Despite advancements in understanding the interplay between systemic lupus erythematosus (SLE), cardiovascular disease and COVID-19, challenges and knowledge gaps persist. This study aimed to characterize the cardiovascular profiles of SLE patients hospitalized with COVID-19 and to evaluate the influence of SLE on the development of cardiovascular complications.
MethodsThis was a multicentre, nationwide observational study in which data were sourced from the SEMI-COVID-19 Registry between March 1, 2020, and March 31, 2021, involving 150 Spanish hospitals. SLE patients were matched with non-SLE patients based on sex, age, and hospitalization date.
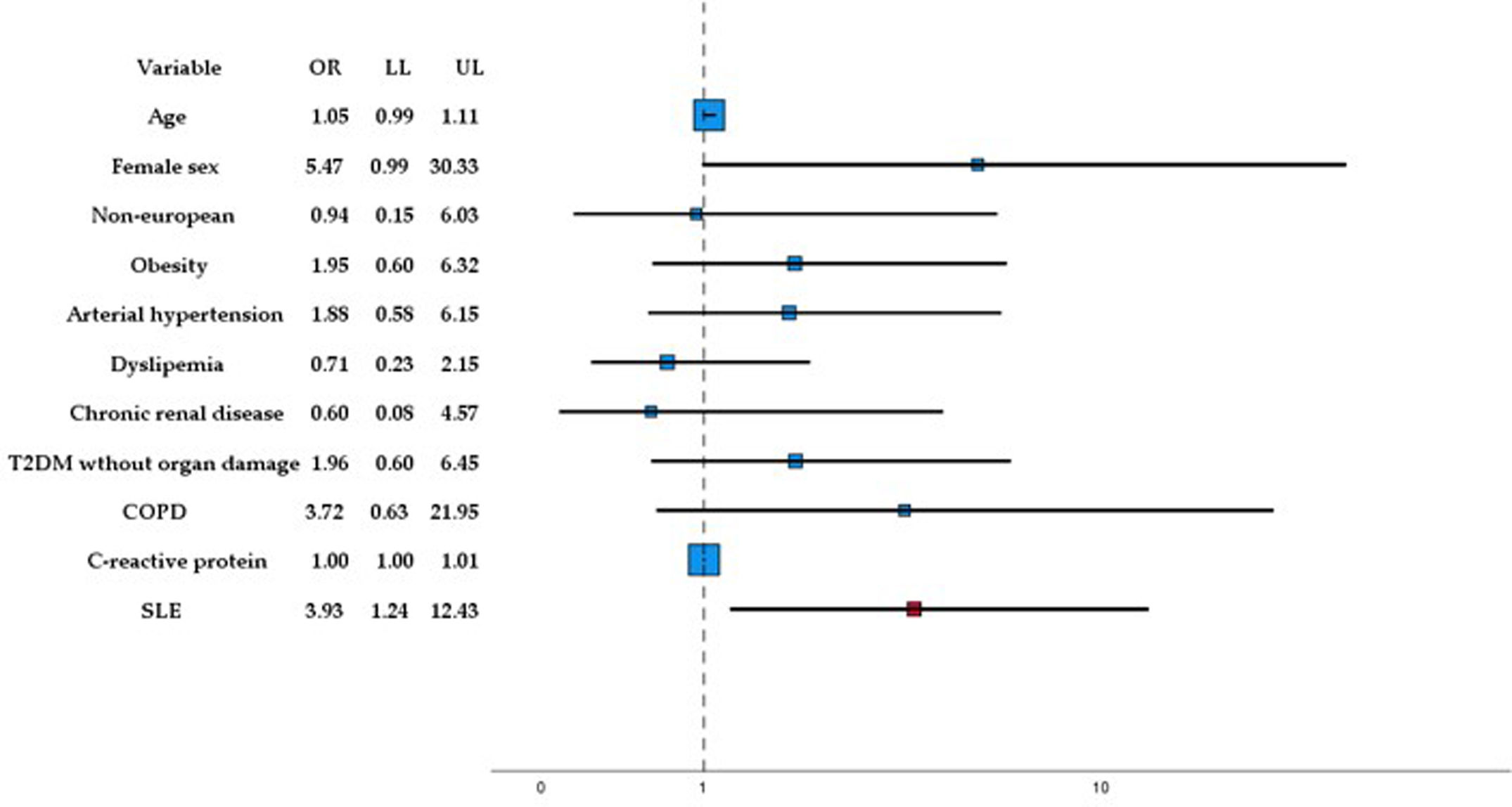
ResultsOf the 20,970 patients included in the SEMI-COVID-19 Registry, 38 were previously diagnosed with SLE. The non-SLE group was composed of 103 patients. The mean age of the SLE patients was 63 years, with 81.6% females and 21.1% non-European patients. SLE patients exhibited a significantly higher frequency of chronic kidney disease (14.4% vs 2.9%; p=0.004), stroke (23.7% vs 2.9%; p<0.001), and increased use of cardiovascular medications. SLE demonstrated an independent association with the occurrence of major cardiovascular events (MACE) (OR: 3.934; 95% CI: 1.247–12.432).
ConclusionsSLE patients hospitalized for COVID-19 are at high risk of having an unfavorable baseline cardiovascular profile and are more prone to MACEs and adverse noncardiovascular outcomes during hospitalization.
A pesar de los avances en la comprensión de la interacción entre el lupus eritematoso sistémico (LES), la enfermedad cardiovascular y la COVID-19, persisten retos y lagunas de conocimiento. El objetivo de este estudio fue caracterizar los perfiles cardiovasculares de los pacientes con LES hospitalizados por COVID-19 y evaluar la influencia del LES en el desarrollo de complicaciones cardiovasculares.
