tyrosine kinase-1 (sFLT-1) as an indirect biomarCker of endothelial dysfunction in sepsis or septic shock from articles published in Pubmed between 2010 and March 2022.
Materials and methodsA systematic review of studies studying sFLT-1 monitoring in intensive care units in adults with sepsis or septic shock vs. controls for sepsis diagnosis and prognosis has been carried out (PROSPERO CRD42023412929 Registry).
ResultsThe endothelial dysfunction of sepsis is one of the keys to the development of the disease. VEGF binds to sFlt-1 acting as a competitive inhibitor of VEGF signalling in endothelial cells and thus neutralizes its pro-inflammatory effects. Endothelial dysfunction is reflected in increased sFLT-1 levels. High values of sFLT-1 were used for the differential diagnosis of sepsis versus other inflammatory pathologies, septic shock versus other types of shock, were elevated over time, estimation of disease prognosis, correlation with sepsis severity, organ dysfunction, and mortality prediction.
ConclusionsIt is evident that sepsis is based on endothelial dysfunction. sFLT-1 is one of the main biomarkers of microvascular alteration and is a predictive diagnostic and prognostic biomarker.
La presente revisión sistemática analiza el papel de soluble fms-like tyrosine kinase-1 (sFLT-1) como biomarcador indirecto de disfunción endotelial en sepsis o shock séptico de artículos publicados en Pubmed entre 2010 y marzo 2022.
Materiales y métodosSe ha realizado una revisión sistemática de estudios estudiando la Monitorización de sFLT-1 en unidades de cuidados intensivos en adultos con sepsis o shock séptico vs controles para diagnóstico y pronóstico de sepsis (Registro PROSPERO CRD42023412929).
ResultadosLa disfunción endotelial de la sepsis resulta una de las claves para el desarrollo de la enfermedad. VEGF se une a sFlt-1 actuando como un inhibidor competitivo de la señalización del VEGF en las células endoteliales y, por lo tanto, neutraliza sus efectos proinflamatorios. La disfunción endotelial se ve reflejada en el aumento de los valores de sFLT-1. Valores elevados de sFLT-1 se emplearon para el diagnóstico diferencial de la sepsis frente otras patologías inflamatorias, shock séptico frente a otros tipos de shock, en un análisis ajustado, estuvieron elevados a lo largo del tiempo, estimación del pronóstico de la enfermedad, correlación con la gravedad de la sepsis, la disfunción orgánica y predicción de mortalidad.
ConclusionesSe evidencia como la sepsis se fundamenta en la disfunción endotelial. sFLT-1 es uno de los principales biomarcadores de alteración microvascular y resulta biomarcador predictivo diagnóstico y pronóstico.
Sepsis is defined as severe, life-threatening organ dysfunction resulting from dysregulation of the immune system in response to infection.1 A recent study by the Institute for Health Metrics and Evaluation (IHME) on the global burden of sepsis estimated an incidence of 48.9 million and mortality of 11 million worldwide in 2017, with a cost of approximately $24 billion annually in the US.2
Sepsis is caused by a generalised reaction from an uncontrolled local lesion, resulting in what is known as SIRS (systemic inflammatory response syndrome) in the SEPSIS-2 classification. This phenomenon is not fully understood, although it will depend primarily on the micro-organism and the nature of the host response.
Currently, the treatment of sepsis is based on a triple therapy consisting of early antibiotic therapy, control of the focus and targeted haemodynamic support.3
Due to the high mortality and economic impact of patients with sepsis,1 as well as the high increase in multidrug-resistant bacteria,4 the search for new markers that are more sensitive and specific than those currently available is of particular interest. As Tan et al.5 describe in their meta-analysis, CRP and PCT are of moderate value for the diagnosis of sepsis in adult patients. For CRP they found an AUC of 0.73 (95%CI, 0.69−0.77), with a sensitivity and specificity of 0.80 (95%CI, 0.63−0.90) and 0.61 (95%CI, 0.50−0.72), respectively. PCT had an AUC of 0.85 (95% CI, 0.82−0.88), with a sensitivity and specificity of 0.80 (95% CI, 0.69−0.87) and 0.77 (95% CI, 0.60−0.88). These results highlight the need to find more discriminating biomarkers.
At the molecular level, endothelial dysfunction has been described as one of the causes of the transition from local infection to systemic effects. Among the multiple causes of endothelial dysfunction, the pathophysiology of vascular endothelial growth factor (VEGF)-mediated neoangiogenesis deficit stands out.6
VEGF is a key regulator of normal angiogenesis, during which it promotes endothelial cell survival, growth and migration. The effects of VEGF are mediated by two receptors: FMS-like tyrosine kinase-1 (FLT-1) receptor or the FLK-1 receptor. Soluble fms-like tyrosine kinase-1 (sFLT-1) can act to sequester VEGF and prevent binding to FLK-1, which is able to transduce a much stronger signal, thus inhibiting VEGF binding and its vasodilator and neoangiogenic effect.7
As mentioned above, neoangiogenesis plays a key role in endothelial dysfunction. This phenomenon arises from the balance between a variety of proangiogenic (VEGF, PlGF) and anti-angiogenic (sFLT-1) factors.6
Given the great difficulty in directly assessing endothelial dysfunction, the goal of modern biochemistry will be to identify indirect biomarkers that allow us to evaluate possible interactions at the molecular level.
