The aim of this study was to evaluate hyperferritinemia could be a predicting factor of mortality in hospitalized patients with coronavirus disease-2019 (COVID-19).
MethodsA total of 100 hospitalized patients with COVID-19 in intensive care unit (ICU) were enrolled and classified into moderate (n=17), severe (n=40) and critical groups (n=43). Clinical information and laboratory results were collected and the concentrations of ferritin were compared among different groups. The association between ferritin and mortality was evaluated by logistic regression analysis. Moreover, the efficiency of the predicting value was assessed using receiver operating characteristic (ROC) curve.
ResultsThe amount of ferritin was significantly higher in critical group compared with moderate and severe groups. The median of ferritin concentration was about three times higher in death group than survival group (1722.25μg/L vs. 501.90μg/L, p<0.01). The concentration of ferritin was positively correlated with other inflammatory cytokines, such as interleukin (IL)-8, IL-10, C-reactive protein (CRP) and tumor necrosis factor (TNF)-α. Logistic regression analysis demonstrated that ferritin was an independent predictor of in-hospital mortality. Especially, high-ferritin group was associated with higher incidence of mortality, with adjusted odds ratio of 104.97 [95% confidence interval (CI) 2.63–4185.89; p=0.013]. Moreover, ferritin had an advantage of discriminative capacity with the area under ROC (AUC) of 0.822 (95% CI 0.737–0.907) higher than procalcitonin and CRP.
ConclusionThe ferritin measured at admission may serve as an independent factor for predicting in-hospital mortality in patients with COVID-19 in ICU.
El objetivo de este estudio fue evaluar si la hiperferritinemia podría ser un factor predictivo de la mortalidad en pacientes hospitalizados con enfermedad por coronavirus de 2019 (COVID-19).
MétodosSe incluyó un total de 100 pacientes hospitalizados con COVID-19 en la unidad de cuidados intensivos (UCI), clasificándose como grupos moderado (n=17), grave (n=40) y crítico (n=43). Se recopiló la información clínica y de laboratorio, comparándose los niveles de ferritina entre los diferentes grupos. Se evaluó la asociación entre ferritina y mortalidad mediante un análisis de regresión logística. Además, se evaluó la eficacia del valor predictivo utilizando la curva ROC (receiver operating characteristic).
ResultadosLa cantidad de ferritina fue significativamente superior en el grupo de pacientes críticos en comparación con el grupo de pacientes graves. La media de concentración de ferritina fue cerca de 3 veces superior en el grupo de muerte que en el grupo de supervivientes (1.722,25μg/L vs. 501,90μg/L, p<0,01). La concentración de ferritina guardó una correlación positiva con otras citoquinas inflamatorias tales como interleucina (IL)-8, IL-10, proteína C reactiva (PRC) y factor de necrosis tumoral (TNF)-α. El análisis de regresión logística demostró que la ferritina era un factor predictivo independiente de la mortalidad intrahospitalaria. En especial, el grupo de ferritina alta estuvo asociado a una mayor incidencia de la mortalidad, con un valor de odds ratio ajustado de 104,97 [intervalo de confianza (IC) del 95% 2,63-4.185,89; p=0,013]. Además, el valor de ferritina tuvo una ventaja de capacidad discriminativa en el área bajo la curva ROC (AUC) de 0,822 (IC 95% 0,737-0,907] superior al de procalcitonina y PRC.
ConclusiónEl valor de ferritina medido durante el ingreso puede servir de factor independiente para prevenir la mortalidad intrahospitalaria en los pacientes de COVID-19 en la UCI.
The coronavirus disease 2019 (COVID-19) remains a worldwide pandemic since it is first reported in Wuhan, China in December 2019.1 As of 19 October 2020, the WHO reported 39,944,882 infected cases and 1,111,998 deaths worldwide due to this rapidly spreading and highly contagious corona virus. Most of these patients have developed mild or moderate symptoms, such as fever and dry cough, and recovered quickly. However, severe patients have developed dyspnea and/or hypoxemia and may progress rapidly to acute respiratory distress syndrome (ARDS), septic shock, coagulopathy, and even multiple organ failure (MOF).2 Most of these severe cases are older patients with complications of hypertension (HP), diabetes mellitus (DM), or coronary heart diseases (CHD). Some severe cases have developed fast, and some patients show severe or critical manifestations since the onset of the disease and have died in a few days. The mortality for severe and critical cases are higher than those of other corona viruses, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).3
Many inflammatory biomarkers, such as interleukin (IL)-2, IL-6, IL-10, and tumor necrosis factor (TNF)-α, in severe patients are higher compared with those in patients with mild or moderate disease.4 Mortality in these severe patients with COVID-19 has been found to be associated with cytokine storm featured with a lot of inflammatory cytokines. However, the clinical and the laboratory markers of severe cases are still needed to predict the severity or mortality of the disease.
Ferritin is an intracellular protein that can store iron, and plays a critical role in inflammatory diseases, such as infection, cancer, or neurodegeneration. As a hallmark of the “hyperferritinemic syndromes”, high circulating ferritin is found in four critical diseases, including macrophage activation syndrome (MAS), adult-onset Still's disease (AOSD), catastrophic anti-phospholipid syndrome (CAPS), and septic shock.5 Some reports revealed that ferritin is an independent risk factor for severity in patients with COVID-19.6–8 However, the relationship of ferritin and mortality in patients is not clear. In this study, we measured the levels of ferritin and other clinical test results in different patients especially in severe and critical patients in intensive care unit (ICU), and conducted a retrospective study to evaluate the prognostic use of ferritin in mortality in hospitalized patients with COVID-19.
