Persistent post-COVID olfactory dysfunction continues to be studied due to the controversy in its pathophysiology and neuroimaging.
Materials and methodsThe patients had confirmed mild COVID-19 infection with olfactory dysfunction of more than one month of evolution and they were compared to controls with normal olfaction, assessed using the Sniffin’ Sticks Olfactory Test and underwent brain, magnetic resonance imaging (MRI) of the olfactory bulb and olfactory function.
ResultsA total of 8 patients and 2 controls participated. The average age of the patients was 34.5 years (SD 8.5), and that of the controls was 28.5 (SD 2.1). The average score in the patients’ olfactory test was 7.9 points (SD 2.2). In brain and olfactory bulb MRI tests, no morphological differences were found. When evaluated by functional MRI, none of the patients activated the entorhinal area in comparison to the controls, who did show activation at this level. Activation of secondary olfactory areas in cases and controls were as follows: orbitofrontal (25% vs 100%), basal ganglia (25% vs 50%) and insula (38% vs 0%) respectively.
ConclusionsThere were no observed morphological changes in the brain MRI. Unlike the controls, none of the patients activated the entorhinal cortex in the olfactory functional MRI.
La alteración olfatoria persistente post-COVID continúa en estudio por la controversia en su fisiopatología y neuroimagen.
Materiales y métodosLas pacientes tuvieron COVID-19 leve confirmada con una disfunción olfatoria de más de un mes de evolución y se compararon con controles con olfacción normal, evaluados mediante el Sniffin’ Sticks Olfatory Test y sometidos a una resonancia magnética (RM) cerebral, del bulbo olfatorio y funcional olfatoria.
ResultadosParticiparon 8 pacientes y 2 controles. La edad media de las pacientes fue 34,5 años (DE 8,5) y la de los controles fue 28,5 (DE 2,1). La puntuación media de los pacientes en el test olfatorio fue de 7,9 puntos (DE 2,2). En las pruebas de RM cerebral y bulbo olfatorio no se encontraron diferencias morfológicas. En la RM funcional ninguna de las pacientes activó el área entorrinal, en contraposición a los controles que sí mostraron activación a este nivel. La activación de áreas olfatorias secundarias en casos y controles fue orbitofrontal (25% vs. 100%), ganglios basales (25% vs. 50%) e ínsula (38% vs. 0%) respectivamente.
ConclusionesNo se apreciaron cambios morfológicos en la RM cerebral ni en el bulbo olfatorio. A diferencia de los controles, ninguna de las pacientes activó la corteza entorrinal en la RM funcional olfatoria.
From the time when SARS-COV-2 emerged in late 2019, olfactory dysfunction has been one of the most characteristic manifestations of this infection. Its prevalence varies according to the studies (13–25%) and reaches up to 50% in the stratified analysis for non-hospitalized patients.1 This suggests that it may be more common in individuals who experienced a mild form of the disease. Moreover, the use of validated tests such as the Sniffin’ Sticks Olfactory Test in COVID-19 patients has revealed a more objective view of reality.2
The pathogenesis of post-COVID anosmia is still controversial. Injury to sustentacular cells of the mucosa through the cellular receptor angiotensin-converting enzyme 2 (ACE2) may explain cases of transient olfactory dysfunction during the time of its regeneration. However, the etiopathogenesis of persistent olfactory dysfunction is debated because olfactory receptors do not express ACE2 receptors.2 Research is ongoing to investigate the potential pathway for the virus to access the central nervous system (CNS), especially in those patients who had a mild COVID infection without a high systemic viral load.
During the acute phase of the disease, case series were published with enhancement at the olfactory bulb in MRI. In later phases, MRI findings in some cases with olfactory bulb atrophy, as well as cortical hypometabolism in PET-CT scans, may suggest neurotropism of the virus, but the literature is still limited and contradictory.3
Our study aimed to provide new evidence regarding alterations of the CNS that occur in patients with persistent olfactory dysfunction after mild COVID-19 disease. To do this, we selected cases with objective hyposmia assessed using the Sniffin’ Stick Olfactory Test and compared them systematically to controls. We examined the findings in structural brain MRI, olfactory bulb MRI, and functional olfactory MRI (fMRI). Odor-stimulated functional MRI has also been used in assessment of OD in neurodegenerative disease and posttraumatic anosmia.4 However, to date, there have been few publications on its use in persistent olfactory dysfunction post-COVID.
Materials and methodsStudy populationOur study is a descriptive, observational study conducted at the University Hospital of Toledo, a tertiary care center serving a population of approximately 478,000 residents. It was conducted following the principles of the Helsinki Declaration and was approved by the Ethics Committee of the University Hospital of Toledo. Informed consent was obtained from all participants.