To date, numerous cohort studies have evaluated this biomarker in sepsis or septic shock. Confirming the relevance of this biomarker could mean a before and after in the early diagnosis of sepsis, as well as being a biomarker for monitoring this pathology, predicting mortality and antibiotic de-escalation, among other roles.
Therefore, the aim of our systematic review is to assess the prognostic value of sFLT-1 at the onset of sepsis in adult patients with sepsis or septic shock.
Material and methodsLiterature retrievalA systematic review of the biomarker sFLT-1 was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statement. The PubMed database was searched from 2010 to March 2022. The following terms were used as a search strategy: Advanced Search sFlt-1 [All Fields] AND ("sepsis"[MeSH Terms] OR "sepsis"[All Fields]). The protocol of the present review was registered in the International Prospective Register of Systematic Reviews "PROSPERO" (CRD42023412929).
Eligibility criteriaThe review questions were developed according to the PICOS framework (Participants, Interventions, Comparisons, Outcomes and Study Design):
Participants: Patients aged 19 years or older with sepsis or septic shock.
Intervention: Diagnostic and prognostic test.
Comparison: No restrictions were applied on the control group.
Outcome: Comparison of biomarkers versus healthy controls, differential diagnosis versus other pathologies, relationship with organ dysfunction, severity and prognosis.
Study design: Clinical trial, quasi-experimental, case-control, cohort.
Inclusion and exclusion criteriaOnly original texts published in English were included. Results were limited to adult patients in sepsis and septic shock, eliminating systematic reviews and meta-analyses. Due to its importance and relationship with sepsis, articles that included patients with COVID 19 pneumonia were included.
Literature screeningPublications were initially screened by title and abstract and subsequently by full text. Decisions regarding inclusion and exclusion of studies were made independently by two reviewers and any discrepancies were resolved by discussion. In cases where a consensus could not be reached, the decision was taken by a third reviewer.
Data extraction and quality assessmentGeneral information such as author, year of publication, number and characteristics of cases and controls, type of biomarker, etc., and information on diagnostic parameters such as relative risk, odds ratio, confidence intervals, area under the curve, sensitivity, specificity, false positives, false negatives, etc. Author and year of publication were documented. The following outcomes of interest were considered: values according to pathologies, organ dysfunction, among others.
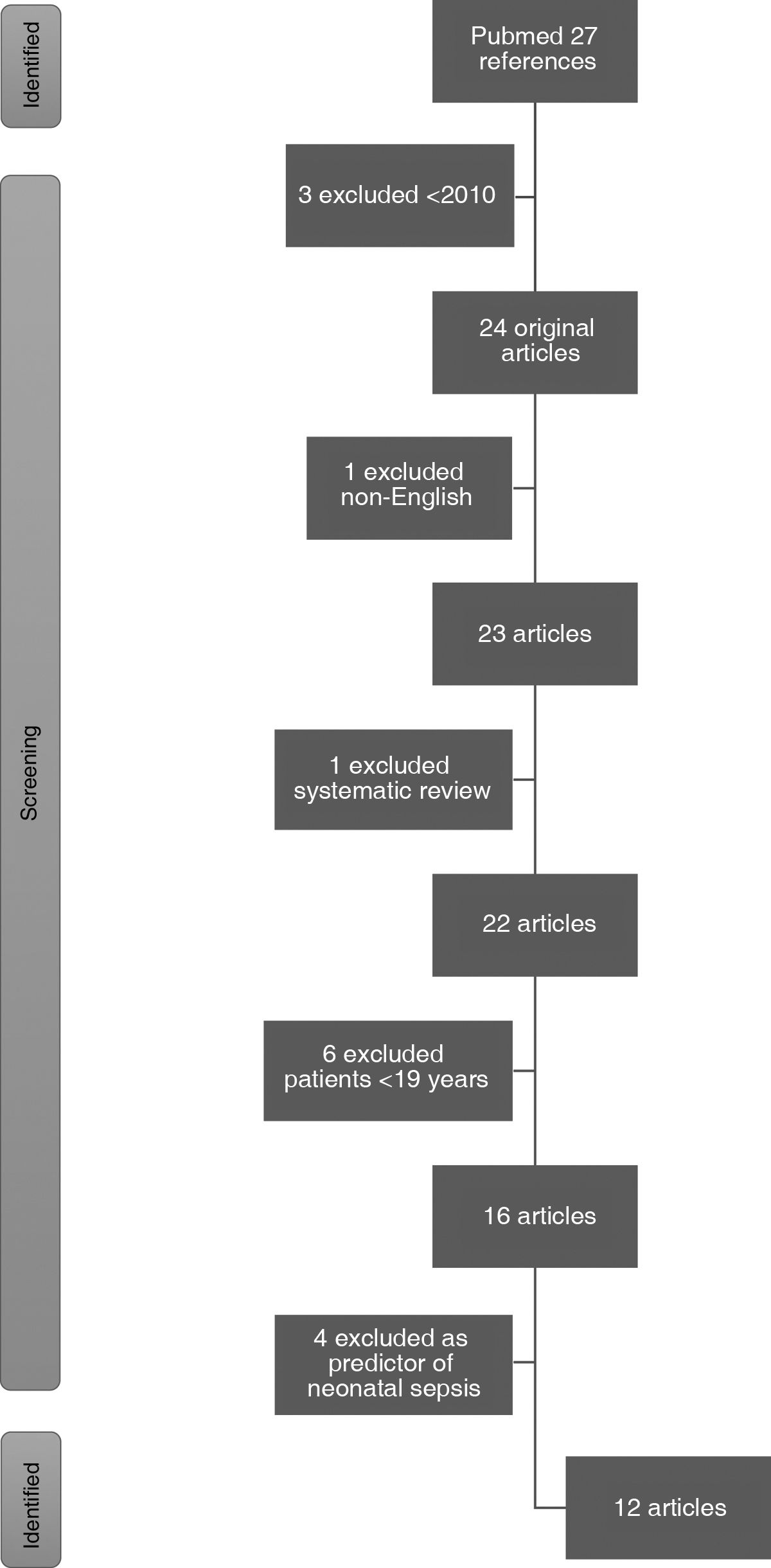
ResultsThe PubMed literature search retrieved a total of 27 articles. Three pre-2010 articles and one non-English article were excluded. One systematic review and 6 articles were excluded because they included patients under the age of 19. Finally, 4 were excluded for analysing the biomarker as a predictor of neonatal sepsis and one for studying acute pancreatitis. Finally, 11 articles were included in this review (Fig. 1).