Materials and methodsPatients’ source and clinical information collectionA total of 100 patients with COVID-19 admitted to Sino-French New City Branch of Tongji Hospital from 30 January to 30 March 2020 were enrolled into this study. The confirmed cases were positive for SARS-CoV-2 nucleic acid through pharyngeal swab test by using the reverse transcription-polymerase chain reaction (RT-PCR) assay twice administered by different physicians. The RT-PCR assay was conducted in accordance with the manufacturer's protocol (Shanghai BioGerm Medical Technology, Co., Ltd.).
Enrolled patients with COVID-19 were classified into three groups in accordance with the guideline for the diagnosis and treatment of 2019 novel coronavirus-infected pneumonia.9 Moderate group showed fever and respiratory symptoms, such as dry cough, with radiological findings of pneumonia. Severe group was defined when any of the following criteria was met: dyspnea, respiration rate (RR)≥30 times/min; oxygen saturation by pulse oximeter≤93% in resting state; partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio≤300mm Hg (lmmHg=0.133kPa). Critical group was defined as: respiratory failure and requiring mechanical ventilation, shock or with other organ failure that requires ICU care. Severe and critical patients were treated in ICU. A retrospective study on the clinical information and the laboratory results was conducted, and verbal consent was obtained before enrollment.
The clinical information, such as symptoms, age, gender, and history, were documented upon admission to the hospital. Laboratory test results, including routine blood test, blood chemistry, coagulation test, and inflammatory markers, were also acquired immediately after admission.
All patients were treated in accordance with the instructions of the guidelines, and some treatments were adjusted individually. All patients’ status and the days from admission to discharge or death were documented. The study was performed according to the Declaration of Helsinki, and all procedures were approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University.
Statistical analysisThe results of continuous variables were presented as median and interquartile range (IQR) values, and categorical variables were presented as counts and percentages. Continuous variables were compared among three groups by using one-way ANOVA on ranks (Kruskal–Wallis test), whereas categorical variables were compared using the chi-square test. The Spearman correlation was used to analyze the relationship between ferritin and other cytokines. The variables with statistical significance were considered for multivariable logistic regression analysis. The regression equation and the coefficient were obtained for each influence factor. The receiver operating characteristic (ROC) analysis was conducted to obtain the best threshold values of the ferritin, procalcitonin (PCT) and C-reactive protein (CRP) to distinguish the death and the survivor with COVID-19. Sensitivity, specificity, and area under the curve (AUC) were observed. Statistical analyses were performed using the IBM SPSS Statistic Version 26.0. p<0.05 was considered statistically significant.
