In December 2019, Wuhan, China, experienced an outbreak of coronavirus disease 2019 (COVID-19). Some patients admitted to our hospital were treated with early prone positioning (PP). Here, we analyzed its clinical significance.
MethodsThis was a retrospective observational study. We defined the early PP group as mild COVID-19 patients who were placed into a prone position within 24h of admission; others served as the control group. We recorded basic data and outcomes of early PP and compared the results to those of controls.
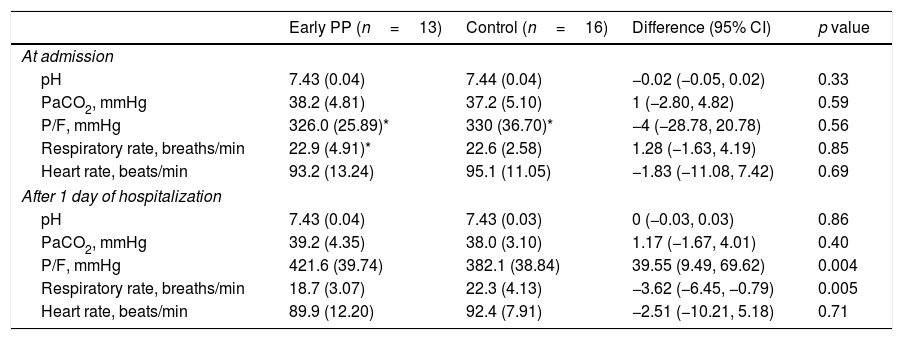
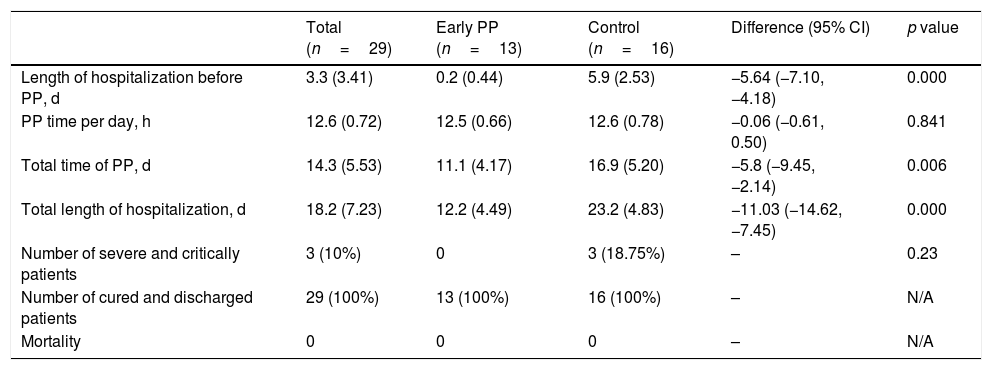
ResultsAfter 1 day of treatment, oxygenation was greater in the early PP group than in the control group (P/F: 421.6±39.74 vs. 382.1±38.84mmHg [1mmHg=0.133kPa], p<0.01). And early PP group spent less total time in prone position (11.1±4.17 vs. 16.9±5.20 days, p<0.01), and required shorter hospitalization duration (12.2±4.49 vs. 23.2±4.83 days, p<0.001).
ConclusionsEarly PP treatment can improve hypoxia and shorten the prone position time and hospitalization duration in mild COVID-19 patients. It is a potential clinically applicable intervention.
En diciembre de 2019, Wuhan, China, experimentó un brote de enfermedad por coronavirus 2019 (COVID-19). Algunos pacientes ingresados en nuestro hospital fueron tratados con posicionamiento temprano en decúbito prono (PP). En este estudio analizamos su significación clínica.
MétodosEstudio retrospectivo observacional en el que definimos el PP temprano como aquellos pacientes con COVID-19 que fueron posicionados en decúbito prono dentro de las 24 horas siguientes a su ingreso, sirviendo el resto de los pacientes como grupo control. Registramos los datos básicos y los resultados de PP temprano, comparando dichos resultados con los de los controles.