The inclusion criteria were as follows: female gender, due to the higher prevalence of COVID-related hyposmia in this population1 and gender differences in olfactory fMRI,5 and an age range between 16 and 50 years to avoid presbyosmia.6 Additionally, a positive COVID-19 test and subjective olfactory dysfunction lasting more than one month after recovering from COVID-19 were required.
The exclusion criteria were: previously diagnosed olfactory or gustatory dysfunction, hospitalization during acute COVID-19 infection, presence of parosmia at the time of evaluation, previously diagnosed or objectively documented relevant neurological or otolaryngological (ENT) disorders observed during the study assessments.
As for the control group, we included women up to 50 years of age who had consulted with neurology for other conditions unrelated to olfactory disturbances. Those who had already experienced COVID-19, had previous olfactory impairments, or obtained a pathological result in the olfactory test were excluded from the study.
The inclusion of both volunteer patients and controls who met the previously mentioned criteria were conducted over a period of 5 months in the second half of 2021.
ProceduresAll participants underwent a clinical interview in which sociodemographic variables related to olfactory disturbances and other symptoms such as parosmias, phantosmias, or brain fog were collected. A neurological examination and an ENT assessment were also conducted. To evaluate olfactory function, the Sniffin’ Sticks Olfactory Test was administered. After meeting the previously mentioned criteria, a brain MRI with olfactory functional study was performed.
Olfactory evaluationThe Sniffin’ Sticks are felt-tip pens that contain a selection of odors described by Hummel et al.6 This test has shown its utility for the study of olfaction in different cultures and populations, including Spain.2 The kit used was the Sniffin’ Sticks 16 odor identification test (n=16). Its application was carried out following standard instructions. To gather additional clinical information, we asked patients to specify with which pens they experienced selective anosmia.
MRI protocolAll participants underwent a 1.5T MRI (Magnetom, Siemens, Erlangen, Germany) with an 8-channel head coil. In addition to conventional brain sequences, sequences dedicated to the olfactory system included sagittal and coronal T2 images with a field of view (FOV) covering the anterior pole of the olfactory bulb to the primary olfactory region.
Orange and anise were the olfactory stimuli used during the olfactory fMRI. The choice of one or the other stimuli was made individually for each patient based on which one they had reported anosmia to in the Sniffin’ Sticks Olfactory Test. Conversely, the controls were randomly stimulated with either orange or anise if they had performed successfully in the olfactory test. This approach aimed to replicate the olfactory test results as faithfully as possible, avoiding artifacts in the diffusion of the aroma.
The analysis of the images obtained by MRI and fMRI were conducted by a neuroradiologist with extensive experience and blinded to the cases of olfactory dysfunction and healthy controls. The processing of fMRI data was carried out using Syngovia MR-NEURO 3D software (VB40, Siemens). The extended version of MRI protocol, acquisition parameters and image evaluation are shown in Supplementary material.
ResultsIn the neuroimaging study, 8 patients who met the previously mentioned criteria participated. Additionally, 2 controls who had not experienced COVID-19 infection and scored within the normal range on the olfactory test also took part in the study.
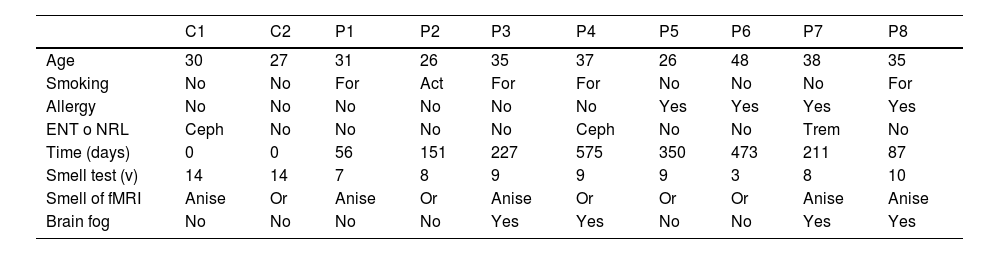
Sociodemographic variables and background of the sampleThe mean age of the patients was 34.5 years (SD 8.5) and 28.5 years (SD 2.1) for the controls. The sociodemographic and clinical variables of the patients and controls are described in Table 1. Patients 5–8 reported allergies to pollen, grass, or ragweed. None of them had allergic rhinitis, diabetes mellitus, head injury, or relevant ENT conditions.