Of the 11 articles finally selected, 10 were prospective observational studies. Of these, one was a case-control study and the remaining nine were cohorts, including two multicentre prospective cohort studies. The remaining article analysed was a clinical trial. As a common denominator, all included studies collected blood samples for biomarkers on admission.
The main aspects analysed were: values versus controls, differential diagnosis versus other pathologies, relationship with organ dysfunction, severity of sepsis and mortality. The number of patients in these studies ranged from 41 to 605, with a minimum age of 18 years, as well as multiple pathologies. To better determine the prognostic value of biomarkers, AUC, OR and statistical significance figures were analysed.
The sFLT-1 levels were analysed using commercially available ELISA (Enzyme-Linked ImmunoSorbent Assay) immunoassay kits. Thresholds 136.2 (96.3–211) were used for healthy controls, with an AUC = 0.70.
Schuetz et al.8 propose the study of biomarkers for the differential diagnosis of shock in the emergency department. Two groups were obtained: sepsis vs. non-septic shock. sFLT-1 (227.4; 142.4–328.2) vs. (136.2; 96.3–211) p < 0.001. E-selectin (53.2; 34.9–79.2) vs. (30.5; 20.0–40.8) p < 0.001. ICAM-1 (2; 152–267) vs. (149; 115–224) p = 0.04. VEGF (271; 91–496) vs. (118; 43–270) p = 0.01. PAI-1 not significant.
In terms of AUC, the following were described as statistically significant: sFLT-1 0.70 (2.01; 1.07–3.79) p = 0.03 and E-selectin 0.74 (3.67; 1.72–7.84) p = 0.001, while the rest of the biomarkers analysed were non-significant.
Day et al.9 identified biomarkers for the differential diagnosis between septic shock and other causes of shock. They found statistical significance in the following biomarkers: sFLT-1 (0.51 vs. 1.48; p = 0.03), E-selectin (1.75 vs. 1.97; p < 0.01), sICAM-1 (2.29 vs. 2.5; p < 0.01), PAI-1 (1.77 vs. 2.06 p < 0.01), IL-6 (1.14 vs. 2.05 p < 0.01) and procalcitonin (-0.8 vs. 0.31 p < 0.01). AUCs were used to discriminate diagnostic ability using clinical parameters as reference (AUC 0.76, 95%CI, 0.67−0.85, p < 0.01), biomarkers (AUC 0.82, 95%CI, 0.75−0.90, p < 0.01) and the joint use of clinical parameters and biomarkers (AUC 0.88, 95%CI, 0.81−0.94, p < 0.01).
Skibsted et al.10 analysed the trend between groups (sepsis, severe sepsis and septic shock) presenting levels for sFLT-1 (p < 0.0001), sE-selectin (p < 0.001), sVCAM-1 (p < 0.002), PAI-1 (p < 0.001) and sICAM-1 (p < 0.001). As for their relationship with the SOFA score, sFLT-1 (r = 0.6) and PAI-1 (r = 0.4), sE-selectin (r = 0.3) all correlated with p < 0.0001 except sICAM-1 (r = 0.15) which showed p = 0.03. All biomarkers correlated with IL-6, especially sFLT-1 (r = 0.63, p < 0.0001). As for the APACHE-II score, correlation was demonstrated with sFLT-1 (r = 0.64), PAI-1 (r = 0.58), sE-selectin (r = 0.31), sVCAM-1 (r = 0.38) all of them p < 0.0001 except sICAM-1 (r = 0.17, p < 0.05).