ResultadosTras un día de tratamiento, la oxigenación fue más alta en el grupo PP temprano que en el grupo control (P/F: 421,6 ± 39,74 vs. 382,1 ± 38,84 mmHg [1 mmHg=0,133 kPa], p < 0,01). El grupo PP temprano pasó menor tiempo total en posición de decúbito prono (11,1 ± 4,17 vs. 16,9 ± 5,20 días, p < 0,01), y requirió menor tiempo de hospitalización (12,2 ± 4,49 vs. 23,2 ± 4,83 días, p < 0,001).
ConclusionesEl tratamiento de PP temprano puede mejorar la hipoxia y reducir el tiempo de posición en decúbito prono en pacientes con COVID-19 leve. Se trata de una intervención potencialmente aplicable desde el punto de vista clínico.
In December 2019, a novel coronavirus (SARS-CoV-2) appeared in Wuhan, China,1 causing the coronavirus disease 2019 (COVID-19) outbreak.2 The epidemic quickly spread globally. At present, it is clear that the main route of infection is through respiratory droplets and transmission via close contact.3 According to the disease stage, the use of traditional Chinese medicine is recommended for COVID-19 treatment.3 However, fewer treatment plans mention prone positioning (PP) in early treatment.
PP therapy is commonly used in patients with mild to severe acute respiratory distress syndrome (ARDS), and it can improve ventilation/blood flow (V/Q) ratios and hypoxia.4,5 Thus, at our hospital, COVID-19 patients who had pulmonary lesions that were similar to those seen in ARDS were treated with PP. However, some patients received the treatment earlier than others. In this study, we assessed the clinical significance of early PP treatment versus later PP.
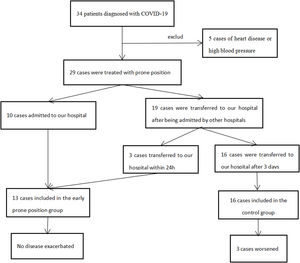
Materials and methodsFrom January 22 to March 13, 2020, 34 patients diagnosed with mild COVID-19 were admitted to our hospital. They were treated in accordance with the COVID-19 Treatment Program,3 PP, interferon atomization, Kelizhi antiviral treatment, Chinese medicine treatment, nasal catheter oxygen therapy, psychological intervention and other treatment measures were given. According to each patient's vital signs, clinical symptoms, chest imaging, arterial blood gas, viral nucleic acid test (NAT) results, and other laboratory tests. Comprehensive assessment of disease changes is essential to provide support for the stepwise respiratory treatment strategy for patients with severe and critical in treatment.3 Patients with clinical symptoms disappeared were tested for viral nuclei acid, and two consecutive negative results on the NAT were considered cured. These patients were discharged and were asked to stay at home for 14 days.
We enrolled the patients with mild COVID-19. However, those who cannot tolerate prone position due to diseases and other factors, such as heart failure and heart diseases, were excluded. The PP treatment consists of the patient resting in bed in the prone position for no less than 2h in the morning, no less than 2h in the afternoon, and no less than 6h at night, for a total PP time 10–14h/d,6 which is carried out simultaneously with other treatment measures, and does not affect the implementation of other treatment measures.
Before the PP procedure, we collected basic information on each group of patients, including age, sex, body temperature, laboratory test at admission, and chest imaging. Then, we observed changes in pH, PaCO2, P/F, Heart rate, and Respiratory rate after 1 day of treatment. The length of hospitalization before PP, total PP time per day, total PP time overall, total hospitalization time, number of severe and critically ill patients, and mortality rate were calculated for each group. The ethics committee reviewed and approved the study protocol (No. 2020010). Informed consent was obtained from the patients or their family members.