MétodosEstudio observacional multicéntrico de ámbito nacional en el que los datos proceden del Registro SEMI-COVID-19 entre el 1 de marzo de 2020 y el 31 de marzo de 2021, en el que participan 150 hospitales españoles. Los pacientes con LES se emparejaron con pacientes sin LES en función del sexo, la edad y la fecha de hospitalización.
ResultadosDe los 20.970 pacientes incluidos en el Registro SEMI-COVID-19, 38 fueron diagnosticados previamente de LES. El grupo sin LES estaba compuesto por 103 pacientes. La edad media de los pacientes con LES era de 63años, con el 81,6% de mujeres y el 21,1% de pacientes no europeos. Los pacientes con LES presentaban una frecuencia significativamente mayor de enfermedad renal crónica (14,4% frente al 2,9%; p=0,004), ictus (23,7% frente al 2,9%; p<0,001) y un mayor uso de medicación cardiovascular. El LES demostró una asociación independiente con la aparición de eventos cardiovasculares mayores (MACE) (OR: 3,934; IC95%: 1,247 a 12,432).
ConclusionesLos pacientes con LES hospitalizados por COVID-19 presentan un alto riesgo de tener un perfil cardiovascular basal desfavorable y son más propensos a sufrir MACE y resultados adversos no cardiovasculares durante la hospitalización.
Systemic lupus erythematosus (SLE) is a complex autoimmune disorder with manifestations impacting various organs, such as the skin, joints, central nervous system, and kidneys.1 Notably, SLE imposes an added burden with an escalated risk of premature cardiovascular disease (CVD), contributing significantly to overall morbidity and mortality.1,2
Compared to the general population, SLE patients, especially younger (aged 19–39 years) versus older patients, have a 2–3-fold increased risk of developing CVD.3 This increased risk is not entirely explained by the prevalence of classical cardiovascular risk factors but rather by complex interactions between the inflammatory activity of these diseases and the treatments received.4 Consequently, SLE is considered an independent and robust risk factor for CVD.5
During the COVID-19 pandemic, SLE patients were a major concern.6,7 SLE disproportionately affects populations already severely impacted by COVID-19, including those from non-white ethnic groups and those with limited economic resources.8,9 The prevalence of COVID-19 infection varies between 0.0% and 18.1% in SLE patients, and the hospitalization rates range from 0.24% to 16.4%.10,11 A decrease in the SLE hospitalization rate was found during the COVID-19 pandemic, and disease flare (38.9%) and infection (22.2%) were considered the main reasons for hospitalizations in SLE patients.10
The inherent immunosuppression and high comorbidity burden of SLE patients suggest that these patients are at high risk for more severe forms of COVID-19.9 As the COVID-19 pandemic unfolded, a shift was observed in the profile of hospitalized patients, with a decreasing prevalence of preexisting CVD. However, the incidence of diagnosed cardiovascular complications has increased, encompassing events such as myocardial infarction, stroke, and pulmonary embolism.12 Early recognition of the intersection between COVID-19 and CVD identified patients with cardiovascular risk factors as more prone to developing severe forms of the disease.13,14 Furthermore, cardiovascular complications are significant contributors to the morbidity and mortality associated with COVID-19.13–15
Despite advancements in understanding the interplay between SLE and COVID-19, challenges and knowledge gaps persist.9 Although data from the Global Rheumatology Alliance provide valuable insights into the outcomes of SLE patients affected by COVID-19, additional dedicated research is still needed to identify specific risk factors and refine the clinical management of this vulnerable population.9,16 The lack of clarity regarding whether this infection is a primary trigger or a risk factor for cardiovascular events in patients with a heightened cardiovascular risk underscores the importance of a detailed evaluation of the cardiovascular profile in SLE patients.2,9,17 Therefore, this study aimed to characterize the cardiovascular profile of SLE patients hospitalized for COVID-19 and to evaluate the influence of SLE on the development of cardiovascular complications in hospitalized COVID-19 patients in the context of other cardiovascular risk factors.
Materials and methodsStudy design and patientsThis was a multicentre, nationwide observational study involving patients diagnosed with SLE who were hospitalized for COVID-19 in Spain between March 1 and March 31, 2021. Patient data were sourced from the SEMI-COVID-19 Registry of the Spanish Society of Internal Medicine, encompassing 150 Spanish hospitals.18 The registry included all consecutive patients aged≥18 years admitted to hospitals with confirmed COVID-19 through polymerase chain reaction (PCR) with reverse transcription (RT-PCR) analysis of nasopharyngeal swabs, sputum samples, or bronchoalveolar lavage fluid.
Inclusion criteria for this study required patients to have a prior diagnosis of SLE according to the criteria in place at the time of the SEMI-COVID-19 Registry's establishment in 2020.18 Specifically, SLE classification was based on the fulfillment of the 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) criteria,19 providing a standardized and widely accepted framework for diagnosis. Exclusion criteria included subsequent admissions of the same patient, denial or withdrawal of informed consent, and any cases without confirmed COVID-19 or lacking an established diagnosis of SLE. These criteria were applied to ensure consistency in patient selection and to enhance the methodological transparency and reliability of the study.
