Hyponatraemia has negative effects on cognitive function and gait stability and is a risk factor for osteoporosis, falls, fractures and hospital mortality. Acute hyponatraemia can lead to neurological dysfunction due to cerebral oedema. Its rapid correction can also be fatal, leading to osmotic demyelination syndrome. For some antiepileptics, thiazides, benzodiazepines or antidepressants this reaction is widely described. Knowing which drugs are most likely to cause hyponatraemia will allow early detection and prevention of its complications, as well as individualising the prescription of these drugs according to the patient's characteristics.
ObjectiveThe main objectives are to identify potential new safety signals related to hyponatraemia and to analyse the cases of hyponatraemia reported to the Spanish Pharmacovigilance System for Medicines for Human Use (SEFV-H).
MethodA disproportionality and a descriptive analysis of individual case safety reports (ICSR) was performed in the SEFV-H database (FEDRA).
ResultsSix hundred and fifty-nine cases of suspected drug-induced hyponatraemia were found (0.6% of the total database). Over the 5 years period studied, there was a 57% increase in the number of hyponatraemia reports in Spain. Most of the reported cases were serious (93%). Patients were most often women (63.7%) and elderly (71.9%). The time to onset ranged from 1 to 7030 days (median, 79 days) and approximately 70% of the total occurred within the first year of treatment. Five hundred and forty-six patients (82.9%) showed complete recovery after the withdrawal of the suspected medicine. Diuretics (reported in 57.7% of the cases), antidepressants (in 25%), drugs acting on renin angiotensin system (in 24%) and antiepileptics (in 20.2%) were the most frequent involved drugs. Disproportionate reporting has been found for almost all the substances most frequently reported, higher for amiloride and oxcarbazepine. Regarding new safety signals, the Reporting Odds Ratio (ROR) (95% CI) was found to be statistically significant for valsartan [7.7 (5.1–11.5)], olmesartan [7.3 (4.7–11.1)], amlodipine [3.4 (2.1–5.4)], pregabalin [2.5 (1.4–4.5)], irbesartan [18.6 (9.6–35.9)], paliperidone [2.7 (1.3–5.7)], ritonavir [2.4 (1.1–5.5)], atosiban [29.7 (8.6–102.2)], melphalan [9.7 (3.5–26.8)] and clozapine [4.4 (1.6–11.8)]. These active ingredients do not include this reaction on their SPC and comply with the EMA criteria for a safety signals.
ConclusionThere are increasing reports of drug-induced hyponatraemia. It can be serious and seems to most often affect women over 65 years of age who take more than 1 medication. The time to onset varies and can be very long, so patient monitoring should be continuous throughout treatment. Hydrochlorothiazide is the drug with the highest number of reported cases in our setting. In terms of disproportionate reporting, diuretics leads the list, followed by antiepileptics as oxcarbazepine and eslicarbazepine. Safety signals were found for several drugs, more plausibly for pregabalin and paliperidone, thus a possible association between these drugs and hyponatraemia/SIAD is identified. This signal must be further studied. Meanwhile healthcare professionals should pay attention to this possibility. The reporting of suspected ADRs is essential to understand the risks associated with medicines once they are on the market.
Conocer qué fármacos pueden causar hiponatremia permitirá detectarla precozmente, prevenir complicaciones e individualizar la prescripción de estos fármacos.
ObjetivosIdentificar posibles nuevas señales de seguridad relacionadas con la hiponatremia y analizar los casos de hiponatremia notificados al Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano (SEFV-H).
MétodoAnálisis descriptivo y de desproporcionalidad en la base de datos del SEFV H.
ResultadosSe encontraron 659 casos de sospechas de hiponatremia de origen medicamentoso (0,6% del total de la base de datos). Durante el periodo de 5 años estudiado, se produjo un aumento del 57% en el número de notificaciones de hiponatremia en España. La mayoría de los casos eran graves (93%), producidos en mujeres (63,7%) y en ancianos (71,9%). El tiempo transcurrido hasta la aparición de la enfermedad osciló entre 1 y 7.030 días (mediana, 79 días) y aproximadamente el 70% del total se produjo durante el primer año de tratamiento. Quinientos cuarenta y seis pacientes (82,9%) mostraron una recuperación completa tras la retirada del medicamento sospechoso. Diuréticos (en el 57,7% de los casos), antidepresivos (25%), fármacos que actúan sobre el sistema renina angiotensina (24%) y antiepilépticos (20,2%) fueron los medicamentos más frecuentemente implicados. Se ha observado una desproporción en la notificación de casi todos los fármacos más frecuentes, mayor en el caso de amilorida y oxcarbazepina. En relación con las nuevas señales de seguridad, el Reporting Odds Ratio (ROR) (IC 95%) fue estadísticamente significativa para: valsartán [7,7 (5,1-11,5)], olmesartán [7,3 (4,7-11,1)], amlodipino [3,4 (2,1-5,4)], pregabalina [2,5 (1,4-4.5)], irbesartán [18,6 (9,6-35,9)], paliperidona [2,7 (1,3-5,7)], ritonavir [2,4 (1,1-5,5)], atosibán [29,7 (8,6-102,2)], melfalán [9,7 (3,5-26,8)] y clozapina [4,4 (1,6-11,8)]. Estos principios activos no incluyen esta reacción en su ficha técnica y cumplen los criterios de la EMA para las señales de seguridad.