SPSS 22.0 statistical software was used for statistical analyses. The measurement data were tested using a t-test and expressed as mean±standard deviation (SD). Categorical variables were tested using chi-square tests. p<0.05 was considered statistically significant.
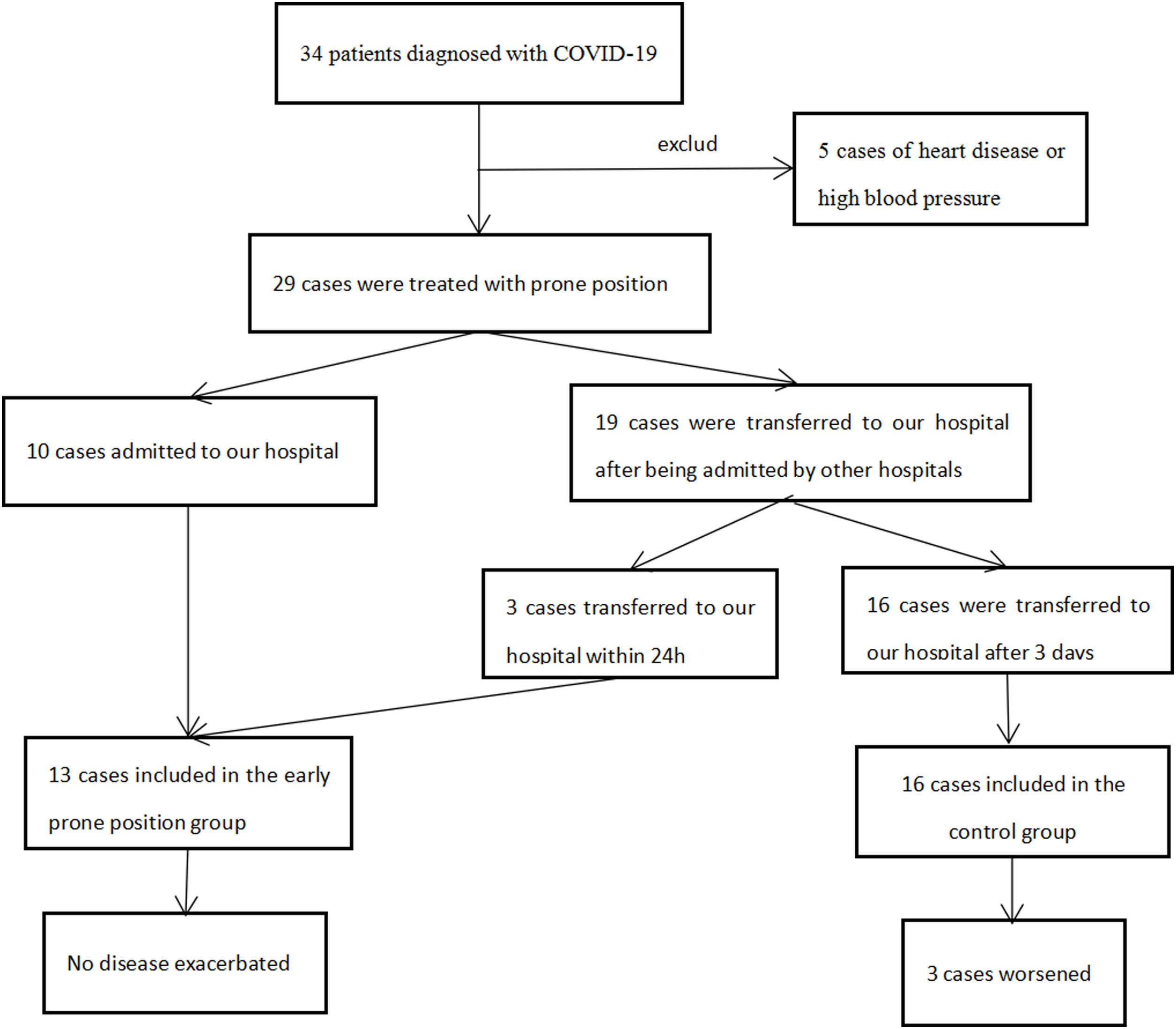
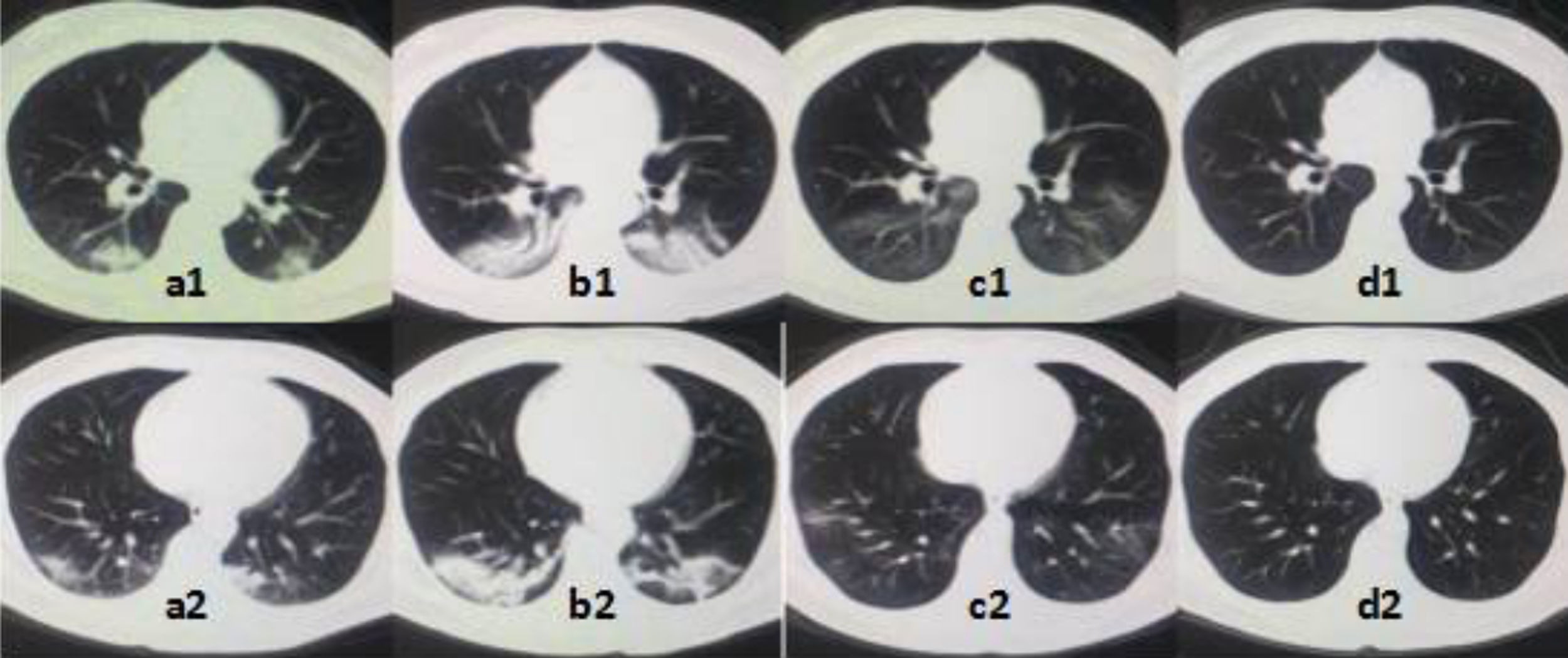
ResultsWe retrospectively collected 29 mild COVID-19 patients who received PP treatment at our hospital (Supplementary Figure 1). Overall 13 patients received PP therapy within 24h of admission and were classified into the early PP group. The others (n=16) received PP therapy after 3 days and were classified into the control group. Imaging changes of treatment (Supplementary Figure 2).