Matching strategyA matching strategy was employed within the SEMI-COVID-19 Registry to identify SLE patients and controls. Each SLE patient was randomly matched with a non-SLE patient based on sex, age, and COVID-19 hospitalization date, aiming for an SLE: non-SLE ratio of 1:3. A match without replacement was applied with a caliper of 0.1. This methodology aimed to minimize the influence of confounding variables such as age, sex, and hospitalization date on the cardiovascular profile, enhancing comparability between groups.
VariablesThe SEMI-COVID-19 Registry retrospectively collects data from the initial admission of patients aged≥18 years with confirmed COVID-19. The data included sociodemographic information, medical history, usual treatments, clinical presentation, clinical status, analytical results, radiological findings, clinical management, in-hospital complications, duration of hospital stay, early readmissions, referral to long-stay or specialized nursing centers, and in-hospital deaths. Detailed information on the justification, objectives, methodology, and preliminary results of the SEMI-COVID-19 Registry is published in Revista Clínica Española.18 Physicians retrospectively collected the data through an online electronic data capture system.
Atherosclerotic cardiovascular diseases included a history of ischemic heart disease (myocardial infarction, acute coronary syndrome, angina, or coronary revascularization), cerebrovascular disease (stroke, transient ischemic attack), or peripheral artery disease (intermittent claudication, revascularization, lower limb amputation, or abdominal aortic aneurysm).20 The nonatherosclerotic cardiovascular diseases included atrial fibrillation and heart failure. Obesity was defined as a body mass index≥30kg/m2. Hypertension, diabetes mellitus, and dyslipidemia were considered if there was a previous clinical diagnosis or if the patient had been receiving pharmacological treatment for these disorders, excluding cases where these medications were prescribed for other indications.21 Moderate to severe kidney disease was defined as an estimated glomerular filtration rate<45ml/min/1.73m2 according to the CKD Epidemiology Collaboration (CKD-EPI) equation.22
Outcome variablesThe primary outcome variable for this study was major cardiovascular events (MACE), a composite outcome variable comprising acute myocardial infarction, stroke, acute decompensated heart failure, venous thromboembolism, ventricular or atrial arrhythmias, pericardial effusion, or aborted cardiac arrest; all newly occurring during hospitalization.17 Secondary outcomes included adult respiratory distress syndrome, need for vasopressors, acute renal failure, bacterial pneumonia, sepsis, disseminated intravascular coagulation, multiorgan failure, need for invasive mechanical ventilation, need for admission to the intensive care unit, hospital readmission, in-hospital death, days in intensive care units, days on invasive mechanical ventilation and in-hospital stay.
Statistical analysisQualitative variables are presented as absolute and relative frequencies and were compared using the Chi-square test or Fisher's exact test, as appropriate. Quantitative variables are expressed as the mean and standard deviation (SD) or median and interquartile range (IQR) and were compared using the independent samples t test or Mann–Whitney U test, as appropriate. To assess the influence of SLE on the occurrence of MACEs, a binary logistic regression analysis was performed, including demographic factors as adjustment variables and well-established cardiovascular risk factors.13 A similar procedure was used to analyze the influence of SLE on secondary outcome variables: in-hospital mortality, ICU admission, invasive mechanical ventilation, adult respiratory distress syndrome, sepsis, disseminated intravascular coagulation, multiorgan failure and hospital readmission. Kaplan–Meier curves were generated to visually represent patients’ in-hospital stays based on their cardiovascular profiles. A significance level of 0.05 (95% confidence level) was assumed. The data were stored and analyzed with the statistics packages SPSS® version 29 and MedCalc® version 23.0.8, both for Windows.
Ethical considerationsThe SEMI-COVID-19 Registry was approved by the Provincial Research Ethics Committee of Malaga (Spain) on March 27, 2020 (Ethics Committee code: SEMI-COVID-19 27/03/20). Informed consent was obtained from all patients. In cases of biosafety issues or patient discharge, verbal informed consent was sought and documented in the medical records. The strict confidentiality of the data and patient anonymity were maintained in accordance with Spanish regulations governing observational studies. Identifiable patient information was removed before analysing the database to ensure that individual patients could not be identified in this study or in the database.
ResultsPatients and demographicsOf the 20,970 patients included in the SEMI-COVID-19 Registry, only 38 (0.001%) were previously diagnosed with SLE. Once the matching strategy was applied according to age, sex and date of diagnosis, the group of non-SLE patients was composed of 103 patients. Table 1 displays the demographic characteristics of the patients in the study.
Patient cardiovascular profiles at admission.