To further assess the accuracy of the different markers extracted in the ED, in-hospital mortality was analysed by AUC. sFLT-1 (0.87; 0.79−0.95), PAI-1 (0.87; 0.78−0.95), sE-selectin (0.77; 0.69−0.85), ICAM (0.71; 0.60−0.81), sVCAM-1 (0.78; 0.68−0.89) all p < 0.05 except IL-6 (0.80; 0.69−0.91, p = 0.54).
Hou et al.11 analysed goal-directed haemodynamic resuscitation in the emergency department vs. treatment as usual. There was no difference in clinical outcome between resuscitation strategies, nor in biomarker profiles of vascular permeability (sFLT-1, VEGF, Ang-2) or haemostasis (vWF, tPA, TM) at 6 h and 24 h for the biomarkers analysed. All previously mentioned biomarkers were significant indicators of mortality, both at baseline and at 6 h and 24 h. Of particular interest is the baseline measurement of biomarkers as predictors of mortality, all of which were shown to have significant AUCs: sFLT-1 0.70 (0.61−0.78), VEGF 0.56 (0.47−0.66), Ang-2 0.67 (0.58−0.76), vWF 0.60 (0.51−0.69), tPA 0.69 (0.61−0.77) and thrombomodulin 0.69 (0.60−0.77).
For patients with compromised immune systems (neutropenic and diabetic), the following conclusions were drawn:
Alves et al.12 conducted a retrospective study comparing 31 patients with sepsis vs. 10 patients with haematological neutropenic septic shock. At fever onset they had no difference in VEGF-A levels (20.7 pg/mL, 7.9–129.3 pg/ml) vs. (20.0 pg/mL, 9.3–158.9 pg/ml; p = 0.9) nor in sFLT-1 levels (47.3 pg/mL, 20.8–117.6 pg/ml) vs. (49.2 pg/mL, 29.6–91.1 pg/ml; p = 0.3) nor in sepsis or septic shock.
However, after 48 h, the levels of both markers were higher in patients with septic shock compared to septic patients (116.0 pg/mL, 42.7–208.4 pg/ml) vs (42.9 pg/mL, 25.9–472.9 pg/ml; p = 0.002). As for correlation with respect to the SOFA score VEGF-A was demonstrated at baseline (Rs = -0.21; p = 0.23) vs. 48 h (Rs = -0.17; p = 0.33) and sFLT-1 at baseline (Rs = 0.3; p = 0.04) vs. 48 h (Rs = 0.25; p = 0.16). Such values are endorsed in the areas under the curve (AUC) at 48 h VEGF (0.76, 0.55−0.97; p = 0.02) and sFLT-1 (0.87, 0.73–1.00; p < 0.01).
In contrast, Fiusa et al.,13 analysing neutropenic fever patients, found no significant VEGF-A and sFLT-1 in terms of risk of developing septic shock. They subsequently compared uncomplicated vs. complicated patients in terms of angiopoietin: Ang-1 (898.8 pg/mL; 77.9−5420 pg/mL) vs. (1220 pg/mL; 32.5–47,924 pg/mL) p = 0.07. Ang-2 (4467 pg/mL; 1289−37.318 pg/mL) vs. (6494 pg/mL, 173−49.611 pg/mL), p = 0.02. Given the antagonistic role of Ang-1 vs. Ang-2, the Ang-1/Ang-2 ratio is analysed showing a ratio (5.3; 0.6–57.1) vs. (1.9; 0.1–64.6) p = 0.01. AUC was estimated at 0.68 (95%CI, 0.55−0.81; p = 0.01). Ratio of 5 had sensitivity 60% (95%CI, 36.1–80.9%) and specificity 77.2% (95%CI, 66.4–85.9%). Positive and negative predictive value 47% and 85%, respectively. Ang-2/Ang-1 ratio above 5 increased the relative risk of death at 28 days by 5.8 (95%CI, 1.96–17.59; p = 0.001) and 2.74 (95%CI, 1.054–7.160; p = 0.03) at higher median levels.
With regard to diabetes, Schuetz et al.14 selected patients with sepsis, severe sepsis and septic shock and divided them according to their underlying pathology (diabetes vs. non-diabetes). First, they compared the role of diabetes and sepsis demonstrating increased E-selectin (71.4, 45.8–116) vs. (45.1, 30.1–68.6; p < 0.0001) and sFLT-1 (192, 103–378) vs. (112, 70–187; p < 0.0001), while ICAM-1 and PAI-1 were not statistically significant in diabetics vs. non-diabetics. Once the positive relationship was established for diabetes, sepsis vs septic shock was analysed, demonstrating E-selectin (119, 71–168) vs (61, 37–83; p < 0.001) and sFLT-1 (370, 243–712) vs (190, 113–351; p = 0.001), with ICAM-1, VCAM and PAI-1 being non-significant. After performing the multivariate study, it was concluded that there was a greater association in the SOFA score in diabetic patients when adjusting for sepsis, among other variables (β 0.09; p = 0.006).