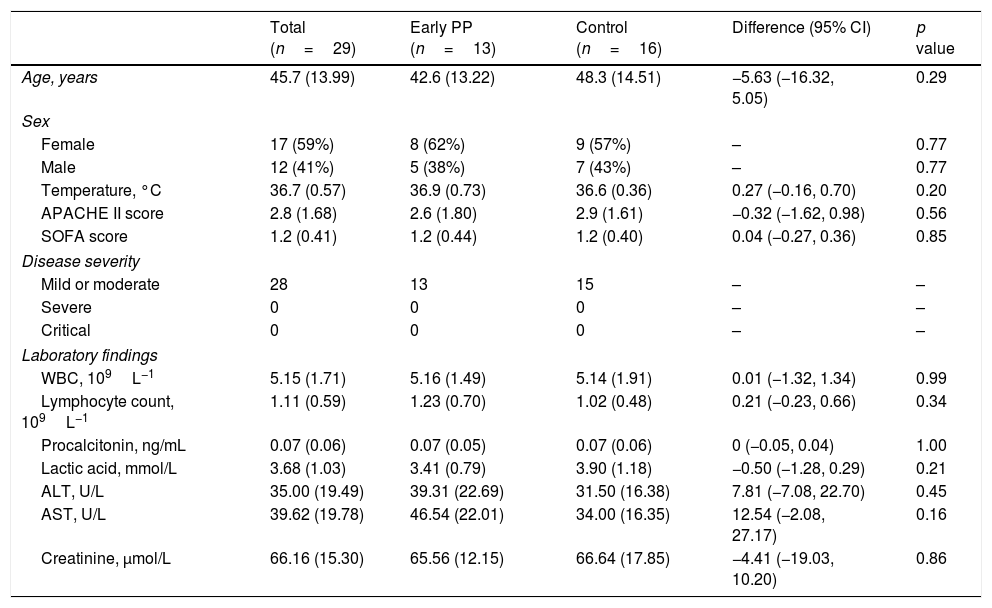
There were no statistically significant differences in basic data between the two groups (Table 1). P/F in both groups increased, and only respiratory rate in the early PP improved after 1 day of hospitalization when compared within group. However, P/F was much higher and respiratory rate was much lower in the early PP group than in controls (Table 2).
Demographic, clinical, and laboratory findings at admission.
| Total (n=29) | Early PP (n=13) | Control (n=16) | Difference (95% CI) | p value | |
|---|---|---|---|---|---|
| Age, years | 45.7 (13.99) | 42.6 (13.22) | 48.3 (14.51) | −5.63 (−16.32, 5.05) | 0.29 |
| Sex | |||||
| Female | 17 (59%) | 8 (62%) | 9 (57%) | – | 0.77 |
| Male | 12 (41%) | 5 (38%) | 7 (43%) | – | 0.77 |
| Temperature, °C | 36.7 (0.57) | 36.9 (0.73) | 36.6 (0.36) | 0.27 (−0.16, 0.70) | 0.20 |
| APACHE II score | 2.8 (1.68) | 2.6 (1.80) | 2.9 (1.61) | −0.32 (−1.62, 0.98) | 0.56 |
| SOFA score | 1.2 (0.41) | 1.2 (0.44) | 1.2 (0.40) | 0.04 (−0.27, 0.36) | 0.85 |
| Disease severity | |||||
| Mild or moderate | 28 | 13 | 15 | – | – |
| Severe | 0 | 0 | 0 | – | – |
| Critical | 0 | 0 | 0 | – | – |
| Laboratory findings | |||||
| WBC, 109L−1 | 5.15 (1.71) | 5.16 (1.49) | 5.14 (1.91) | 0.01 (−1.32, 1.34) | 0.99 |
| Lymphocyte count, 109L−1 | 1.11 (0.59) | 1.23 (0.70) | 1.02 (0.48) | 0.21 (−0.23, 0.66) | 0.34 |
| Procalcitonin, ng/mL | 0.07 (0.06) | 0.07 (0.05) | 0.07 (0.06) | 0 (−0.05, 0.04) | 1.00 |
| Lactic acid, mmol/L | 3.68 (1.03) | 3.41 (0.79) | 3.90 (1.18) | −0.50 (−1.28, 0.29) | 0.21 |
| ALT, U/L | 35.00 (19.49) | 39.31 (22.69) | 31.50 (16.38) | 7.81 (−7.08, 22.70) | 0.45 |
| AST, U/L | 39.62 (19.78) | 46.54 (22.01) | 34.00 (16.35) | 12.54 (−2.08, 27.17) | 0.16 |
| Creatinine, μmol/L | 66.16 (15.30) | 65.56 (12.15) | 66.64 (17.85) | −4.41 (−19.03, 10.20) | 0.86 |
PP=prone positioning, APACHE II=acute physiology and chronic health evaluation II, SOFA=sequential organ failure assessment, CI=confidence interval, WBC=white blood cell count, ALT=alanine aminotransferase, and AST=aspartate transaminase.