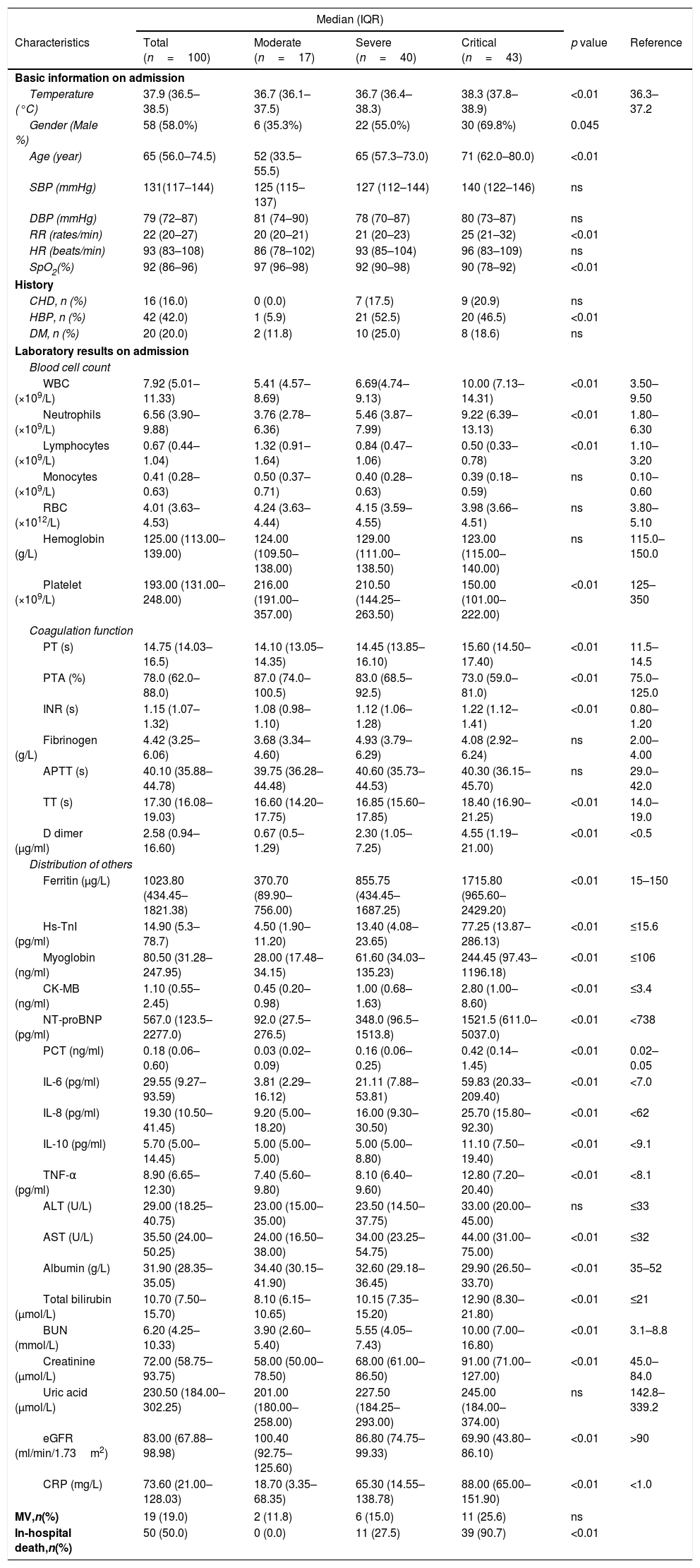
ResultsDemographic and clinical characteristicsA total of 100 patients with confirmed COVID-19 were enrolled and classified into moderate (n=17), severe (n=40) and critical groups (n=43) according to the guidelines for COVID-19. The median age of the patients was 65 years old, and males accounted for 58.0% of the enrolled patients. The median body temperature of the patients was 37.9°C when they were admitted to the hospital. HP (42.0%), DM (20.0%), and CHD (16.0%) were the most common complications (Table 1).
Clinical characteristics and laboratory results in patients with COVID-19.
| Median (IQR) | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total (n=100) | Moderate (n=17) | Severe (n=40) | Critical (n=43) | p value | Reference |
| Basic information on admission | ||||||
| Temperature (°C) | 37.9 (36.5–38.5) | 36.7 (36.1–37.5) | 36.7 (36.4–38.3) | 38.3 (37.8–38.9) | <0.01 | 36.3–37.2 |
| Gender (Male %) | 58 (58.0%) | 6 (35.3%) | 22 (55.0%) | 30 (69.8%) | 0.045 | |
| Age (year) | 65 (56.0–74.5) | 52 (33.5–55.5) | 65 (57.3–73.0) | 71 (62.0–80.0) | <0.01 | |
| SBP (mmHg) | 131(117–144) | 125 (115–137) | 127 (112–144) | 140 (122–146) | ns | |
| DBP (mmHg) | 79 (72–87) | 81 (74–90) | 78 (70–87) | 80 (73–87) | ns | |
| RR (rates/min) | 22 (20–27) | 20 (20–21) | 21 (20–23) | 25 (21–32) | <0.01 | |
| HR (beats/min) | 93 (83–108) | 86 (78–102) | 93 (85–104) | 96 (83–109) | ns | |
| SpO2(%) | 92 (86–96) | 97 (96–98) | 92 (90–98) | 90 (78–92) | <0.01 | |
| History | ||||||
| CHD, n (%) | 16 (16.0) | 0 (0.0) | 7 (17.5) | 9 (20.9) | ns | |
| HBP, n (%) | 42 (42.0) | 1 (5.9) | 21 (52.5) | 20 (46.5) | <0.01 | |
| DM, n (%) | 20 (20.0) | 2 (11.8) | 10 (25.0) | 8 (18.6) | ns | |
| Laboratory results on admission | ||||||
| Blood cell count | ||||||
| WBC (×109/L) | 7.92 (5.01–11.33) | 5.41 (4.57–8.69) | 6.69(4.74–9.13) | 10.00 (7.13–14.31) | <0.01 | 3.50–9.50 |
| Neutrophils (×109/L) | 6.56 (3.90–9.88) | 3.76 (2.78–6.36) | 5.46 (3.87–7.99) | 9.22 (6.39–13.13) | <0.01 | 1.80–6.30 |
| Lymphocytes (×109/L) | 0.67 (0.44–1.04) | 1.32 (0.91–1.64) | 0.84 (0.47–1.06) | 0.50 (0.33–0.78) | <0.01 | 1.10–3.20 |
| Monocytes (×109/L) | 0.41 (0.28–0.63) | 0.50 (0.37–0.71) | 0.40 (0.28–0.63) | 0.39 (0.18–0.59) | ns | 0.10–0.60 |
| RBC (×1012/L) | 4.01 (3.63–4.53) | 4.24 (3.63–4.44) | 4.15 (3.59–4.55) | 3.98 (3.66–4.51) | ns | 3.80–5.10 |
| Hemoglobin (g/L) | 125.00 (113.00–139.00) | 124.00 (109.50–138.00) | 129.00 (111.00–138.50) | 123.00 (115.00–140.00) | ns | 115.0–150.0 |
| Platelet (×109/L) | 193.00 (131.00–248.00) | 216.00 (191.00–357.00) | 210.50 (144.25–263.50) | 150.00 (101.00–222.00) | <0.01 | 125–350 |
| Coagulation function | ||||||
| PT (s) | 14.75 (14.03–16.5) | 14.10 (13.05–14.35) | 14.45 (13.85–16.10) | 15.60 (14.50–17.40) | <0.01 | 11.5–14.5 |
| PTA (%) | 78.0 (62.0–88.0) | 87.0 (74.0–100.5) | 83.0 (68.5–92.5) | 73.0 (59.0–81.0) | <0.01 | 75.0–125.0 |
| INR (s) | 1.15 (1.07–1.32) | 1.08 (0.98–1.10) | 1.12 (1.06–1.28) | 1.22 (1.12–1.41) | <0.01 | 0.80–1.20 |