| SLE patients(n=38) | Non-SLE patients(n=103) | p | |
|---|---|---|---|
| Demographics | |||
| Mean age±SD | 63.0±13.3 | 63.9±12.9 | 0.720 |
| Female gender (%) | 31 (81.6) | 82 (79.6) | 0.500 |
| Ethnic group/non-European (%) | 8 (21.1) | 14 (13.6) | 0.301 |
| Current pregnancy (%) | 0 (0) | 2 (1.9) | 1.000 |
| Cardiovascular risk factors | |||
| Arterial hypertension (%) | 20 (52.6) | 52 (50.5) | 0.851 |
| Former smoker (%) | 13 (34.2) | 27 (26.2) | 0.698 |
| Smoker (%) | 1 (2.6) | 3 (2.9) | |
| Dyslipemia (%) | 20 (52.6) | 38 (36.9) | 0.123 |
| Obesity (%) | 9 (21.8) | 26 (26.5) | 0.797 |
| Chronic obstructive pulmonary disease (%) | 2 (5.3) | 7 (6.8) | 1.000 |
| Moderate-severe chronic renal disease (%) | 7 (18.4) | 3 (2.9) | 0.004 |
| Diabetes mellitus without target organ damage (%) | 9 (23.7) | 19 (18.4) | 0.635 |
| Previous cardiovascular disease | |||
| Auricular fibrillation (%) | 6 (15.8) | 13 (12.6) | 0.781 |
| Acute myocardial infarction (%) | 5 (13.2) | 4 (3.9) | 0.059 |
| Angina (%) | 2 (5.3) | 2 (1.9) | 0.294 |
| Congestive heart failure (%) | 6 (15.8) | 6 (5.8) | 0.086 |
| Stroke without sequelae or transient cerebral ischemia (%) | 9 (23.7) | 3 (2.9) | <0.001 |
| Stroke with sequelae (%) | 2 (5.3) | 3 (2.9) | 0.611 |
| Peripheral vascular disease (%) | 4 (10.5) | 3 (2.9) | 0.085 |
| Diabetes mellitus with target organ damage (%) | 2 (5.3) | 3 (2.9) | 0.611 |
| Cardio-metabolic medications | |||
| Metformin (%) | 6 (15.8) | 17 (16.5) | 1.000 |
| Anti-vitamin K (%) | 8 (21.1) | 5 (4.9) | 0.012 |
| Direct oral anticoagulants (%) | 3 (7.9) | 8 (7.8) | |
| Acetylsalicylic acid (%) | 15 (39.5) | 15 (14.6) | 0.002 |
| Statins (%) | 18 (47.4) | 29 (28.2) | 0.044 |
| Aniotensin-converting enzyme inhibitors (%) | 4 (10.5) | 16 (15.5) | 0.590 |
| Angiotensin II receptor antagonists (%) | 12 (31.6) | 22 (21.4) | 0.267 |
| Dipeptidyl peptidase-4 inhibitors (%) | 2 (5.3) | 7 (6.8) | 1.000 |
| Sodium-glucose cotransporter type 2 inhibitors (%) | 4 (10.5) | 4 (3.9) | 0.211 |
| Insulin (%) | 6 (15.8) | 5 (4.9) | 0.069 |
| Vital signs at admission | |||
| Mean Heart rate (heartbeats/minute)±SD | 90.6±13.2 | 92.3±17.3 | 0.605 |
| Mean Systolic blood pressure (mmHg)±SD | 128.0±18.9 | 128.6±21.4 | 0.885 |
| Mean Diastolic blood pressure (mmHg)±SD | 76.6±18.4 | 75.5±13.9 | 0.693 |
| Mean Temperature (°C)±SD | 37.3±0.9 | 36.8±0.9 | 0.007 |
| Mean Pulsioximetry oxygen saturation (%)±SD | 92.1±7.2 | 93.6±4.0 | 0.073 |
| Mean C-reactive protein at admission (mg/L)a±SD | 123.2±105.9 | 69.6±63.9 | <0.001 |
The average age of the SLE patients was 63 years, 81.6% were female, 21.1% were of non-European ethnicity, and none were pregnant upon admission. No significant differences were observed in these demographic characteristics compared to those of the control group.
Cardiovascular profileOur patients’ cardiovascular profiles, expressed in terms of cardiovascular risk factors, preexisting cardiovascular disease, and cardiometabolic medication use upon admission, are presented in Table 1. Notably, these results highlighted a significantly higher frequency of moderate to severe chronic kidney disease (14.4% vs 2.9%; p=0.004), as well as a higher incidence of stroke (23.7% vs 2.9%; p<0.001), the use of vitamin K antagonists (21.1% vs 4.9%; p=0.012), acetylsalicylic acid (39.5% vs 14.6%; p=0.002), and statins (47.4% vs 28.2%; p=0.044), and elevated levels of inflammation measured by C-reactive protein (123.2mg/L vs 69.6mg/L; p≤0.001) in the SLE group.
Notably, in the SLE group, four patients had undergone renal transplantation. In the non-SLE group, none of the patients were taking oral immunosuppressive drugs, whereas oral corticosteroids were administered to 25 (65.8%) SLE patients and 4 (3.9%) non-SLE patients (p<0.001). Hydroxychloroquine was exclusively administered to 20 patients in the SLE group (52.6%), and biologics were administered to 6 patients (15.8%) and 1 patient (1.0%) in the non-SLE group (p=0.002). In both groups, no patient received highly active antiretroviral therapy, had moderate to severe liver disease, or was receiving GLP-1 drug therapy.