Skibsted et al.15 proposed the monitoring of StO2 in three phases: baseline, occlusion phase and recovery phase after tourniquet release. A multivariate study was performed in the three phases comparing the biomarkers sE-selectin, sVCAM-1, ICAM-1, IL-6, sFLT-1 and PAI-1. Only the latter two were statistically significant: sFLT-1 (r = -0.08, p < 0.001) and PAI-1 (r = -0.06, p < 0.001). However, when studied by subgroups, none of the biomarkers were statistically significant in the sepsis group (Tables 1 and 2).
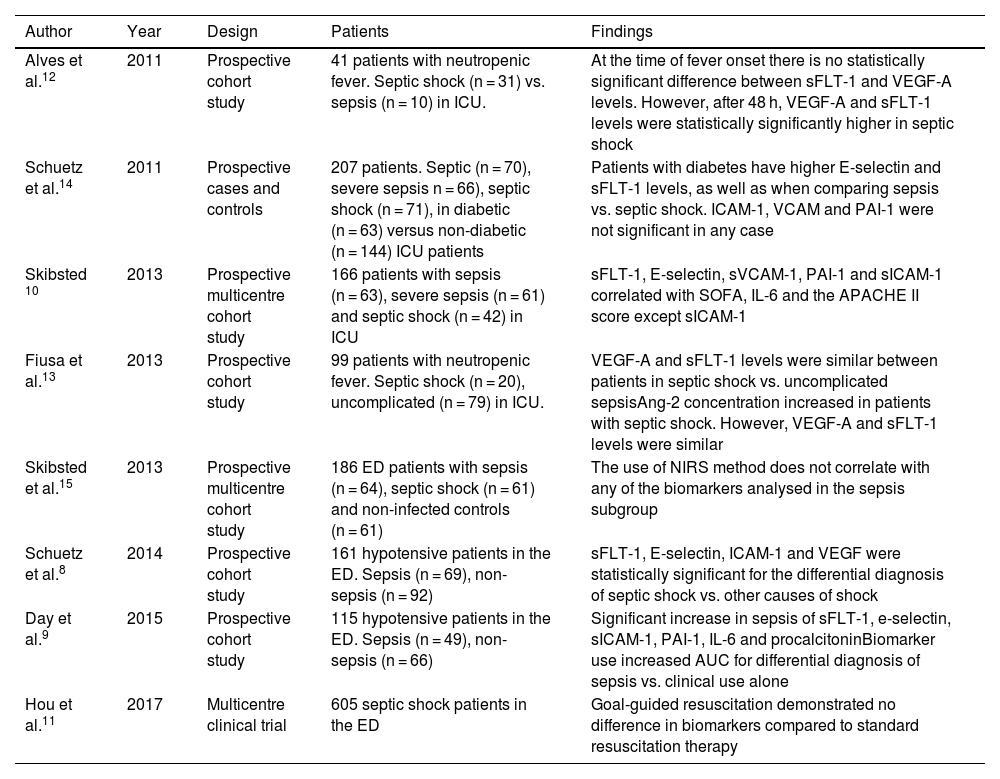
sFLT-1 as a biomarker for severity classification.
| Author | Year | Design | Patients | Findings |
|---|---|---|---|---|
| Alves et al.12 | 2011 | Prospective cohort study | 41 patients with neutropenic fever. Septic shock (n = 31) vs. sepsis (n = 10) in ICU. | At the time of fever onset there is no statistically significant difference between sFLT-1 and VEGF-A levels. However, after 48 h, VEGF-A and sFLT-1 levels were statistically significantly higher in septic shock |
| Schuetz et al.14 | 2011 | Prospective cases and controls | 207 patients. Septic (n = 70), severe sepsis n = 66), septic shock (n = 71), in diabetic (n = 63) versus non-diabetic (n = 144) ICU patients | Patients with diabetes have higher E-selectin and sFLT-1 levels, as well as when comparing sepsis vs. septic shock. ICAM-1, VCAM and PAI-1 were not significant in any case |
| Skibsted 10 | 2013 | Prospective multicentre cohort study | 166 patients with sepsis (n = 63), severe sepsis (n = 61) and septic shock (n = 42) in ICU | sFLT-1, E-selectin, sVCAM-1, PAI-1 and sICAM-1 correlated with SOFA, IL-6 and the APACHE II score except sICAM-1 |
| Fiusa et al.13 | 2013 | Prospective cohort study | 99 patients with neutropenic fever. Septic shock (n = 20), uncomplicated (n = 79) in ICU. | VEGF-A and sFLT-1 levels were similar between patients in septic shock vs. uncomplicated sepsisAng-2 concentration increased in patients with septic shock. However, VEGF-A and sFLT-1 levels were similar |
| Skibsted et al.15 | 2013 | Prospective multicentre cohort study | 186 ED patients with sepsis (n = 64), septic shock (n = 61) and non-infected controls (n = 61) | The use of NIRS method does not correlate with any of the biomarkers analysed in the sepsis subgroup |
| Schuetz et al.8 | 2014 | Prospective cohort study | 161 hypotensive patients in the ED. Sepsis (n = 69), non-sepsis (n = 92) | sFLT-1, E-selectin, ICAM-1 and VEGF were statistically significant for the differential diagnosis of septic shock vs. other causes of shock |
| Day et al.9 | 2015 | Prospective cohort study | 115 hypotensive patients in the ED. Sepsis (n = 49), non-sepsis (n = 66) | Significant increase in sepsis of sFLT-1, e-selectin, sICAM-1, PAI-1, IL-6 and procalcitoninBiomarker use increased AUC for differential diagnosis of sepsis vs. clinical use alone |
| Hou et al.11 | 2017 | Multicentre clinical trial | 605 septic shock patients in the ED | Goal-guided resuscitation demonstrated no difference in biomarkers compared to standard resuscitation therapy |
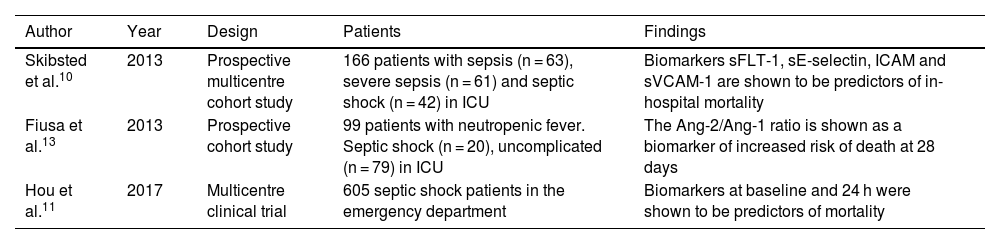
sFLT-1 as a biomarker of mortality.