ConclusionesCada vez se notifican más casos de hiponatremia al SEFV-H. Esta puede ser grave y parece afectar con mayor frecuencia a mujeres mayores de 65 años que toman más de 1 medicamento. El tiempo de aparición varía y puede ser muy largo, por lo que la monitorización del paciente debe ser continua durante todo el tratamiento. Hidroclorotiazida es el fármaco con mayor número de casos notificados en nuestro medio. En términos de desproporción, los diuréticos encabezan la lista, seguidos de antiepilépticos como oxcarbazepina y eslicarbazepina. Se encontraron señales de seguridad para varios fármacos, más plausibles para pregabalina y paliperidona, por lo que se identifica una posible asociación entre estos fármacos y la hiponatremia/SIAD. Estas señales deben seguir estudiándose. Mientras tanto, los profesionales sanitarios deben prestar atención a esta posibilidad. La notificación de sospechas de reacciones adversas es esencial para comprender los riesgos asociados a los medicamentos una vez comercializados.
Hyponatraemia, defined as a plasma sodium concentration below 135mEq/L, is the most common water–electrolyte disorder in the population.1 Clinically, it presents with a series of neurological manifestations resulting from the production of cerebral oedema, the severity of which depends not only on sodium levels but also on the time of onset. Thus, if it is acute (onset <48h), symptoms vary from headache and nausea in mild cases, to disorientation, cerebral herniation, coma and death. If it appears gradually (>48h), the organism sets in motion a cerebral adaptation to the oedema that makes the symptoms subtle. Although apparently asymptomatic, it has a negative effect on cognitive function and gait stability, constituting a risk factor for osteoporosis, falls, fractures and hospital mortality even when the sodium deficit is moderate. In addition, excessively rapid correction of natraemia in such cases may cause a potentially fatal osmotic demyelination syndrome.1
Hyponatraemia is classified into hypovolaemic, euvolemic and hypervolaemic, with differentiated aetiology and management. Among the causes of hyponatraemia are sodium losses; water gain greater than sodium gain characteristic of pathologies such as cirrhosis, heart failure, renal failure or pregnancy and some endocrine alterations such as hypothyroidism, glucocorticoid deficiency or, the most frequent cause, the syndrome of inappropriate antidiuresis (SIAD).1,2 In SIAD there is an abnormal production of antidiuretic hormone (ADH). In addition to various pathological conditions, this syndrome can be caused by drugs which, either stimulate ADH secretion or increase renal sensitivity to ADH.3 For some drugs this reaction is widely described, such as some antiepileptics, thiazides, benzodiazepines or antidepressants.1,3
Knowing which drugs are most likely to cause hyponatraemia will allow early detection of its onset and prevention of its complications, as well as individualising the prescription of these drugs according to the patient's characteristics.
ObjectivesThe main objective is to identity potential new safety signals related to hyponatraemia. Secondary objectives are to analyse the cases of hyponatraemia reported to the Spanish Pharmacovigilance System for Medicines for Human Use (SEFV-H), its main characteristics, the drugs most involved and the evolution of the reporting of this adverse reaction over the studied years.
MethodsA disproportionality analysis of individual case safety reports (ICSR) was performed in the SEFV-H database to explore hyponatraemia and to identify potential new safety signals related to this adverse drug reaction (ADR).
Data source and study periodWe used FEDRA, the Spanish Pharmacovigilance database, to identify all the hyponatremia cases reported from 1 January 2016 to 31 Decembre 2020. FEDRA includes all ADRs reported to the SEFV-H by healthcare professionals since 1983. It also includes cases reported by the pharmaceutical industry and by citizens, since 2013. It includes both spontaneously reported cases (cases seen by health professionals during their usual clinical practice for which reporting is mandatory) and cases from observational studies. Reports are evaluated by qualified personnel at the Regional Pharmacovigilance Centres and coded according to international standards with the Medical Dictionary for Regulatory Activities (MedDRA).4 Medicines are coded using a national ‘nomenclature’ that codes each active ingredient and includes also the World Health Organisation's Anatomical Therapeutic Chemical Classification (ATC) system.
The database currently contains more than 490,000 spontaneous reports of suspected adverse drug reactions.
Cases identificationA case of hyponatraemia was considered if it included any of the adverse reactions listed in the Standardised MedDRA Query (SMQ) “Hyponatraemia/SIADH” (MedDRA v23.1). This SMQ includes the following Preferred Terms (PT): “Antidiuretic hormone abnormality”, “Blood antidiuretic hormone abnormal”, “Blood antidiuretic hormone increased”, “Blood sodium abnormal”, “Blood sodium decreased”, “Ectopic antidiuretic hormone secretion”, “Hyponatraemia”, “Hyponatraemic coma”, “Hyponatraemic encephalopathy”, “Hyponatraemic syndrome”, “Hypoosmolar state”, “Inappropriate antidiuretic hormone secretion”, “Neonatal hyponatraemia”, “Osmotic demyelination syndrome”, “Rapid correction of hyponatraemia”. All types of patients, without restriction by sex or age, were included in the descriptive study.
As an exploratory study, no specific drug was searched for, but all drugs involved were analysed.
Only valid spontaneous reports were included in the study, i.e. reports from research studies were not included, and duplicate cases were removed.
Data analysisA descriptive study was carried out on the variables of interest: sex, age, adverse reactions, severity of reaction, suspected active ingredients and time to onset of the adverse reaction.