Demographic, background, and olfactory characteristics of the participants.
| C1 | C2 | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 30 | 27 | 31 | 26 | 35 | 37 | 26 | 48 | 38 | 35 |
| Smoking | No | No | For | Act | For | For | No | No | No | For |
| Allergy | No | No | No | No | No | No | Yes | Yes | Yes | Yes |
| ENT o NRL | Ceph | No | No | No | No | Ceph | No | No | Trem | No |
| Time (days) | 0 | 0 | 56 | 151 | 227 | 575 | 350 | 473 | 211 | 87 |
| Smell test (v) | 14 | 14 | 7 | 8 | 9 | 9 | 9 | 3 | 8 | 10 |
| Smell of fMRI | Anise | Or | Anise | Or | Anise | Or | Or | Or | Anise | Anise |
| Brain fog | No | No | No | No | Yes | Yes | No | No | Yes | Yes |
C: control. P: patient. ENT o NRL: Otorhinolaryngological or neurological background. For: former smoker. Act: active smoker. Ceph: headache. Trem: tremor. Or: orange.
As observed in Table 1, the average duration of olfactory impairment until the patients’ consultation was 266.3 days (SD 177.8). Patient 4 had the longest duration, with 575 days at the time of the study.
The average score on the Sniffin’ Stick Olfactory Test for patients was 7.9 points (SD 2.2). In the case of the controls, both individuals had an identical score within the normal range. Regarding symptoms associated with post-COVID olfactory dysfunction, 50% reported symptoms consistent with brain fog.
MRI of the olfactory bulbIn all the patients, a preserved olfactory bulb morphology was observed when compared to that of the controls. Additionally, none of them exhibited signal alterations or any type of hyperintensity at this level upon contrast injection (Supplementary material).
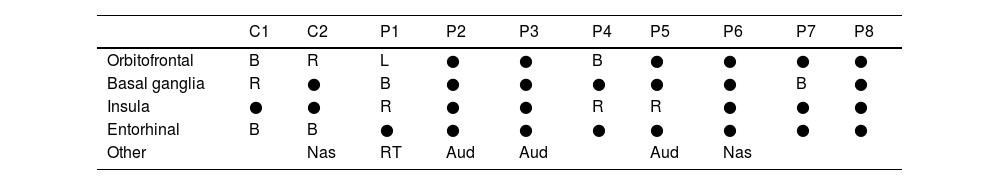
Functional olfactory MRIAs we can see in Table 2, orbitofrontal activity was not observed in 75% of COVID-19 patients, and in those who did show activity, it was either on the left (patient 1) or bilateral (patient 4) (Fig. 1). Activation of this area did occur in both controls.
Areas of activation in olfactory functional MRI.
| C1 | C2 | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Orbitofrontal | B | R | L | ● | ● | B | ● | ● | ● | ● |
| Basal ganglia | R | ● | B | ● | ● | ● | ● | ● | B | ● |
| Insula | ● | ● | R | ● | ● | R | R | ● | ● | ● |
| Entorhinal | B | B | ● | ● | ● | ● | ● | ● | ● | ● |
| Other | Nas | RT | Aud | Aud | Aud | Nas |
C: control. P: patient. R: right. L: left. B: bilateral. Nas: nasal area. RT: right thalamus. Aud: auditory area. ●: no activation.
fMRI patterns of brain activation. Control 2 is a 27-year-old woman with a normal olfactory test who, when stimulated with the scent of orange in the 3D T1 fMRI sequence, showed activation in the entorhinal, nasal areas and right orbitofrontal cortex in sagittal, coronal and axial planes (A–C) marked with a white arrow. Patient 4 is a 37-year-old woman with hyposmia for 575 days at the time of evaluation. When stimulated with the same scent of orange in the 3D T1 fMRI sequence, bilateral orbitofrontal activation was observed in sagittal, coronal and axial planes, but not entorhinal (D–F) limited by white arrows.
Activation in the basal ganglia was observed in 25% of the cases (all bilateral) and in 50% of the controls. In 38% of the patients, activity was seen in the insula, all of them on the right side (Supplementary material). This signal was not observed in any of the controls.
Regarding entorhinal activity, as reflected in Table 2, bilateral uptake was observed in both controls, unlike the cases where none showed activation at this level (Fig. 1).
DiscussionBased on previous literature, post-viral loss of smell is predominantly observed among female patients, and this has been reflected in the percentage of participants in many post-COVID studies published to date.2,4,7,8 The increase in the number of affected individuals in this population could be attributed to gender-related variations in the inflammatory process and the greater willingness of women to voluntarily participate in studies. However, in addition to the observed difference in prevalence, there has also been reported potential dissimilarity in olfaction studies with neuroimaging.5 Therefore, we matched cases and controls based on these characteristics to ensure a more precise comparison.