| Author | Year | Design | Patients | Findings |
|---|---|---|---|---|
| Skibsted et al.10 | 2013 | Prospective multicentre cohort study | 166 patients with sepsis (n = 63), severe sepsis (n = 61) and septic shock (n = 42) in ICU | Biomarkers sFLT-1, sE-selectin, ICAM and sVCAM-1 are shown to be predictors of in-hospital mortality |
| Fiusa et al.13 | 2013 | Prospective cohort study | 99 patients with neutropenic fever. Septic shock (n = 20), uncomplicated (n = 79) in ICU | The Ang-2/Ang-1 ratio is shown as a biomarker of increased risk of death at 28 days |
| Hou et al.11 | 2017 | Multicentre clinical trial | 605 septic shock patients in the emergency department | Biomarkers at baseline and 24 h were shown to be predictors of mortality |
The following is a description of the 3 articles obtained by applying the aforementioned inclusion criteria that are related to SARS-CoV-2 infection. It was decided to include them because they exhibited a pathology of bilateral viral interstitial pneumonia, which eventually leads to adult respiratory distress syndrome and a variety of bacterial infectious complications. This endothelial dysfunction arises from microcirculatory dysfunction due to the infiltration of endothelial cells expressing ACE2.
Greco et al.16 compared sFLT-1 levels in infected subjects vs. healthy controls, at levels of 90.3 pg/mL (AUC 0.902, sensitivity 83.89%, specificity 86.67%, p < 0.001). sFLT-1 appeared associated with the extent and severity of disease. Third day (122.4 ± 6.9 vs. 155.6 ± 10.6 pg/mL, p = 0.01), second week 199.4 ± 32.4 pg/mL, third week 199.0 ± 12.0 pg/mL. In control patients we observed an sFLT-1 concentration of 78.9 ± 2.5 pg/mL. Levels of sFLT-1 and lymphocytosis, neutrophilia, CRP, LDH, ferritin and procalcitonin were grouped by quartiles, showing an increase in COVID-19 deaths. The use of CA 15.3 as a marker of fibrosis progression was analysed, showing serum levels of CA 15.3 of patients admitted to ICU compared to patients who were hospitalised in ward and control subjects (42.6 ± 3.3 vs. 25.7 ± 1.5 U/mL vs. control patients 14.5 ± 0.3 U/mL p < 0.0001).
Espino-y-Sosa et al.17 compared severe vs. non-severe COVID-19 positive pregnant women. The comparison of sFLT-1 levels showed statistical significance (1424; 1054–2099 vs. 6119; 2099–7900, p = 0.0001), with an sFLT-1/ANG-II ratio (0.92; 0.25–2.03 vs. 14.27; 4.47–42.46 p = 0.0001). Along these lines, ORs for severe sepsis of sFLT-1 (1.01; 1.00–1.01 p < 0.0001) and sFLT-1/ANG-II (1.31; 1.09–1.56 p = 0.003) were found. Finally, an sFLT-1/ANG-II ratio ≥ 3.06 gave an AUC for severe pneumonia 0.96 with sensitivity 0.96 (95%CI, 0.88–1.0), specificity 0.886 (95%CI, 0.80−0.972), PPV 8.48 (95%CI, 3.97–18) and NPV 0.045 (95%CI, 0.01−0.31). Similarly, the AUCs for the ratio ≥ 3.06 of sFLT-1/ANG-II for risk of ICU admission, intubation, viral sepsis and maternal death were also significant.