We performed a case/non-case study with a disproportionality analysis. Cases were all reports of “hyponatremia/SIADH”. The non-cases used as controls included all other adverse drug reaction reports recorded in FEDRA for the same period. The case/non-case analysis calculated reporting odds ratios (RORs) and their 95% confidence intervals (CIs) as a measure of disproportionality between a drug and a particular adverse drug reaction. The ROR was calculated using a two by two table, ROR=ad/cb (where a=exposed cases; b=exposed noncases; c=nonexposed cases; and d=nonexposed noncases). According to the European Medicines Agency recommendations, to generate a safety signal, the lower bound of the 95% CI of the ROR is required to be ≥1 and the number of individual cases to be ≥3.
Thus, in order to identify possible signals, for the drugs involved in 3 or more cases with a lower bound of the 95% CI of the ROR ≥1, a search was performed both in their summary of product characteristics (SPC) and in the literature to check whether hyponatraemia/SIAD is a known adverse reaction for the drug.
ResultsDuring the study period, 108,660 spontaneous reports of ADRs were received by the SEFV-H. Six hundred and eighty-five of these reports described hyponatraemia or SIAD, of which 26 were eliminated as duplicates. Thus, 659 cases (0.6% of the total database) were finally included in the study. Interestingly, over the 5 years period studied, there was a 57% increase in the number of hyponatraemia reports received (Fig. 1).
The main characteristics of the cases identified are presented in Table 1. Most of the reported cases came from hospitals and were reported by physicians. Most of the patients were women (420; 63.7%) and older than 65 years (474; 71.9%) with ages between 0 and 102 years [median: 77; IQR (85–64)]. Most of the cases were serious (614; 93%); more than half required hospital admission (356; 54%) and 14 patients died (2.1%). Notably, in the case of infants, children and adolescents, all cases were considered serious.
Characteristics of drug-induced hyponatraemia case reports (SEFV-H, 2016–2020).
| Characteristics | Cases (n=659) |
|---|---|
| Origin of reports,n(%)* | |
| Hospital | 453 (68.7) |
| Out-of-hospital | 69 (10.5) |
| Unknown | 181 (27.5) |
| Sex,n(%) | |
| Female | 420 (63.7) |
| Male | 232 (35.2) |
| Unknown | 7 (1.1) |
| Age (years), median [range] | 77 [0–102] |
| Age groups,n(%) | |
| Newborns (0–27 days) | 5 (0.8) |
| Infants (28 days–23 months) | 6 (0.9) |
| Children (2 years–11 years) | 7 (1.1) |
| Adolescents (12–17 years) | 4 (0.6) |
| Adults (18–65 years) | 150 (22.8) |
| >65 years | 474 (71.9) |
| Unknown | 13 (2.0) |
| Seriousness,n(%) | |
| Non-serious | 45 (7) |
| Serious | 614 (93) |
| Requiring admission | 356 (54.0) |
| Medically significant | 170 (25.8) |
| Life-threatening | 42 (6.3) |
| Prolonged hospitalization | 32 (4.9) |
| Fatal | 14 (2.1) |
| Outcome,n(%) | |
| Recovered/recovering | 546 (82.9) |
| Recovered with sequalae | 6 (0.9) |
| Not recovered | 26 (3.9) |
| Fatal | 14 (2.1) |
| Unknown | 67 (10.2) |
| Number of drugs, median [range] | 3 [1–30] |
| Number of drug,n(%) | |
| 1 | 184 (27.9) |
| 2–4 | 309 (46.9) |
| 5–10 | 128 (19.4) |
| >10 | 38 (5.8) |
| Type of suspected drugs (1st ATC level) | |
| Cardiovascular system | 578 (46.0) |
| Nervous system | 381 (30.3) |
| Antineoplastic/immunomodulating agents | 77 (6.1) |
| Alimentary tract/metabolism | 72 (5.7) |
| Antiinfectives (systemics) | 66 (5.3) |
| Hormones (excl. sex hormones and insulins) | 24 (1.9) |
| Musculo-skeletal system | 15 (1.2) |
| Blood and blood forming organs | 15 (1.2) |
| Genito urinary system/sex hormones | 12 (1.0) |
| Various | 6 (0.5) |
| Antiparasitics/insecticides/repellents | 5 (0.4) |
| Sensory organs | 5 (0.4) |
| Respiratory system | 1 (0.1) |
| Time to onset (months) | |
| ≤0.5 | 235 (28.4) |
| 0.5–1 | 94 (11.3) |
| 1–3 | 98 (11.8) |
| 3–12 | 165 (19.9) |
| >12 | 237 (28.6) |
| Personal medical history*,n(%) | |
| Hypertension | 69 (8.3) |
| Dyslipidemia | 32 (3.8) |
| Diabetes mellitus | 30 (3.6) |
| Atrial fibrillation | 21 (2.5) |
| Depression | 17 (2.0) |
| Chronic kidney disease (CKD) | 14 (1.7) |
| Hypothyroidism | 12 (1.4) |
| Osteoporosis | 11 (1.3) |
| Chronic obstructive pulmonary disease (COPD) | 10 (1.2) |
| Obesity | 8 (1.0) |
| Hyponatremia | 8 (1.0) |
The time to onset ranged from 1 to 7030 days (median, 79 days) and approximately 70% of the total occurred within the first year. Five hundred and forty-six patients (82.9%) showed complete recovery after the withdrawal of the suspected medicine.
The total number of drugs taken by an individual patient varies between 1 and 30 (median: 3), with most of the patients taking more than 1 (475; 72.1%). The most common reasons for taking the drugs were hypertension, followed by depression, epilepsy/seizures and heart failure.
Information on the patient's medical history was scarce, with only 207 (31%) cases providing this information, but the five most frequently encountered comorbidities were hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation and depression.