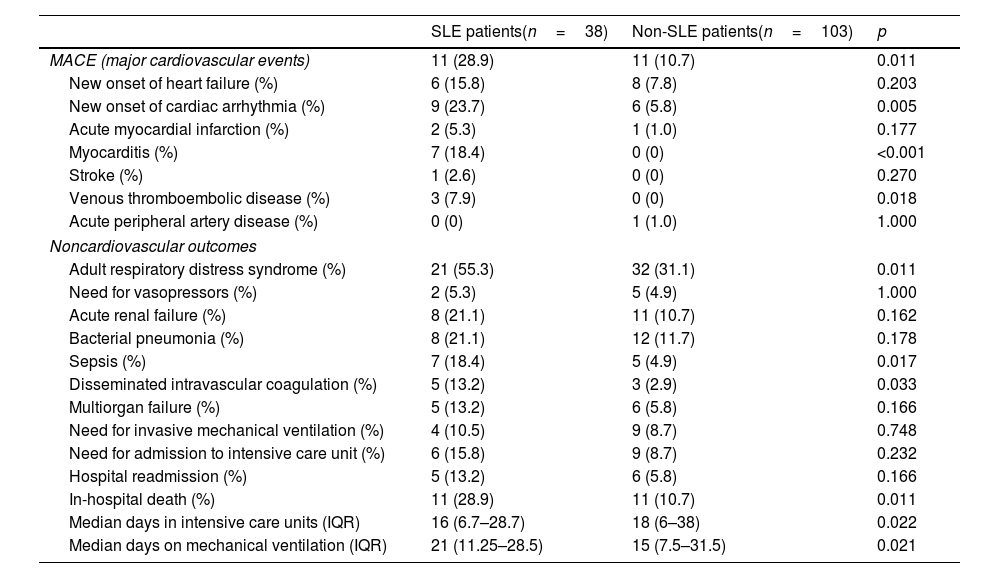
MACE and noncardiovascular outcomesCompared to those in the non-SLE group, SLE patients in the SLE group exhibited a significantly higher incidence of MACEs during hospitalization (28.9% vs 10.7%; p=0.011), primarily in the form of new cardiac arrhythmias (23.7% vs 5.8%; p=0.005), myocarditis (18.4% vs 0%; p<0.001), and venous thromboembolic disease (7.9% vs 0%; p=0.018). Conversely, it was significantly more common for SLE patients to present with adult respiratory distress syndrome (55.3% vs 31.1%; p=0.011), disseminated intravascular coagulation (13.2% vs 2.9%; p=0.033), sepsis (18.4% vs 44.9%; p=0.017), in-hospital mortality (28.9% vs 10.7%; p=0.011), or more days of mechanical ventilation (21 vs 15 days; p=0.021). The distributions of cardiovascular and noncardiovascular outcomes according to group are presented in Table 2.
Cardiovascular and noncardiovascular outcomes.
| SLE patients(n=38) | Non-SLE patients(n=103) | p | |
|---|---|---|---|
| MACE (major cardiovascular events) | 11 (28.9) | 11 (10.7) | 0.011 |
| New onset of heart failure (%) | 6 (15.8) | 8 (7.8) | 0.203 |
| New onset of cardiac arrhythmia (%) | 9 (23.7) | 6 (5.8) | 0.005 |
| Acute myocardial infarction (%) | 2 (5.3) | 1 (1.0) | 0.177 |
| Myocarditis (%) | 7 (18.4) | 0 (0) | <0.001 |
| Stroke (%) | 1 (2.6) | 0 (0) | 0.270 |
| Venous thromboembolic disease (%) | 3 (7.9) | 0 (0) | 0.018 |
| Acute peripheral artery disease (%) | 0 (0) | 1 (1.0) | 1.000 |
| Noncardiovascular outcomes | |||
| Adult respiratory distress syndrome (%) | 21 (55.3) | 32 (31.1) | 0.011 |
| Need for vasopressors (%) | 2 (5.3) | 5 (4.9) | 1.000 |
| Acute renal failure (%) | 8 (21.1) | 11 (10.7) | 0.162 |
| Bacterial pneumonia (%) | 8 (21.1) | 12 (11.7) | 0.178 |
| Sepsis (%) | 7 (18.4) | 5 (4.9) | 0.017 |
| Disseminated intravascular coagulation (%) | 5 (13.2) | 3 (2.9) | 0.033 |
| Multiorgan failure (%) | 5 (13.2) | 6 (5.8) | 0.166 |
| Need for invasive mechanical ventilation (%) | 4 (10.5) | 9 (8.7) | 0.748 |
| Need for admission to intensive care unit (%) | 6 (15.8) | 9 (8.7) | 0.232 |
| Hospital readmission (%) | 5 (13.2) | 6 (5.8) | 0.166 |
| In-hospital death (%) | 11 (28.9) | 11 (10.7) | 0.011 |
| Median days in intensive care units (IQR) | 16 (6.7–28.7) | 18 (6–38) | 0.022 |
| Median days on mechanical ventilation (IQR) | 21 (11.25–28.5) | 15 (7.5–31.5) | 0.021 |
Of the 3 cases with venous thromboembolic disease, 2 were deep venous thrombosis of the lower limbs and 1 was pulmonary thromboembolism. No patients underwent into extracorporeal membrane oxygenation, and there were no instances of aborted cardiac arrest in the studied cohort.