Changes on vital signs and arterial blood gas from admission to 1 day of hospitalization.
| Early PP (n=13) | Control (n=16) | Difference (95% CI) | p value | |
|---|---|---|---|---|
| At admission | ||||
| pH | 7.43 (0.04) | 7.44 (0.04) | −0.02 (−0.05, 0.02) | 0.33 |
| PaCO2, mmHg | 38.2 (4.81) | 37.2 (5.10) | 1 (−2.80, 4.82) | 0.59 |
| P/F, mmHg | 326.0 (25.89)* | 330 (36.70)* | −4 (−28.78, 20.78) | 0.56 |
| Respiratory rate, breaths/min | 22.9 (4.91)* | 22.6 (2.58) | 1.28 (−1.63, 4.19) | 0.85 |
| Heart rate, beats/min | 93.2 (13.24) | 95.1 (11.05) | −1.83 (−11.08, 7.42) | 0.69 |
| After 1 day of hospitalization | ||||
| pH | 7.43 (0.04) | 7.43 (0.03) | 0 (−0.03, 0.03) | 0.86 |
| PaCO2, mmHg | 39.2 (4.35) | 38.0 (3.10) | 1.17 (−1.67, 4.01) | 0.40 |
| P/F, mmHg | 421.6 (39.74) | 382.1 (38.84) | 39.55 (9.49, 69.62) | 0.004 |
| Respiratory rate, breaths/min | 18.7 (3.07) | 22.3 (4.13) | −3.62 (−6.45, −0.79) | 0.005 |
| Heart rate, beats/min | 89.9 (12.20) | 92.4 (7.91) | −2.51 (−10.21, 5.18) | 0.71 |
In the control group, three patients became severe and performed non-invasive ventilation, however, none of them had received PP treatment before this exacerbation. Compared to the control group, the early PP group started the treatment earlier, and had significantly shorter total PP time and total length of hospitalization (Table 3).
Outcomes.
| Total (n=29) | Early PP (n=13) | Control (n=16) | Difference (95% CI) | p value | |
|---|---|---|---|---|---|
| Length of hospitalization before PP, d | 3.3 (3.41) | 0.2 (0.44) | 5.9 (2.53) | −5.64 (−7.10, −4.18) | 0.000 |
| PP time per day, h | 12.6 (0.72) | 12.5 (0.66) | 12.6 (0.78) | −0.06 (−0.61, 0.50) | 0.841 |
| Total time of PP, d | 14.3 (5.53) | 11.1 (4.17) | 16.9 (5.20) | −5.8 (−9.45, −2.14) | 0.006 |
| Total length of hospitalization, d | 18.2 (7.23) | 12.2 (4.49) | 23.2 (4.83) | −11.03 (−14.62, −7.45) | 0.000 |
| Number of severe and critically patients | 3 (10%) | 0 | 3 (18.75%) | – | 0.23 |
| Number of cured and discharged patients | 29 (100%) | 13 (100%) | 16 (100%) | – | N/A |
| Mortality | 0 | 0 | 0 | – | N/A |
PP=prone positioning and N/A, not applicable.