Drugs involvedOf 2508 drugs included in the reports, 1257 were considered suspected of causing hyponatraemia (individually or by interaction with others). Almost all of the suspected drugs were from the cardiovascular system, followed by the nervous system (Table 1). More specifically, the most commonly reported pharmacological group were diuretics, overall hydrochlorothiazide, which is the most frequent drug, included in more than 25% of the cases. We found a statistically significant disproportionate reporting of “hyponatremia/SIADH” for all active substances most frequently reported, except for ramipril and sacubitril (Table 2). The highest disproportion was found for amiloride [ROR (95% CI): 106.0 (59.2–190.1)], a potassium-sparing diuretic, followed by oxcarbazepine [ROR (95% CI): 81.3 (55.0–120.2)].
Pharmacological groups most frequently involved (≥10% of the cases) in hyponatremia reported to the SEFV-H and their reporting odds ratios (2016–2020).
| Pharmacological groups | Cases involved n=659 | ROR (95% CI) | ADR in SPC |
|---|---|---|---|
| Diuretics,n(%) | 380 (57.7) | ||
| Thiazides, 226 (34.3%) | |||
| Hydrochlorothiazide | 185 (28.1) | 42.8 (35.8–51.3) | Yes |
| Chlorthalidone | 22 (3.3) | 67.5 (40.8–111.6) | Yes |
| Indapamide | 19 (2.9) | 45.9 (27.4–76.8) | Yes |
| Loop diuretics, 95 (14.4%) | |||
| Furosemide | 72 (10.9) | 39.8 (30.6–51.8) | Yes |
| Torasemide | 23 (3.5) | 58.5 (36.1–94.8) | Yes |
| Potassium-sparing, 59 (9.0%) | |||
| Spironolactone | 34 (5.2) | 30.8 (21.3–44.6) | Yes |
| Amiloride | 20 (3.0) | 106.0 (59.2–190.1) | Yes |
| Eplerenone | 4 (0.6) | 15.4 (5.5–43.2) | Yes |
| Antidepressants,n(%) | 165 (25.0) | ||
| SSRIs, 88 (13.4%) | |||
| Sertraline | 35 (5.3) | 14.4 (10.2–20.3) | Yes |
| Escitalopram | 18 (2.7) | 8.7 (5.7–13.2) | Yes |
| Paroxetine | 13 (2.0) | 5.9 (3.4–10.5) | Yes |
| Citalopram | 13 (2.0) | 13.0 (7.4–23.0) | Yes |
| Fluoxetine | 8 (1.2) | 10.5 (5.9–19.0) | Yes |
| Non SSRIs, 77 (11.7%) | |||
| Mirtazapine | 15 (2.3) | 7.8 (4.8–12.6) | Yes |
| Venlafaxine | 14 (2.1) | 9.3 (5.6–15.5) | Yes |
| Duloxetine | 12 (1.8) | 4.7 (2.6–8.4) | Yes |
| Trazodone | 11 (1.7) | 3.9 (2.1–7.0) | Yes |
| Vortioxetine | 5 (0.8) | 2.6 (1.1–5.8) | Yes |
| Desvenlafaxine | 8 (1.2) | 7.0 (3.6–13.7) | Yes |
| Amitriptyline | 5 (0.8) | 3.8 (1.9–7.8) | Yes |
| Clomipramine | 3 (0.5) | 10.8 (3.3–34.8) | Yes |
| Drugs acting on renin angiotensin system,n(%) | 158 (24.0) | ||
| ARB, 104 (15.8%) | |||
| Losartan | 28 (4.2) | 21.3 (14.3–31.9) | Yes |
| Valsartan | 26 (3.9) | 7.7 (5.1–11.5)* | No |
| Olmesartan | 23 (3.5) | 7.3 (4.7–11.1)* | No |
| Candesartan | 12 (1.8) | 18.6 (10.2–34.1) | Yes |
| Irbesartan | 10 (1.5) | 18.6 (9.6–35.9)* | No |
| Telmisartan | 5 (0.8) | 7.2 (2.9–17.7) | Yes |
| ACEi, 50 (7.6%) | |||
| Enalapril | 35 (5.3) | 4.3 (3.1–6.0) | Yes |
| Lisinopril | 6 (0.9) | 6.4 (2.8–14.6) | Yes |
| Ramipril | 4 (0.6) | 1.3 (0.5–6.6) | Yes |
| Perindopril | 3 (0.5) | 8.6 (2.8–27.6) | Yes |
| Renin neprilysin inhibitors, 4 (0.6%) | |||
| Sacubitril | 3 (0.5) | 1.9 (0.6–5.9) | Yes |
| Antiepileptic drugs,n(%) | 133 (20.2) | ||
| Oxcarbazepine | 37 (5.6) | 81.3 (55.0–120.2) | Yes |
| Eslicarbazepine | 34 (5.2) | 40.0 (27.4–58.3) | Yes |
| Carbamazepine | 21 (3.2) | 24.5 (15.4–39.0) | Yes |
| Pregabalin | 12 (1.8) | 2.5 (1.4–4.5)* | No |
| Valproate | 9 (1.4) | 4.2 (2.2–7.9) | Yes |
| Levetiracetam | 7 (1.1) | 3.2 (1.6–6.4) | Yes |
| Gabapentin | 7 (1.1) | 3.3 (1.6–7.0) | Yes |
ADR: adverse drug reaction; SPC: summary of product characteristics; ACEi: angiotensin-converting enzyme inhibitors; ARB: angiotensin II receptor blockers; NA: not applicable; SSRIs: selective serotonin reuptake inhibitors; ROR: reporting odds ratio; CI: confidence interval.