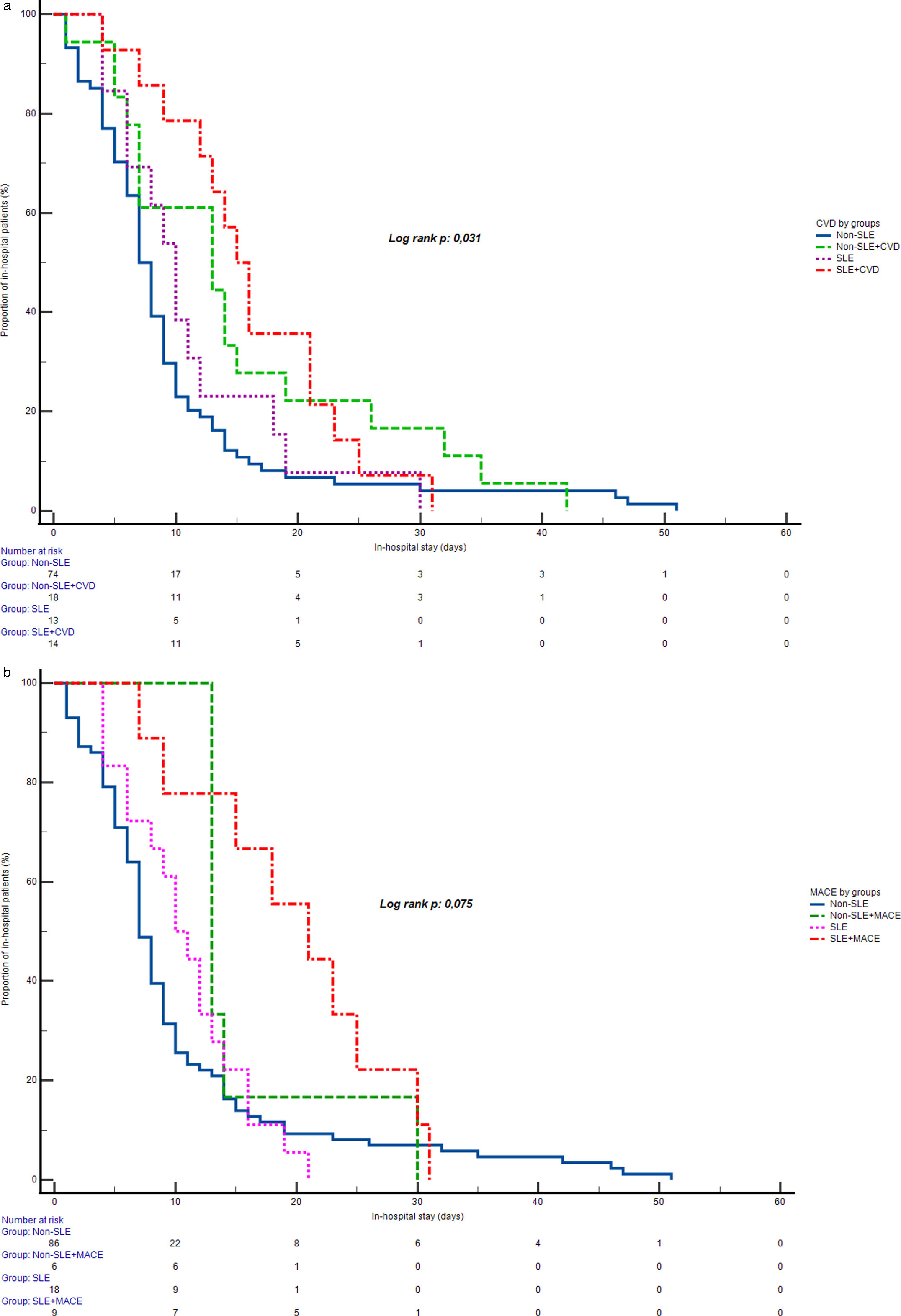
Among the surviving patients (71.1% in the SLE group vs. 89.3% in the non-SLE group), no statistically significant difference in in-hospital stay was observed between groups with or without MACE (Log Rank test, p=0.073), as indicated by similar median in-hospital stay durations across subgroups. However, a statistically significant difference in in-hospital stay emerged when comparing groups by prior CVD status (Log Rank test, p=0.031). The longest median in-hospital stay was observed in SLE patients with prior CVD (median of 15 days), followed by non-SLE patients with CVD (median of 13 days), SLE patients without CVD (median of 10 days), and non-SLE patients without CVD (median of 7 days). Fig. 1 displays the in-hospital stays across these groups.
Multivariate analysisFig. 2 illustrates the results of the multivariate analysis regarding MACEs, our primary outcome variable. SLE demonstrated a significant association with the occurrence of MACEs (OR: 3.934; 95% CI: 1.247–12.432), independent of age, sex, ethnic group, and the presence of obesity, arterial hypertension, diabetes mellitus without target organ damage, smoking, moderate-severe chronic kidney disease, chronic obstructive pulmonary disease, dyslipidemia, and C-reactive protein levels at admission.
In the multivariate analysis of the secondary outcomes, the same adjustment variables were used for the primary outcome, MACE. A statistically significant and independent relationship was found between SLE and the development of sepsis (OR: 6.880; 95% CI: 1.558–30.386) and between SLE and in-hospital mortality (OR: 3.187; 95% CI: 1.046–9.711). However, no statistically significant relationships were detected between SLE and the need for mechanical ventilation (OR: 1.089; 95% CI: 0.225–4.646), adult respiratory distress syndrome (OR: 2.162; 95% CI: 0.873–5.359), disseminated intravascular coagulation (OR: 5.601; 95% CI: 0.955–32.851), multiorgan failure (OR: 2.207; 95% CI: 0.557–8.857), intensive care unit admission (OR: 1.506; 95% CI: 0.407–5.569), or hospital readmission (OR: 1.262; 95% CI: 0.250–6.367) once adjusted for the remaining variables in the model.
DiscussionThis investigation of the cardiovascular profiles of SLE patients and COVID-19 patients hospitalized in Spain through analysis of the SEMI-COVID-19 Registry has the potential to shed light on crucial aspects of the interaction between these medical conditions. Patients with autoimmune diseases had previously been analyzed as a collective group in the SEMI-COVID-19 Registry23; however, this study left pending the individual analysis of autoimmune diseases such as SLE, and specific dimensions, particularly in terms of cardiovascular aspects, were not addressed.