| Fibrinogen (g/L) | 4.42 (3.25–6.06) | 3.68 (3.34–4.60) | 4.93 (3.79–6.29) | 4.08 (2.92–6.24) | ns | 2.00–4.00 |
| APTT (s) | 40.10 (35.88–44.78) | 39.75 (36.28–44.48) | 40.60 (35.73–44.53) | 40.30 (36.15–45.70) | ns | 29.0–42.0 |
| TT (s) | 17.30 (16.08–19.03) | 16.60 (14.20–17.75) | 16.85 (15.60–17.85) | 18.40 (16.90–21.25) | <0.01 | 14.0–19.0 |
| D dimer (μg/ml) | 2.58 (0.94–16.60) | 0.67 (0.5–1.29) | 2.30 (1.05–7.25) | 4.55 (1.19–21.00) | <0.01 | <0.5 |
| Distribution of others | ||||||
| Ferritin (μg/L) | 1023.80 (434.45–1821.38) | 370.70 (89.90–756.00) | 855.75 (434.45–1687.25) | 1715.80 (965.60–2429.20) | <0.01 | 15–150 |
| Hs-TnI (pg/ml) | 14.90 (5.3–78.7) | 4.50 (1.90–11.20) | 13.40 (4.08–23.65) | 77.25 (13.87–286.13) | <0.01 | ≤15.6 |
| Myoglobin (ng/ml) | 80.50 (31.28–247.95) | 28.00 (17.48–34.15) | 61.60 (34.03–135.23) | 244.45 (97.43–1196.18) | <0.01 | ≤106 |
| CK-MB (ng/ml) | 1.10 (0.55–2.45) | 0.45 (0.20–0.98) | 1.00 (0.68–1.63) | 2.80 (1.00–8.60) | <0.01 | ≤3.4 |
| NT-proBNP (pg/ml) | 567.0 (123.5–2277.0) | 92.0 (27.5–276.5) | 348.0 (96.5–1513.8) | 1521.5 (611.0–5037.0) | <0.01 | <738 |
| PCT (ng/ml) | 0.18 (0.06–0.60) | 0.03 (0.02–0.09) | 0.16 (0.06–0.25) | 0.42 (0.14–1.45) | <0.01 | 0.02–0.05 |
| IL-6 (pg/ml) | 29.55 (9.27–93.59) | 3.81 (2.29–16.12) | 21.11 (7.88–53.81) | 59.83 (20.33–209.40) | <0.01 | <7.0 |
| IL-8 (pg/ml) | 19.30 (10.50–41.45) | 9.20 (5.00–18.20) | 16.00 (9.30–30.50) | 25.70 (15.80–92.30) | <0.01 | <62 |
| IL-10 (pg/ml) | 5.70 (5.00–14.45) | 5.00 (5.00–5.00) | 5.00 (5.00–8.80) | 11.10 (7.50–19.40) | <0.01 | <9.1 |
| TNF-α (pg/ml) | 8.90 (6.65–12.30) | 7.40 (5.60–9.80) | 8.10 (6.40–9.60) | 12.80 (7.20–20.40) | <0.01 | <8.1 |
| ALT (U/L) | 29.00 (18.25–40.75) | 23.00 (15.00–35.00) | 23.50 (14.50–37.75) | 33.00 (20.00–45.00) | ns | ≤33 |
| AST (U/L) | 35.50 (24.00–50.25) | 24.00 (16.50–38.00) | 34.00 (23.25–54.75) | 44.00 (31.00–75.00) | <0.01 | ≤32 |
| Albumin (g/L) | 31.90 (28.35–35.05) | 34.40 (30.15–41.90) | 32.60 (29.18–36.45) | 29.90 (26.50–33.70) | <0.01 | 35–52 |
| Total bilirubin (μmol/L) | 10.70 (7.50–15.70) | 8.10 (6.15–10.65) | 10.15 (7.35–15.20) | 12.90 (8.30–21.80) | <0.01 | ≤21 |
| BUN (mmol/L) | 6.20 (4.25–10.33) | 3.90 (2.60–5.40) | 5.55 (4.05–7.43) | 10.00 (7.00–16.80) | <0.01 | 3.1–8.8 |
| Creatinine (μmol/L) | 72.00 (58.75–93.75) | 58.00 (50.00–78.50) | 68.00 (61.00–86.50) | 91.00 (71.00–127.00) | <0.01 | 45.0–84.0 |
| Uric acid (μmol/L) | 230.50 (184.00–302.25) | 201.00 (180.00–258.00) | 227.50 (184.25–293.00) | 245.00 (184.00–374.00) | ns | 142.8–339.2 |
| eGFR (ml/min/1.73m2) | 83.00 (67.88–98.98) | 100.40 (92.75–125.60) | 86.80 (74.75–99.33) | 69.90 (43.80–86.10) | <0.01 | >90 |
| CRP (mg/L) | 73.60 (21.00–128.03) | 18.70 (3.35–68.35) | 65.30 (14.55–138.78) | 88.00 (65.00–151.90) | <0.01 | <1.0 |
| MV,n(%) | 19 (19.0) | 2 (11.8) | 6 (15.0) | 11 (25.6) | ns | |
| In-hospital death,n(%) | 50 (50.0) | 0 (0.0) | 11 (27.5) | 39 (90.7) | <0.01 | |
Data are expressed as median and interquartile range for non-normally distributed data, or n (%). p values denoted the comparison among three groups of patients with COVID-19.