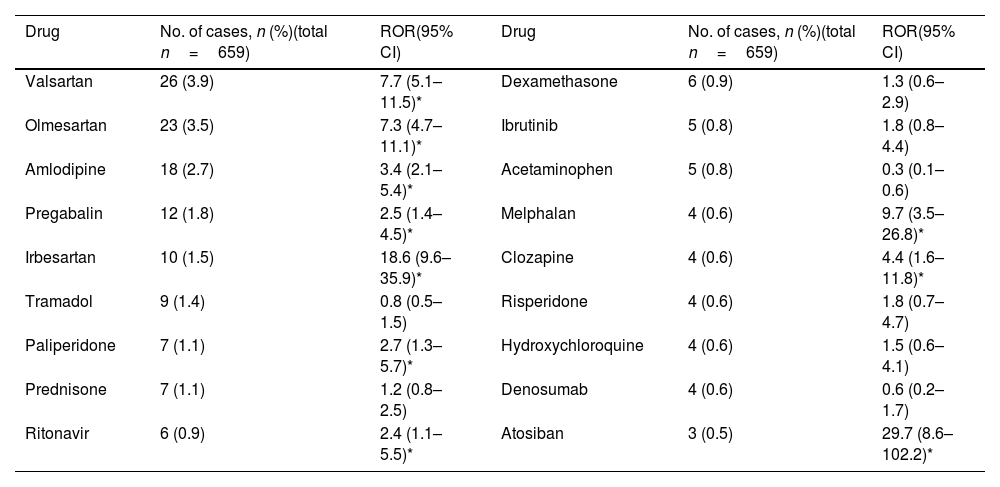
With regard to new safety signals, valsartan, olmesartan, amlodipine, pregabalin, irbesartan, paliperidone, ritonavir, atosiban, melphalan and clozapine do not include this reaction on their SPC and comply with the EMA recommendations for a safety signals (Table 3).
Disproportionate analysis of drugs reported within three or more cases, for which hyponatremia/syndrome of inappropriate antidiuresis (SIAD) is not included in its summary of product characteristics (SPC) (SEFV-H, 2016–2020).
| Drug | No. of cases, n (%)(total n=659) | ROR(95% CI) | Drug | No. of cases, n (%)(total n=659) | ROR(95% CI) |
|---|---|---|---|---|---|
| Valsartan | 26 (3.9) | 7.7 (5.1–11.5)* | Dexamethasone | 6 (0.9) | 1.3 (0.6–2.9) |
| Olmesartan | 23 (3.5) | 7.3 (4.7–11.1)* | Ibrutinib | 5 (0.8) | 1.8 (0.8–4.4) |
| Amlodipine | 18 (2.7) | 3.4 (2.1–5.4)* | Acetaminophen | 5 (0.8) | 0.3 (0.1–0.6) |
| Pregabalin | 12 (1.8) | 2.5 (1.4–4.5)* | Melphalan | 4 (0.6) | 9.7 (3.5–26.8)* |
| Irbesartan | 10 (1.5) | 18.6 (9.6–35.9)* | Clozapine | 4 (0.6) | 4.4 (1.6–11.8)* |
| Tramadol | 9 (1.4) | 0.8 (0.5–1.5) | Risperidone | 4 (0.6) | 1.8 (0.7–4.7) |
| Paliperidone | 7 (1.1) | 2.7 (1.3–5.7)* | Hydroxychloroquine | 4 (0.6) | 1.5 (0.6–4.1) |
| Prednisone | 7 (1.1) | 1.2 (0.8–2.5) | Denosumab | 4 (0.6) | 0.6 (0.2–1.7) |
| Ritonavir | 6 (0.9) | 2.4 (1.1–5.5)* | Atosiban | 3 (0.5) | 29.7 (8.6–102.2)* |
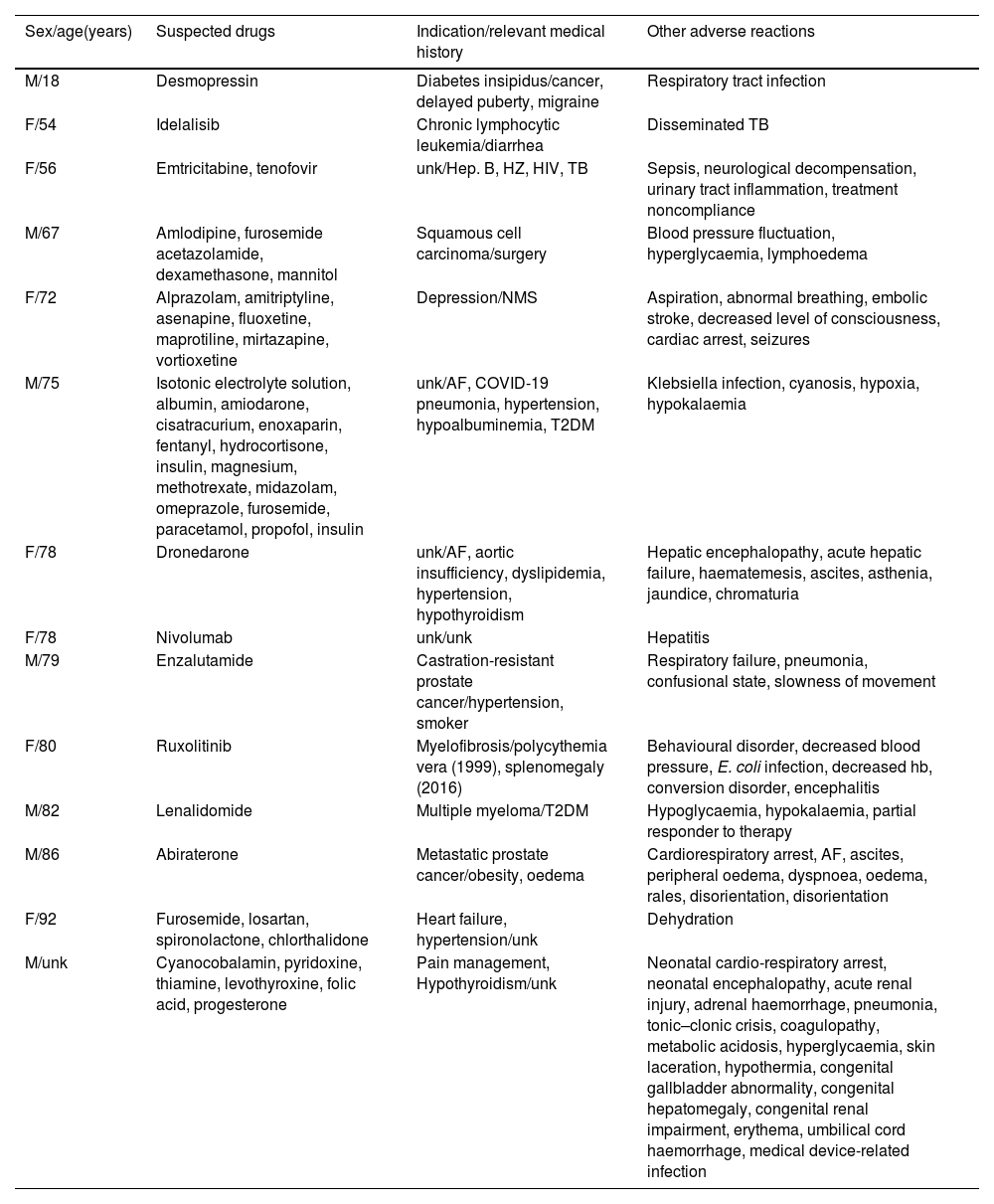
In fatal cases, antineoplastic and immunosuppressive agents were the most common pharmacological groups involved (six cases), with the most common indication being neoplastic processes (Table 4). Most of these are complex cases in which the underlying pathology or other complications appear to have been the cause of death.
Characteristics of fatal cases.
| Sex/age(years) | Suspected drugs | Indication/relevant medical history | Other adverse reactions |
|---|---|---|---|
| M/18 | Desmopressin | Diabetes insipidus/cancer, delayed puberty, migraine | Respiratory tract infection |
| F/54 | Idelalisib | Chronic lymphocytic leukemia/diarrhea | Disseminated TB |
| F/56 | Emtricitabine, tenofovir | unk/Hep. B, HZ, HIV, TB | Sepsis, neurological decompensation, urinary tract inflammation, treatment noncompliance |
| M/67 | Amlodipine, furosemide acetazolamide, dexamethasone, mannitol | Squamous cell carcinoma/surgery | Blood pressure fluctuation, hyperglycaemia, lymphoedema |