It is important to note that it was difficult to find studies with which to compare our results because of the few investigations focused on patients with SLE and COVID-19, and of those available, many have not been performed in hospitalized patients, have not used a control group of non-SLE patients, or have not focused on cardiovascular aspects.9,10,24–27
After matching in our study, only moderate-severe chronic kidney disease, as cardiovascular risk factor, and the presence of stroke, as previous cardiovascular disease, were significantly more common in patients with SLE than in non-SLE patients. However, other authors have reported that, compared to those in nonhospitalized SLE patients, several comorbidities, including congestive heart failure (17% vs 4%), renal failure (30% vs 9%), complicated hypertension (31% vs 10%), complicated diabetes (22% vs 8%), peripheral vascular disorders (12% vs 5%) and coagulopathy (15% vs 7%), were significantly more common among COVID-19-hospitalized SLE patients.11 Our results could be related to the balance of variables sought through the matching strategy with non-SLE patients, which provides more reliable information similar to real-life conditions. These results are also in line with evidence showing a higher prevalence of stroke and chronic kidney disease (probably related to lupus nephritis) in SLE patients than in the general population.3,28
While other research has shown that black and Hispanic SLE patients have worse clinical outcomes,29 this was not observed in our study, possibly due to the coverage and organization of the Spanish health care system and the lower influence of social health disparities.
SLE patients with COVID-19 infection appear to develop more severe manifestations of COVID-19 infection than did matched controls,30 with higher rates of hospitalization, invasive ventilation requirement or death,10,11 especially in those with lupus nephritis and cardiovascular disease.11,31 However, this increased risk cannot be explained solely by comorbidities.30 In this regard, our results indicate that hospitalized patients with COVID-19 and SLE have an increased risk of developing MACE and that this risk is independent of age, sex, ethnicity, level of inflammation (measured by C-reactive protein), and other cardiovascular risk factors. These findings have clinical implications, as early identification of high-risk SLE patients would allow healthcare professionals to implement appropriate interventions and close monitoring to prevent complications and improve patient outcomes.
This study has several limitations. First, the small sample size of SLE patients (only 38 out of 20,970 participants) may impact the generalizability of our findings, potentially reflecting a subset of patients with more severe conditions requiring hospitalization. Second, the observational nature of the study inherently introduces the potential for bias and confounding factors that cannot be entirely controlled, limiting our ability to establish causal relationships. Despite employing a matching strategy to minimize confounding, certain unmeasured variables could still influence the results, necessitating cautious interpretation. Third, data were collected retrospectively from the SEMI-COVID-19 Registry, which may lead to issues related to data accuracy and completeness, including the potential for missing or imprecise information. Additionally, the lack of specific cardiac or metabolic biomarkers, such as troponins, natriuretic peptides, or lipids, limited the comprehensiveness of our cardiovascular analysis. We also lacked data on cumulative organ damage and disease activity assessments based on standardized SLE indices, which could have provided further insight into patient risk profiles.
Nevertheless, our study benefits from the collection of real-world data from 150 hospitals throughout Spain and contributes significantly to the understanding of the pattern of cardiovascular risk factors, underlying cardiovascular disease and clinical evolution in SLE patients hospitalized with COVID-19. The application of a matching strategy aimed at reducing imbalances in potential confounding variables between the SLE patients and control participants improved the robustness of our findings. A focus on the cardiovascular profile and complications of a specific subgroup of patients with little existing scientific evidence underscores the uniqueness of the study and its potential contribution to clinical decision making in SLE patients.
These efforts align with the fundamental goals of reporting factors associated with cardiovascular outcomes in patients with COVID-19, supporting future pandemic preparedness, and developing potential therapeutic strategies to improve cardiovascular care and outcomes in patients admitted with COVID-19.7,13,16 Ultimately, this research could open new perspectives for personalized patient care by exploring the potential impact of individual clinical phenotypes on cardiovascular prognosis.
ConclusionsSLE patients hospitalized with COVID-19 have an unfavorable baseline cardiovascular profile and are more prone to MACEs and adverse noncardiovascular outcomes during hospitalization. These findings accentuate the necessity for early identification of high-risk SLE patients, enabling healthcare professionals to implement tailored interventions and closely monitor patients during COVID-19 admission.
Authors’ contributionsConceptualization: H.H.-N., M.R.B.-L., A.L.-S., M.R.R., J.A.A.G., A.G.U., M.C., M.L.T.M., A.M.M., J.L.B.P., J.D.T.P., J.A.M.O., J.L.A., J.M.C.R., R.G.S., M.L.L.R., B.R.B., J.F.-S., F.A.M., N.V.L., R.V.B., A.P.G., J.-M.R.-R., R.G.-H.; SEMI-COVID-19 Network. Methodology: H.H.-N., M.R.B.-L., A.L.-S., M.R.R., J.A.A.G., A.G.U., M.C., M.L.T.M., A.M.M., J.L.B.P., J.D.T.P., J.A.M.O., J.L.A., J.M.C.R., R.G.S., M.L.L.R., B.R.B., J.F.-S., F.A.M., N.V.L., R.V.B., A.P.G., J.-M.R.-R., R.G.-H.; SEMI-COVID-19 Network. Software: H.H.-N., M.R.B.-L., A.L.-S. Validation: H.H.-N., M.R.R., M.L.T.M., A.M.M., J.L.B.P., J.A.M.O., J.L.A., J.M.C.R., R.G.S., M.L.L.R., B.R.B., J.F.-S., F.A.M., N.V.L., R.V.B., A.P.G., J.-M.R.-R., R.G.-H.; SEMI-COVID-19 Network. Formal Analysis: R.G.-H. Investigation: H.H.-N., M.R.B.-L., A.L.-S., M.R.R., J.A.A.G., A.G.U., M.C., M.L.T.M., A.M.M., J.L.B.P., J.D.T.P., J.A.M.O., J.L.A., J.M.C.R., R.G.S., M.L.L.R., B.R.B., J.F.-S., F.A.M., N.V.L., R.V.B., A.P.G., J.-M.R.-R., R.G.-H.; SEMI-COVID-19 Network. Resources: A.A.; H.H.-N., J.G.O., M.R.R., M.L.T.M., A.M.M., J.L.B.P., J.A.M.O., J.L.A., J.M.C.R., R.G.S., M.L.L.R., B.R.B., J.F.-S., F.A.M., N.V.L., R.V.B., A.P.G., J.-M.R.-R., R.G.-H.; SEMI-COVID-19 Network. Data Curation: H.H.-N., M.R.B.-L., A.L.-S.; SEMI-COVID-19 Network. Writing—Original Draft Preparation: H.H.-N., M.R.B.-L, A.L.-S. Writing—Review and Editing: H.H.-N., M.R.B.-L., A.L.-S, R.G.-H. Supervision: R.G.-H. Project Administration: R.G.-H. All authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
AuthorshipsDr. Ricardo Gómez-Huelgas and Dr. José Manuel Ramos-Rincón have contributed equally to this work and share the final authorship.