Anatomical studies of deceased COVID-19 patients have confirmed that the virus causes inflammatory reactions characterized by deep airway and alveolar damage.7 The lungs show diffuse alveolar damage and pulmonary hyaline membrane formation, consistent with ARDS manifestations.8 PP treatment improves the hypoxic state of ARDS patients,6 and thus we used it as a clinical treatment for mild COVID-19 patients.
The PP is a natural position that is well tolerated by patients. In addition, unlike many patients with mild to severe ARDS that have been reported in the literature,6 mild COVID-19 patients are awake, can perform PP on their own, and do not require invasive ventilation or analgesic sedation.9 The procedure is simple and easy for mild COVID-19 patients to perform, is noninvasive and safe, does not increase the workload of medical staff, and does not generate additional costs. It protects the interests of patients and is clinically feasible.
In this retrospective and controlled analysis of 29 mild COVID-19 cases, there were no statistically significant differences in sex, age, laboratory results, or lung imaging results at the time of admission between the two groups. The P/F values of both groups were increased after 1 day of hospitalization, indicating that the treatment effectively improved hypoxia. However, early PP led to more significant improvement.
In clinically diagnosed COVID-19 patients, early lesions of ARDS may appear in the lungs, including diffuse alveolar injury and pulmonary hyaline membrane formation. This in turn may cause consolidation of the lower lobe of the lung and cause pulmonary V/Q imbalance, manifested as hypoxemia. The PP can improve the V/Q ratio by changing the gravity-dependent region of the lung.4 This will improve the symptoms of respiratory distress caused by hypoxia, and reduce respiratory rate. Therefore, the early PP can significantly improve hypoxemia in patients with mild COVID-19. At the same time, clinical observations in the present study indicated that PP did not affect pH, PaCO2, or heart rate, and had only a small effect on hemodynamics.
None of our 29 mild COVID-19 patients died; all patients were cured and discharged, the mortality is lower than other reports.10 However, although the difference was not statistically significant, there were more severe disease patients in the control group, so the early PP may prevent the disease from becoming aggravated. The proportion of severe patients was 10.3%, lower than related reports.10
In our study, both groups received the same total duration of treatment each day. The only difference was that the early PP group started it sooner after admission than did controls, and their total PP time and total length of hospitalization were significantly shorter than those of controls. Hence, early PP had a positive effect on mild COVID-19 patients.
This study had several limitations. First, the small number of cases may have skewed our results. Second, the retrospective design only provided low quality of evidence. Therefore, a randomized controlled trial with larger samples is required to provide high quality of evidence.
ConclusionsThe treatment of mild COVID-19 patients with early PP can correct early hypoxia, shorten the time of hospitalization, and have a positive effect on clinical outcomes. The procedure is simple and safe, does not increase costs or the workload of medical staff, and is worthy of clinical application.
Conflict of interestWe declare that we have no conflicts of interest to disclose.
Author contributionsXL and FZ conceived and designed the study, had full access to all data, and take responsibility for the integrity of the data and the accuracy of the data analyses. XL, HL, and JD drafted the paper. XL, HL, XZ, and QL collected the data and conducted the analyses. All authors agree to be accountable for all aspects of the work. All authors critically revised the manuscript for important intellectual content and gave final approval for this version to be published.
FundingThis study was supported by the Dazhou Science and Technology Bureau (20ZDYF001).
Authors Xiaoyi Liu and Hui Liu contributed equally. We thank the Dazhou Science and Technology Bureau for financial support.