| F/72 | Alprazolam, amitriptyline, asenapine, fluoxetine, maprotiline, mirtazapine, vortioxetine | Depression/NMS | Aspiration, abnormal breathing, embolic stroke, decreased level of consciousness, cardiac arrest, seizures |
| M/75 | Isotonic electrolyte solution, albumin, amiodarone, cisatracurium, enoxaparin, fentanyl, hydrocortisone, insulin, magnesium, methotrexate, midazolam, omeprazole, furosemide, paracetamol, propofol, insulin | unk/AF, COVID-19 pneumonia, hypertension, hypoalbuminemia, T2DM | Klebsiella infection, cyanosis, hypoxia, hypokalaemia |
| F/78 | Dronedarone | unk/AF, aortic insufficiency, dyslipidemia, hypertension, hypothyroidism | Hepatic encephalopathy, acute hepatic failure, haematemesis, ascites, asthenia, jaundice, chromaturia |
| F/78 | Nivolumab | unk/unk | Hepatitis |
| M/79 | Enzalutamide | Castration-resistant prostate cancer/hypertension, smoker | Respiratory failure, pneumonia, confusional state, slowness of movement |
| F/80 | Ruxolitinib | Myelofibrosis/polycythemia vera (1999), splenomegaly (2016) | Behavioural disorder, decreased blood pressure, E. coli infection, decreased hb, conversion disorder, encephalitis |
| M/82 | Lenalidomide | Multiple myeloma/T2DM | Hypoglycaemia, hypokalaemia, partial responder to therapy |
| M/86 | Abiraterone | Metastatic prostate cancer/obesity, oedema | Cardiorespiratory arrest, AF, ascites, peripheral oedema, dyspnoea, oedema, rales, disorientation, disorientation |
| F/92 | Furosemide, losartan, spironolactone, chlorthalidone | Heart failure, hypertension/unk | Dehydration |
| M/unk | Cyanocobalamin, pyridoxine, thiamine, levothyroxine, folic acid, progesterone | Pain management, Hypothyroidism/unk | Neonatal cardio-respiratory arrest, neonatal encephalopathy, acute renal injury, adrenal haemorrhage, pneumonia, tonic–clonic crisis, coagulopathy, metabolic acidosis, hyperglycaemia, skin laceration, hypothermia, congenital gallbladder abnormality, congenital hepatomegaly, congenital renal impairment, erythema, umbilical cord haemorrhage, medical device-related infection |
AF: atrial fibrillation; F: female; M: male; Hep. B: hepatitis B; HZ: Herpes Zóster; HIV: human immunodeficiency virus; TB: tuberculosis; NMS: neuroleptic malignant syndrome; T2DM: diabetes mellitus type 2; unk: unknown.