Along the same lines, Torres-Torres et al.18 analysed the relationship between sFLT-1 and COVID-19 severity (non-severe vs. severe). The sFLT-1 levels were higher in patients with severe COVID (1789; 1171–3286 vs. 4050; 2099−11,490 p = 0.002). There was a significant association between sFLT-1, pneumonia OR 1.817 (95%CI, 1.365–2.418; p < 0.0001), ICU admission OR 2.195 (95%CI, 1.582–3.047), viral sepsis OR 2.318 (95%CI, 1.407–3.820) and maternal death OR of 5.504 (95%CI, 1.079–28.076). The AUCs were all significant, with pneumonia severity 0.715 (0.582−0.828), PPV 38.7 (9.7–61.3), NPV 45.2 (29–64.5) (Table 3).
sFLT-1 as a diagnostic and severity biomarker in SARS-CoV-2 pneumonia.
| Author | Year | Design | Patients | Findings |
|---|---|---|---|---|
| Greco et al.16 | 2021 | Prospective cohort study | 262 patients hospitalised for COVID pneumonia vs. 101 healthy controls | Serum sFLT-1 levels were significant for the diagnosis of COVID-19 pneumonia vs. healthy subjects, as well as for the stratification of severity. As for CA 15.3, it was significant for predicting pulmonary fibrosis |
| Espino-y-Sosa et al.17 | 2021 | Prospective multicentre cohort study | 80 COVID-positive pregnant women. Severe (n = 25) and non-severe (n = 55) COVID in the emergency department | Increased sFLT-1 and sFLT1/ANG-II ratio are described as predictors of severity as well as complications |
| Torres-Torres et al.18 | 2022 | Prospective cohort study | 113 pregnant women COVID + . Non-severe COVID (n = 82), severe COVID (n = 31) in emergency department | sFLT-1 levels were associated with disease severity, as well as increased complications |
Early diagnosis and prompt intensive initial treatment are key to improving the prognosis of sepsis. Certain biomarkers that can potentially be used for this purpose have been widely described and most are already available in routine clinical practice. However, these acute phase reactants are also elevated in diseases of non-infectious aetiology, making it imperative to identify new biomarkers with sufficient sensitivity and specificity for this purpose.19
SFLT-1 is part of the balance of angiogenic factors, with low levels in people without associated endothelial pathology.
As previously described in detail, sFLT-1 is identified as significant in patients with septic shock compared to other types of shock or in patients with mild sepsis (Sepsis-2 criteria). These results are described extensively highlighting the differential diagnosis of shock in the ED between sepsis vs. non-sepsis,8 other causes of shock9 and differences between sepsis, severe sepsis and septic shock. In this last article, it is worth mentioning the correlation with IL-6, as well as the APACHE-II score, a score already validated for sepsis to predict the severity and prognosis of patients in intensive care units.10 These 3 articles are grouped together as they are highly consistent in that they refer to the statistical and clinical significance of the increase in SFLT-1 in comparative studies of sepsis/septic shock vs non-septic shock.
One of the strengths of this review is the detailed description of each study's multivariate analysis of pathologies adjusted for comorbidities, as it clearly demonstrates the role of previous medical conditions as a determinant of elevated markers of endothelial dysfunction.8–10 Along these lines, another article identified diabetes as a factor in addition to endothelial dysfunction and found that, in these patients, there is an additional significant increase in sFLT1.14 In this case, the prognosis of these patients was not analysed by performing a multivariate analysis of diabetes-biomarker-prognosis, which is why its study would be of great clinical interest.
One of the exclusion criteria in many of the articles is predominantly immunosuppression secondary to tumour, haematological, diabetic, iatrogenic, etc. pathology. For this reason, the articles described below are particularly interesting for the future use of sFLT-1.
Firstly, sepsis was compared with septic shock in neutropenic patients with haematological causes and showed no differences in sFLT-1 levels; however, the increase was significant at 48 h in patients with septic shock. Interestingly, there was a correlation between SOFA and sFLT-1.12
The sFLT-1 levels were not significant and did not agree with the previous article, although the Ang-1/Ang-2 ratio, which was of interest, was significant.13 Further studies in this population are required as the results of studies in immunocompromised patients are contradictory. Similarly, one of the limitations of this review focuses on the unique role of febrile neutropenia secondary to chemotherapy in cancer, as other non-iatrogenic causes of febrile neutropenia are not studied.
Biomarkers in diabetic patients with sepsis, severe sepsis and septic shock are compared in the article.14 The results show that sFLT-1 is positive in diabetic patients and in those with more severe sepsis. This suggests that diabetes is an added factor to endothelial dysfunction in sepsis.
In terms of mortality, it is worth highlighting the predictive capacity of sFLT-1 for in-hospital mortality when analysed in the ED,10 as well as its level at baseline, at 6 h, at 24 h, regardless of the haemodynamic resuscitation strategy employed (the change in biomarkers did not prove to be differential).11 The Ang-1/Ang-2 ratio showed a significant ratio for the relative risk of death at 28 days, but not sFLT-1.13
The COVID-19 pandemic has shown that SARS-CoV-2 induces endothelial dysfunction, which can lead to viral interstitial pneumonia. Studies have shown that sFLT-1 levels, a biomarker of endothelial dysfunction, are elevated in patients with COVID-19, and that higher levels are associated with more severe disease.