SBP indicates systolic blood pressure; DBP diastolic pressure; RR respiratory rate; HR heart rate; SpO2 percutaneous oxygen saturation; CHD coronary heart disease; HBP high blood pressure; DM diabetic mellitus; WBC white blood cells; RBC red blood cells; PT prothrombin time; PTA prothrombin activity; INR international normalized ratio; APTT activated partial thromboplastin time; TT plasma thrombin time; Hs-TnI high sensitive troponin I; CK-MB creatine kinase-MB; NT-proBNP N-terminal pro-B-type natriuretic peptide; PCT procaicltonin; IL-6/8/10 interleukin-6/8/10; TNF-α tumor necrosis factor-α; ALT alanine aminotransferase; AST aspartate aminotransferase; BUN urea nitrogen; eGFR ovulated glomerular filtration rate; CRP C reactive protein; MV mechanical ventilation; ns not significant.
As expected, markers of inflammation, such as neutrophils, myocardia injury markers, and inflammatory cytokines (IL-6, IL-8, IL-10, and TNF-α), were significantly higher in critical group compared with moderate and severe groups. Ferritin levels in critical patients were significantly higher than those in moderate and severe patients.
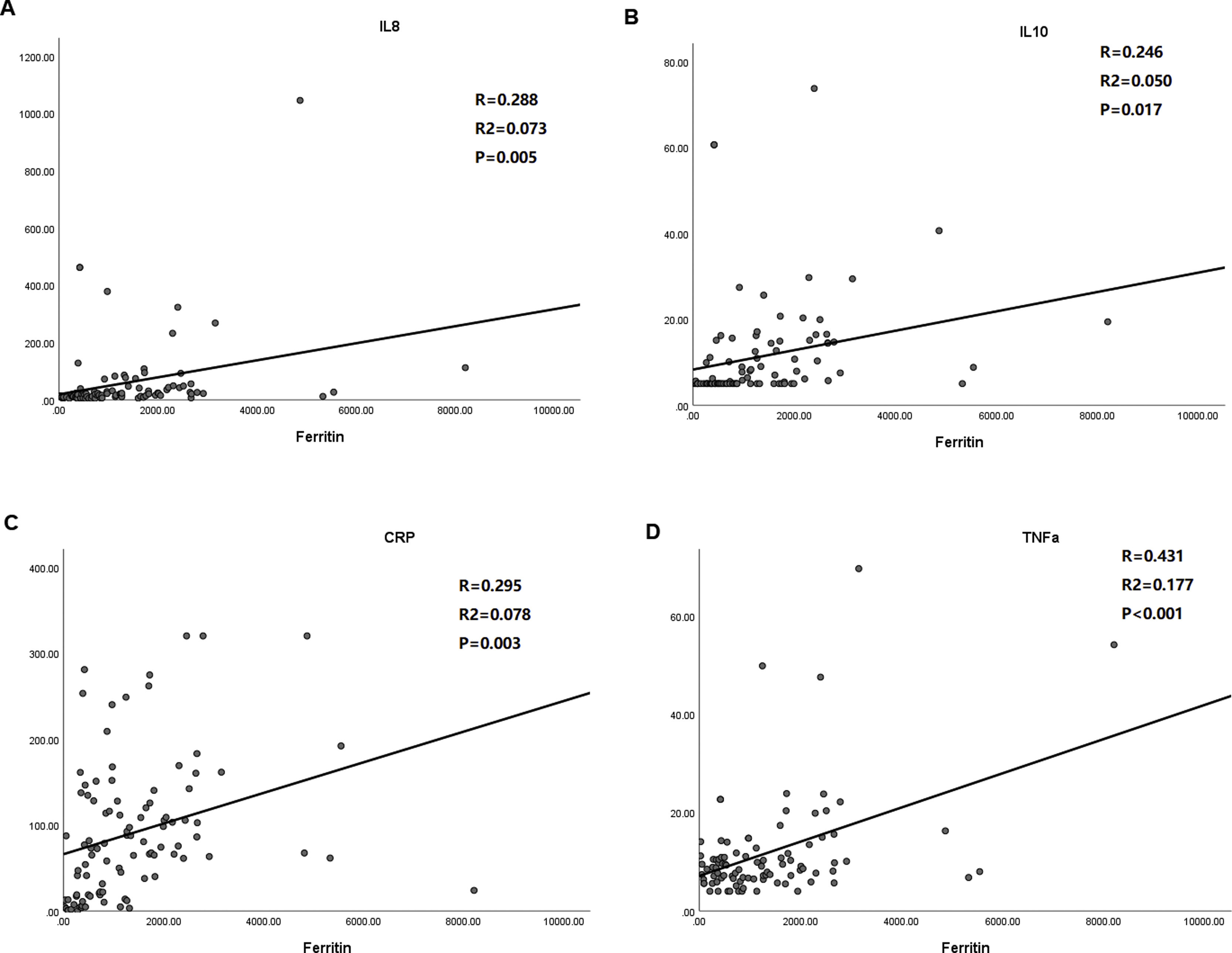
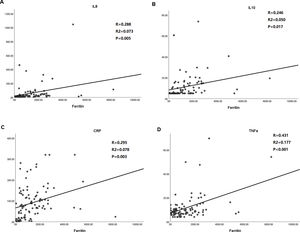
Significant positive correlation between ferritin and inflammatory cytokines, including IL-8 (r=0.288, p=0.005), IL-10 (r=0.246, p=0.017), CRP, (r=0.295, p=0.003) and TNF-α (r=0.431, p<0.001), were evaluated in enrolled patients with COVID-19 (Fig. 1).