The number of hyponatraemia cases reported to SEFV-H has remained relatively stable between 2016 and 2018, experiencing a notable increase especially since 2019. The increase in the prescription of certain SARS-CoV-2 drugs does not seem to explain this finding, nor is it justified by an increase in the reporting of ADR during the pandemic, which overall decreased by 17% compared to 2019.5 Nor have we found any security alerts or news that could have driven this increase. However, some studies that showed that patients with COVID-19 presented more often with hyponatremia on admission to an emergency department compared to controls.6 Hyponatremia seems to be related with the pneumonia this patient suffered, but this is only an hypothesis. Only five of the cases in our study had information that the patient was suspected of having COVID-19, but this could explain the observed increase.
Almost 90% of the cases for which this information was available were reported by physicians and comes from the hospital, which can be explained by the severity of the reported cases. Citizen participation in hyponatraemia reporting is low (5%); the diagnosis of hyponatraemia requires analytical determinations and, moreover, citizens generally report less than health professionals in our country.
The ratio of drug-related hyponatraemia cases is higher in women (1.8:1), with a median age of 77 years, similar to data published in prospective studies of hyponatremia.7,8 Female sex has not been shown to be an independent risk factor for the development of drug-induced hyponatraemia, at least in a cross-over study with antidepressants9; the possibility that lower body mass index may act as a confounding factor has been described, but a higher prescription of hyponatraemia-causing drugs in women has been shown and could be the explanation. This is the case with thiazides and with antidepressants.10 In addition, older patients were most frequently involved (72%). The impaired water-excretory capacity, higher sensitivity to osmotic stimuli, hormones impact in osmotic regulation have been proposed as risk factors presents in this age group.10 A higher number of drugs, with the more risk of drug-drug interactions increases the risk of suffering an ADR.
The most frequently reported comorbidities were: hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, depression, chronic kidney disease, hypothyroidism and hyponatraemia. There is no known association between hyponatraemia and personal history of hypertension, heart failure and chronic kidney disease, it therefore seems more likely that the development of hyponatraemia in this type of patient is caused by medication, as the most frequently implicated pharmacological groups in this study and described in the literature are diuretics.8,10 However, a personal history of hyponatraemia and diabetes mellitus does increase the risk of ADR.
The high frequency of serious cases is not rare, due to the characteristics of the reaction. In 444 (67.3%) patients, the reaction required hospitalisation or was life-threatening or fatal. Although pharmacovigilance regulations in Spain call for the reporting of any suspected ADR, reporting is encouraged especially for serious cases. On the other hand, hyponatraemia, when symptomatic, is a life-threatening medical emergency, which also explains why most of the cases identified and reported are serious. Fourteen cases were fatal, for patients between 18 and 92 years old, with a male/female ratio of 1. The pharmacological group most implicated in these cases was antineoplastic and immunosuppressive agents, the most frequent indication being neoplastic processes. These agents can cause hyponatraemia by a multifactorial mechanism, which includes both SIAD and urinary sodium loss as a consequence of the nephrotoxicity inherent to many of them. Hyponatraemia due to cancer is not uncommon and is associated with a poor prognosis. Heart failure, lung disease and infections are some of the causes that, along with medication, can trigger the condition in these fatal cases. Whether patients died as a result of hyponatraemia or with hyponatraemia is difficult to answer. Of the 14 fatal cases presented here, only in one case was death related to vortioxetine-associated hyponatraemia; in the remaining cases hyponatraemia could be a complication of the pathology from which the patients died.
The time to onset of hyponatremia varies and can be very long, so monitoring should be extended over the entire prescribed period of the drug. The development of hyponatraemia cannot be ruled out even after years of treatment.
Drugs involved and signal identificationThe most commonly implicated drugs were diuretics and antidepressants, followed by agents acting on the renin–angiotensin system, as expected and as described in previous studies.8,11 Individually, the drug most frequently reported to be associated with hyponatraemia was hydrochlorothiazide. Thiazides are diuretics that act on the Na-Cl cotransporter, inhibiting sodium reabsorption in the distal tubule, being responsible for one in four admissions for hyponatraemia, especially during the first month of starting treatment.3,11 Hydrochlorothiazide is followed in decreasing order of frequency by furosemide, oxcarbazepine, enalapril and sertraline. Taking into account the disproportionality in reporting, the drug with the highest ROR is amiloride [ROR (95% CI): 106.0 (59.2–190.1)], a potassium-sparing diuretic, followed by oxcarbazepine [ROR (95% CI): 81.3 (55.0–120.2)]. This means that hyponatraemia is reported 106 times more with amiloride than with any other drug in this database; 80 times more with oxcarbazepine.