In particular, studies have shown that:
- •
In non-pregnant women with COVID-19, sFLT-1 levels increase with disease severity and are associated with an increased risk of mortality.16
- •
In pregnant women with COVID-19, sFLT-1 levels also increase with disease severity and are associated with an increased risk of severe pneumonia, ICU admission, intubation, viral sepsis and maternal death.17
- •
In pregnant women with COVID-19, the sFLT-1/ANG-II ratio also increases with disease severity but is not associated with an increased risk of pre-eclampsia.18
These findings suggest that sFLT-1 may be a useful biomarker for the assessment of the severity of COVID-19 and for the identification of patients at increased risk of complications.
Skibsted et al. compare the use of biomarkers versus bedside oxygenation analysis with NIRS systems, dismissing this procedure because of the discrepancy with biomarkers and the technical difficulty.15
As regards the limitations of the study, we could mention the lack of statistical analysis due to the great variability of the data offered by the different publications studied. Although a priori this might seem to be a limiting factor for generalisation, it is interesting that all studies relate sFLT-1 to sepsis, severity and prognosis from different angles. Schuetz et al.8 discuss the study of biomarkers for the differential diagnosis of shock in the emergency department, the lack of discrimination of the studies in relation to the primary focus of sepsis,8,9 the lack of description between the different causes of sepsis, as well as differentiating the causes of other types of shock in the control groups.9
Day et al.9 identified biomarkers for the differential diagnosis between septic shock and other causes of shock. However, the studies had patient selection biases, such as a small, single-centre population, as well as restrictive inclusion criteria. In addition, most studies only measured biomarkers at a single point in time, which could limit their clinical utility.
It is important to note that biomarkers do not measure endothelial damage directly but indirectly. This could lead to a confounding bias in which indirect biomarkers are significant and in situ endothelial dysfunction does not occur, although the latter option is not very feasible.
Finally, all the articles are significant, so carrying out the study on our own sample could indicate whether there is publication bias. As a result of the present study, we can assert that it would be of great interest to carry out studies in the near future that would allow us not only to specify the prognostic values, but also to include them in scores for predicting nosocomial complications and morbidity and mortality.
The role of sFLT-1 in endothelial dysfunction is clear after a detailed synthesis of all articles. Regarding the role of microvascular pathology, it is obvious that it is part of many pathologies, among which sepsis stands out. In this sense, studies show directionality between infectious and non-infectious pathology and severity according to the biomarkers used. The publication of heterogeneous studies could be a handicap, but we interpret this as an advantage as it shows different types of patients (emergency department, ICU, neutropenic, diabetic, COVID-19 and pregnant women) in which the role of sFLT-1 in endothelial dysfunction in sepsis is demonstrated, which is beneficial for generalisation to the whole population. Interestingly, the results do not depend on the time of sample collection, but the relationship between biomarkers and severity remains unchanged. All of this points to the importance of incorporating sFLT-1 into clinical practice, as it adds information to current clinical criteria and biomarkers.
New theories favour the use of drugs whose therapeutic targets are specific to inflammation, the immune response or endothelial dysfunction. The first of these is the most developed to date, especially in autoimmune pathologies, increasing the number of therapeutic targets beyond corticosteroids. With this in mind, one potential study that could be considered is the role of sFLT-1 in patients on active hydrocortisone treatment for refractory septic shock, assuming a bias towards more severe patients. In this way, the role of anti-inflammatory treatments as predictors of biomarker change could be analysed.
ConclusionThe serum levels of sFlt-1 are important in the differential diagnosis between sepsis and other pathologies, in determining the severity of the infectious process and in assessing mortality in patients with septic shock.
Their determination and analysis are viable for widespread clinical use, serving as a nexus for the identification of new biomarkers (or new combinations or correlations between already known biomarkers) for the early diagnosis of endothelial dysfunction, as it will represent a "before and after" in the prognosis of organ dysfunction, thus allowing faster, more accurate and thus more effective treatment.
FundingFunding from Caja Rural de Soria for the writing of the article. The Instituto de Salud Carlos III (PI18/01238, CIBERINFEC CB21/13/00051), Junta de Castilla y León (GRS 2425/A/21 GRS 2399/A/ 21, GRS 1922/A/19, GRS 2057/A/19), Fundación Ramón Areces (CIVP19A5953).
Authors' contributionMiguel Ugalde Azpiazu: conception and design of the study, acquisition of data, analysis and interpretation of the data, drafting of the article, final approval of the version presented.
Alberto Caballero: conception and design of the study, drafting of the article, critical revision of the intellectual content, final approval of the version presented.
Marta Martín: design and execution of the literature search strategy, data acquisition.
Eduardo Tamayo: data acquisition, data analysis and interpretation, drafting of the article, critical revision of the intellectual content, final approval of the version to be submitted.
Olga de la Varga: data acquisition, data analysis and interpretation, drafting of the article, critical revision of the intellectual content, final approval of the submitted version.
Conflict of interestThe authors declare that there are no potential conflicts of interest with regard to the research and/or authorship of this manuscript.
Institute of Health Sciences of Castilla y León (ICSCYL).