Informed consentInformed consent was obtained from all subjects involved in the study.
Ethics approvalThis study was conducted according to the guidelines of the Declaration of Helsinki and approved by Provincial Research Ethics Committee of Málaga (Spain). Ethics Committee code: SEMI-COVID-19 27-03-20.
FundingM. Rosa Bernal-Lopez was supported by “Miguel Servet Type II” program (CPII/00014) from the ISCIII-Madrid (Spain), co-funded by the Fondo Europeo de Desarrollo Regional-FEDER. M. Rosa Bernal Lopez (“Nicolas Monardes” program, C1-0005-2020) was supported by Consejeria de Salud, Junta de Andalucía, and Halbert Hernandez-Negrin (PREDOC-00826) was supported by Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía-Sevilla (Spain).
Conflicts of interestThe authors declare that there are no conflicts of interest.
Data availabilityThe datasets generated during the present study are not publicly available due to ethical or privacy restrictions but may be requested for reasonable reasons from the author for correspondence.
We gratefully acknowledge all the investigators who participate in the SEMI-COVID-19 Registry.
Halbert Hernández-Negrín1,2, María Rosa Bernal-López1–3, Almudena López-Sampalo1,2, Manuel Rubio-Rivas4, Josefa Andrea Aguilar-García5, Angie Gómez-Uranga6, María Carnevali7, María Luisa Taboada-Martínez8, Antonio Muiño-Miguez9, José Luis Beato-Perez10, José David Torres-Peña3,11, José Ángel Martín-Oterino12, José Loureiro-Amigo13, José Manuel Casas-Rojo14, Ricardo Gil-Sánchez15, Manuel Lorenzo López-Reboiro16, Berta Román-Bernal17, Joaquim Fernandez-Sola18, Francisco Amorós-Martínez19, Natalia Vicente-López20, Reina Valle-Bernad21, Alejandro Pérez-González22, José-Manuel Ramos-Rincón23, Ricardo Gómez-Huelgas1,2,3* on behalf of the SEMI-COVID-19 Network
1Internal Medicine Clinical Management Unit, Hospital Regional Universitario de Málaga, Instituto de Investgación Biomédica de Málaga (IBIMA-Plataforma BIONAND), Avenida Carlos Haya S/N, 29010 Málaga, Spain
2Faculty of Medicine, Universidad de Málaga, Campus Teatinos, 29010 Málaga, Spain.
3Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición (CIBERobn), Instituto de Salud Carlos III, 28029 Madrid, Spain.
4Internal Medicine Department. Hospital Universitario de Bellvitge. L’Hospitalet de Llobregat, Barcelona, Spain.
5Internal Medicine Department. Hospital Costa del Sol de Marbella, Málaga, Spain.
6Internal Medicine Department. Hospital Universitario de San Juan, Alicante, Spain.
7Internal Medicine Department, 12 de Octubre University Hospital, Madrid, Spain.
8Internal Medicine Department. Hospital Universitario de Cabueñes, Gijón, Asturias, Spain.
9Internal Medicine Department. Hospital Universitario Gregorio Marañón, Madrid, Spain.
10Internal Medicine Department. Complejo Hospitalario Universitario de Albacete, Albacete, Spain.
11Lipids and Atherosclerosis Unit, Department of Internal Medicine, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain.
12Internal Medicine Department. Complejo Asistencial Universitario de Salamanca, Salamanca, Spain.
13Internal Medicine Department. Moisès Broggi Hospital, Sant Joan Despí, Barcelona, Spain.
14Internal Medicine Department. Hospital Universitario Infanta Cristina, Parla, Madrid, Spain.
15Internal Medicine Department. Hospital Universitario La Fe, Valencia, Spain.
16Internal Medicine Department. Hospital Público de Monforte de Lemos, Lugo, Spain.
17Internal Medicine Department. Hospital Dr. José Molina Orosa, Lanzarote, Spain.
18Internal Medicine Department. Hospital Clínic de Barcelona, IDIBAPS, Barcelona, Spain.
19Internal Medicine Department. Hospital Universitario del Vinalopo, Alicante, Spain.
20Internal Medicine Department. Hospital Universitario del Sureste, Arganda del Rey, Madrid, Spain.21Internal Medicine Department. Hospital Sierrallana. Torrelavega, Cantabria, Spain.
22Internal Medicine Department. Hospital Comarcal de la Axarquía, Vélez-Málaga, Málaga, Spain.
23Department of Clinical Medicine. Miguel Hernandez University of Elche, Alicante, Spain