The disproportionality analyses show signals of disproportionate reporting between hyponatraemia/SIAD and valsartan, olmesartan, and irbesartan (angiotensin II receptor blockers); amlodipine (calcium channel blocker); pregabalin (gamma-aminobutyric acid analogue); paliperidone and clozapine (antipsychotics); ritonavir (antiviral); melphalan (antineoplastic) and atosiban (oxytocin antagonist) exposure. Without claiming to be exhaustive, as each new safety signal must be analysed in depth and on a case-by-base basis, some aspects of the identified drugs are highlighted. Although an association has been described between treatment with angiotensin II receptor blockers (ARBs) and the risk of admission for hyponatremia, no scientific literature has been found to support the finding of hyponatraemia as a possible adverse effect of treatment with this drug alone, but in combination with thiazides.12 In the majority of cases in this study, the ARBs were used in combination with other hyponatremic drugs, most commonly diuretics. The same happens with amlodipine, a calcium channel blocker, which in almost all cases found was combined with a diuretic, hydrochlorothiazide in most cases, even when two cases have been found in the literature.13,14 The common use of this group of drugs together could lead to a co-prescription bias or even a competition bias, since diuretics are often associated with hyponatremia.15 Pregabalin is an antiepileptic that exerts its action by binding to the α2-δ protein of voltage-dependent calcium channels in the CNS. Although the mechanism by which it occurs is unknown, there have been cases of pregabalin-induced SIAD published in recent years.16–20 Paliperidone and clozapine are antipsychotics. Other antipsychotics have been associated with hyponatraemia. Several mechanisms have been postulated: involvement of D2 receptors, ADH stimulation, or a serotoninergic.21 Several cases have also been published.22-26
With regard to ritonavir, hyponatraemia can be a complication of treatment with some antiretroviral therapies. Cases should be analysed in detail on a case-by-case basis, as ritonavir is always administered with other drugs as an enhancer (due to its potent inhibitory activity on cytochrome P450 enzymes).27
In the case of atosiban, a synthetic peptide that acts as a competitive antagonist of human oxytocin receptors, it is indicated to delay imminent preterm labour in adult pregnant women. Hyponatraemia is not known or expected for this drug but, in 2013, the Spanish Medicines Agency warned about a contamination of atosiban batches with desmopressin, which could explain the reported cases.28 The cases presented here were reported after this alert, but we cannot rule out other contamination (we have not heard of it) or reporting due to notoriety bias (bias that occurs when healthcare professionals increase their reporting of an adverse reaction after news or warnings about it are published).
Melphalan is an antineoplastic drug. Although some case reports have been found in the literature,29 hyponatraemia can occur as a result of cancer or other cancer treatments. Establishing an association with the drug is challenging.
Further studies would be necessary in order to assess this signal for one of each drug here identify. Also, the involvement of several drugs, leading to interactions, cannot be ruled out; even quality defects, as in the case of atosiban.
Although known, drug-induced hyponatraemia often goes unnoticed by physicians because they do not consider the drug in the differential diagnosis. In addition, after the marketing of medicines, some reactions are unknown. This reinforces the importance of suspecting hyponatraemia as an adverse drug reaction and reporting it to SEFV-H. Identifying the drug as a risk factor would prevent or minimise the severity of this adverse reaction. The seriousness of this reaction and the difficulty involved in its treatment make its knowledge and prevention of the disease of utmost interest.
The main limitation of this study is that, due to underreporting, pharmacovigilance databases cannot capture all ADRs that have occurred. This, together with the lack of data on the consumption of the drug in question, makes it impossible to accurately estimate the real incidence of reported ADRs and, consequently, to compare drugs or to have an idea of the real impact on users of each medicine. Furthermore, the number of cases reported to the database largely relies on a series of factors, such as the involved medication (commercialization time, use in the clinical setting, current knowledge on the drug and so forth), and the reporting person's profile (available time, knowledge, expertise, and degree of commitment with routine pharmacovigilance activities, etc.). As the reported data are based on suspicions, further studies may be needed to confirm some of the conclusions drawn.
Despite those limitations, pharmacovigilance systems provide essential information to identify potential new risks associated with the use of medicines. In addition, disproportionality measures in pharmacovigilance databases for new drug-ADR associations have been shown to provide an indication of the direction and magnitude of the risk of an ADR.30
ConclusionsThe number of reports of drug-associated hyponatraemia in Spain has doubled between 2017 and 2020. Most of the cases come from the hospital setting and have been reported by physicians. The patient profile most frequently involved is a woman over 65 years of age taking two or more drugs. Almost all reported hyponatraemias are serious, although most are reversible. The time to onset of drug-induced hyponatraemia varies and can be very long, so patient monitoring should be continuous throughout treatment. The pharmacological groups with the most reported cases are diuretics, antidepressants, renin–angiotensin system inhibitors and anti-epileptic drugs. Hydrochlorothiazide is the drug with the highest number of reported cases of hyponatraemia in our setting, which is consistent with what was previously known. In terms of disproportionate reporting, diuretics leads also the list, followed by some antiepileptics (oxcarbazepine, eslicarbazepine). Safety signals were found for several drugs, more plausibly for pregabalin and paliperidone, thus a possible association between these drugs and hyponatraemia/SIAD is identified. This signal must be further studied, including a qualitative analysis of the ICSR involved. Healthcare professionals should pay attention to this possibility. The reporting of suspected ADRs is essential to understand the risks associated with medicines once they are on the market.
Ethical considerations and statementsThe authors have followed the ethical guidelines for publication; the study was approved by the Clinical Research Ethics Committee (CEIC) of the Valladolid East Health Area on January, 2022 (PI 22-2548).
FEDRA is the Spanish Pharmacovigilance System of Human Medicines (SEFVH) database and is managed by the Spanish Medicines and Health Products Agency (AEMPS). The information comes from a variety of sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases. The discussion and conclusions of this study are the authors’ responsibility and do not represent the opinion of the SEFV-H or the AEMPS.
FundingThe authors declare that they have not received any funding for this work.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors would like to thank healthcare professionals for their collaboration with the Spanish Pharmacovigilance System for Medicinal Products for Human Use (SEFV-H) with the reporting of suspected adverse drug reactions.