Correlation analysis of ferritin level with that of inflammatory cytokines including IL-8, IL-10, TNF-α and CRP. A. Ferritin vs. IL-8; B. Ferritin vs. IL-10; C. Ferritin vs. CRP; D. Ferritin vs. TNF-α. Pearson correlation was used. IL-8/10 indicates interleukin-8/10; TNF-α tumor necrosis factor-α; CRP C reactive protein.
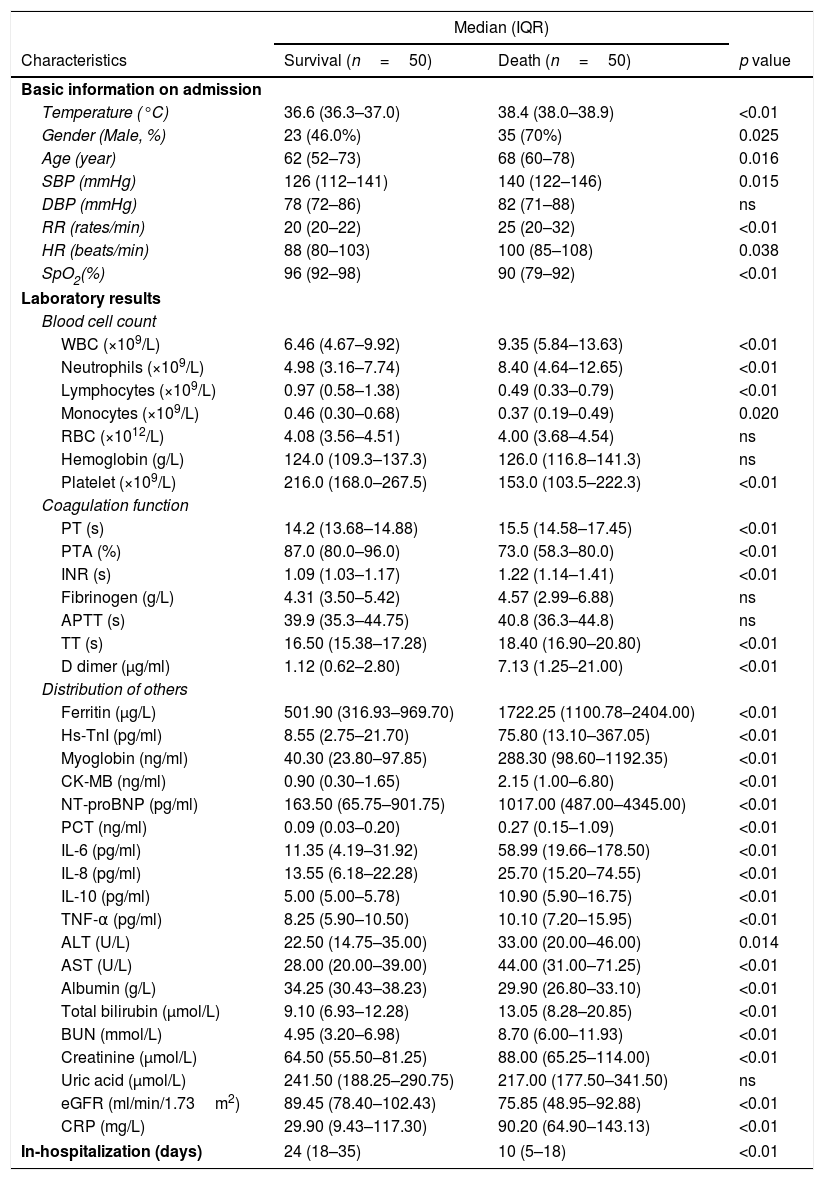
Compared with that in the survival group, the clinical characteristics and laboratory test results at admission were evaluated in the death group (Table 2). In death group, the body temperature was higher when patients admitted in the hospital. The time from admission to death or discharge was lower than that in the survival group, which reflected that the illness was more serious in the death group. The deceased patients were older than the patients who survived the disease, and most of them were male. However, history, such as CHD, HP or DM, and mechanical ventilation rate did not show statistical difference between the two groups. The routine blood tests and the inflammation markers, such as PCT, CRP, IL-6, IL-8, IL-10, and TNF-α, in the death group were higher than those in the survival group. Notably, the median (IQR) concentration of ferritin was significantly higher in death group than survival group.