Furthermore, all our participants experienced mild forms of COVID-19 and were not hospitalized. Most brain imaging publications thus far have focused on moderate to severe forms of COVID-19. However, recent studies with a majority of patients having mild forms provide new evidence of brain damage in these individuals, highlighting the fundamental need for further research into the pathology that occurs in this class of patients.7–9
So far, very few studies have assessed the long-term brain alterations that occur after SARS-CoV-2 infection. In the available literature, the average time from the onset of symptoms to the neuroimaging study varied widely: from 14 days in the earliest study to approximately 330 days in the longest study.3,9 In our study, the time elapsed from the onset of olfactory dysfunction to the assessment moment was 266.3 days. This extended period of symptoms could favor the notion that the findings established in neuroimaging tests are due to persistent lesions caused by the virus rather than transient inflammation or edema of the olfactory mucosa or bulb, as observed in other studies during the acute phase.3 This could be supported by the fact that the patients in our sample, when compared to the controls, did not exhibit alterations in the morphology of the bulb or hyperintensities in different sequences or with contrast administration, as seen in other studies.8 However, the availability of olfactory bulb images in post-COVID patients is still limited.
Systematically, a distinctive feature of our study was the use of specific Sniffin’ Sticks for each patient during olfactory stimulation in fMRI, instead of inhaled scents of similar neutral odor for all participants, as had been done in other studies.4 This way, based on the possibility that deficits may be personalized for a specific odor,2 we obtained images directly extrapolated from the clinical experience of olfactory dysfunction and avoided the possibility of odorant contamination in the ventilation systems during the resting phase of fMRI.
When we receive an olfactory stimulus through the olfactory receptors in the mucosa, this information is transmitted to the olfactory bulb. Subsequently, the information is processed in the primary olfactory cortex (piriform cortex, entorhinal cortex, and amygdala) and relayed to secondary areas (such as the orbitofrontal cortex, insula, thalamus, or parahippocampal gyrus).
In our sample, there was no activation of the entorhinal cortex (EC) in any of the patients, which significantly contrasts with the controls, where signal at this level was observed. This differs from the absence of activation percentage reported by Yildirim et al., which was at 61%.4 However, recent longitudinal studies showed hypoconnectivity in patients with post-COVID syndrome in the parahippocampal gyrus,7 closely related to the EC, and a reduction in gray matter volume in both the parahippocampal gyrus and the EC.8
Due to its strategic position, olfactory information reaches the EC directly through projections from mitral cells in the olfactory bulb and indirectly from other olfactory cortical areas, including the piriform cortex. These intricate local networks suggest an important role in odor discrimination, top-down control involvement, and local processing of olfactory information into multimodal associative memories.10 EC lesions have been described to affect the discrimination of well-learned specific odors that require high olfactory acuity, while simple odor discrimination remains intact. This could explain why most patients in the sample exhibit olfactory dysfunction in the range of hyposmia, as olfactory acuity is required in olfactory testing. However, sufficient olfaction remained for identifying basic odors and achieving a score above the anosmia threshold.
Regarding the activation of some secondary olfactory areas, orbitofrontal activity was not observed in 75% of the cases. This finding are consistent with recent publications that demonstrate reduced perfusion, as well as hypoconnectivity and gray matter reduction in the orbitofrontal area of these patients.7,8 We also observed insula activation in 38% of the patients, as seen in a study where post-COVID olfactory dysfunction patients exhibited increased connectivity in the right insula, related to enhanced connections in the right thalamus,9 similar to what we can observe in patient one.
Nevertheless, we must emphasize that the results of this study should be interpreted with caution due to limitations in sample size, being a single-center study, and the limited number of studies available for comparison.
ConclusionsIn our study, patients with persistent post-COVID olfactory dysfunction did not show differences compared to the controls in terms of morphology or hyperintensities in the MRI of the olfactory or cerebral bulb.
Consistent with previous literature, in olfactory fMRI, bilateral entorhinal area activation was observed in controls, but no signal at this level was detected in any of the patients with olfactory dysfunction. This structure is part of the primary olfactory cortex and may play a crucial role in the discrimination of highly specific odors. Some differences in the activation of secondary olfactory areas such as the orbitofrontal and insular cortex were also noted. Future studies with a larger sample size will need to confirm these findings in patients with persistent olfactory dysfunction following mild COVID-19 infection.
Ethical considerationsOur study was conducted following the principles of the Helsinki Declaration and was approved by the Ethics Committee of the University Hospital of Toledo. Informed consent was obtained from all participants.
Conflict of interestThe authors declared no potential conflicts of interest and no financial support with respect to the research, authorship, and/or publication of this article.
Acknowledgments to the departments of Neurology and Neuroradiology at the University Hospital of Toledo, as well as the participating authors, for their scientific curiosity and willingness to move it forward.