Comparison of the clinical information and laboratory results in patients with COVID-19 depending on in-hospital outcomes.
| Median (IQR) | |||
|---|---|---|---|
| Characteristics | Survival (n=50) | Death (n=50) | p value |
| Basic information on admission | |||
| Temperature (°C) | 36.6 (36.3–37.0) | 38.4 (38.0–38.9) | <0.01 |
| Gender (Male, %) | 23 (46.0%) | 35 (70%) | 0.025 |
| Age (year) | 62 (52–73) | 68 (60–78) | 0.016 |
| SBP (mmHg) | 126 (112–141) | 140 (122–146) | 0.015 |
| DBP (mmHg) | 78 (72–86) | 82 (71–88) | ns |
| RR (rates/min) | 20 (20–22) | 25 (20–32) | <0.01 |
| HR (beats/min) | 88 (80–103) | 100 (85–108) | 0.038 |
| SpO2(%) | 96 (92–98) | 90 (79–92) | <0.01 |
| Laboratory results | |||
| Blood cell count | |||
| WBC (×109/L) | 6.46 (4.67–9.92) | 9.35 (5.84–13.63) | <0.01 |
| Neutrophils (×109/L) | 4.98 (3.16–7.74) | 8.40 (4.64–12.65) | <0.01 |
| Lymphocytes (×109/L) | 0.97 (0.58–1.38) | 0.49 (0.33–0.79) | <0.01 |
| Monocytes (×109/L) | 0.46 (0.30–0.68) | 0.37 (0.19–0.49) | 0.020 |
| RBC (×1012/L) | 4.08 (3.56–4.51) | 4.00 (3.68–4.54) | ns |
| Hemoglobin (g/L) | 124.0 (109.3–137.3) | 126.0 (116.8–141.3) | ns |
| Platelet (×109/L) | 216.0 (168.0–267.5) | 153.0 (103.5–222.3) | <0.01 |
| Coagulation function | |||
| PT (s) | 14.2 (13.68–14.88) | 15.5 (14.58–17.45) | <0.01 |
| PTA (%) | 87.0 (80.0–96.0) | 73.0 (58.3–80.0) | <0.01 |
| INR (s) | 1.09 (1.03–1.17) | 1.22 (1.14–1.41) | <0.01 |
| Fibrinogen (g/L) | 4.31 (3.50–5.42) | 4.57 (2.99–6.88) | ns |
| APTT (s) | 39.9 (35.3–44.75) | 40.8 (36.3–44.8) | ns |
| TT (s) | 16.50 (15.38–17.28) | 18.40 (16.90–20.80) | <0.01 |
| D dimer (μg/ml) | 1.12 (0.62–2.80) | 7.13 (1.25–21.00) | <0.01 |
| Distribution of others | |||
| Ferritin (μg/L) | 501.90 (316.93–969.70) | 1722.25 (1100.78–2404.00) | <0.01 |
| Hs-TnI (pg/ml) | 8.55 (2.75–21.70) | 75.80 (13.10–367.05) | <0.01 |
| Myoglobin (ng/ml) | 40.30 (23.80–97.85) | 288.30 (98.60–1192.35) | <0.01 |
| CK-MB (ng/ml) | 0.90 (0.30–1.65) | 2.15 (1.00–6.80) | <0.01 |
| NT-proBNP (pg/ml) | 163.50 (65.75–901.75) | 1017.00 (487.00–4345.00) | <0.01 |
| PCT (ng/ml) | 0.09 (0.03–0.20) | 0.27 (0.15–1.09) | <0.01 |
| IL-6 (pg/ml) | 11.35 (4.19–31.92) | 58.99 (19.66–178.50) | <0.01 |
| IL-8 (pg/ml) | 13.55 (6.18–22.28) | 25.70 (15.20–74.55) | <0.01 |
| IL-10 (pg/ml) | 5.00 (5.00–5.78) | 10.90 (5.90–16.75) | <0.01 |
| TNF-α (pg/ml) | 8.25 (5.90–10.50) | 10.10 (7.20–15.95) | <0.01 |
| ALT (U/L) | 22.50 (14.75–35.00) | 33.00 (20.00–46.00) | 0.014 |
| AST (U/L) | 28.00 (20.00–39.00) | 44.00 (31.00–71.25) | <0.01 |
| Albumin (g/L) | 34.25 (30.43–38.23) | 29.90 (26.80–33.10) | <0.01 |
| Total bilirubin (μmol/L) | 9.10 (6.93–12.28) | 13.05 (8.28–20.85) | <0.01 |
| BUN (mmol/L) | 4.95 (3.20–6.98) | 8.70 (6.00–11.93) | <0.01 |
| Creatinine (μmol/L) | 64.50 (55.50–81.25) | 88.00 (65.25–114.00) | <0.01 |
| Uric acid (μmol/L) | 241.50 (188.25–290.75) | 217.00 (177.50–341.50) | ns |
| eGFR (ml/min/1.73m2) | 89.45 (78.40–102.43) | 75.85 (48.95–92.88) | <0.01 |
| CRP (mg/L) | 29.90 (9.43–117.30) | 90.20 (64.90–143.13) | <0.01 |
| In-hospitalization (days) | 24 (18–35) | 10 (5–18) | <0.01 |
Data are expressed as median and interquartile range for non-normally distributed data, or n (%). p values denoted the comparison between survival and death cases.
SBP indicates systolic blood pressure; DBP diastolic pressure; RR respiratory rate; HR heart rate; SpO2 percutaneous oxygen saturation; WBC white blood cells; RBC red blood cells; PT prothrombin time; PTA prothrombin activity; INR international normalized ratio; APTT activated partial thromboplastin time; TT plasma thrombin time; Hs-TnI high sensitive troponin I; CK-MB creatine kinase-MB; NT-proBNP N-terminal pro-B-type natriuretic peptide; PCT procaicltonin; IL-6/8/10 interleukin-6/8/10; TNF-α tumor necrosis factor-α; ALT alanine aminotransferase; AST aspartate aminotransferase; BUN urea nitrogen; eGFR ovulated glomerular filtration rate; CRP C reactive protein; ns not significant.
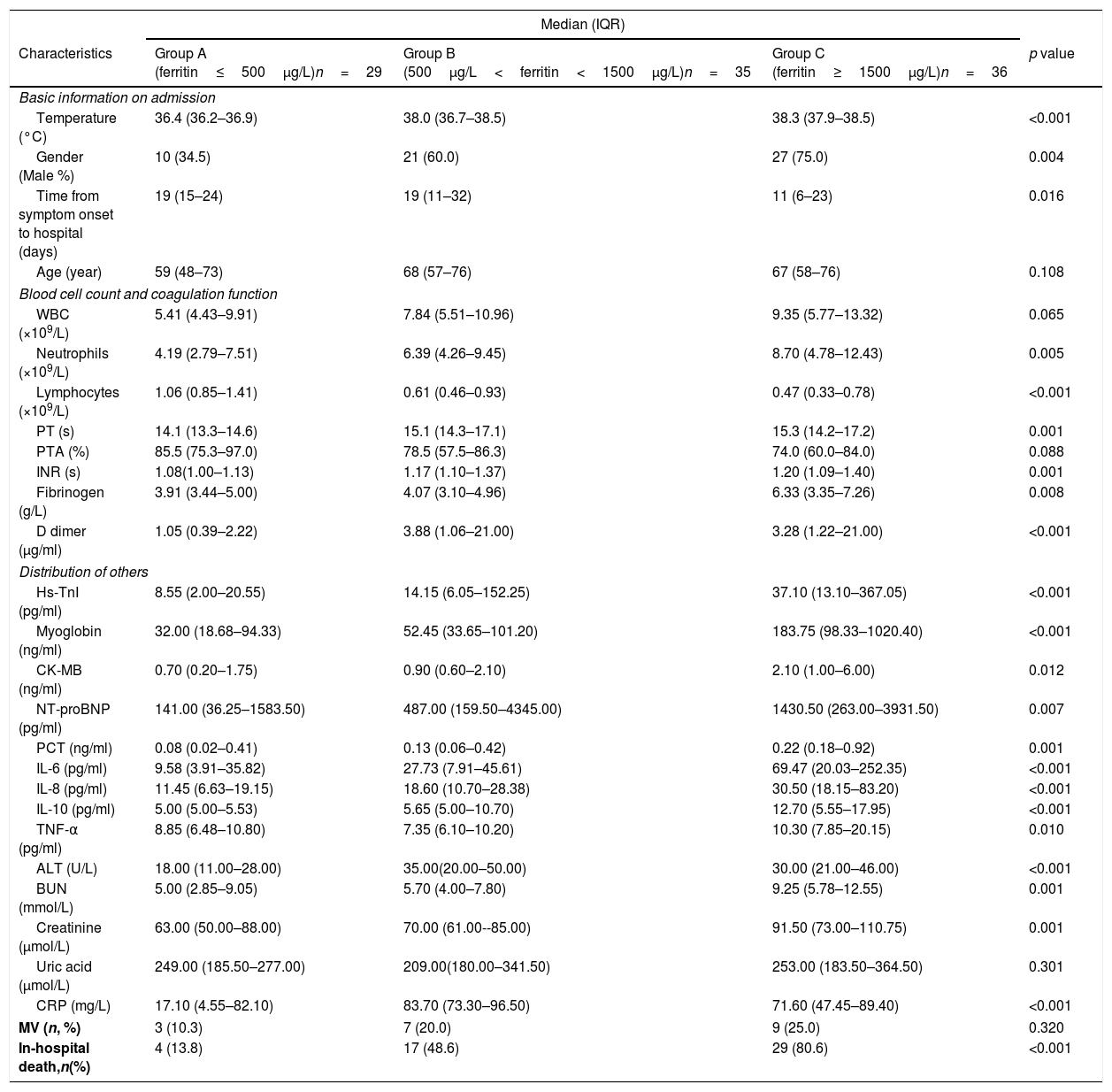
Enrolled Patients were further divided into three groups based on ferritin concentrations: group A (ferritin≤500μg/L), group B (500μg/L<ferritin<1500μg/L), and group C (ferritin≥1500μg/L). Table 3 presents baseline information and inflammatory markers were investigated among these three groups. Concentrations of inflammatory markers and in-hospital mortality rate showed significantly statistics difference (all p<0.001). Moreover, patients who had higher ferritin concentration were more likely to develop death in hospital.
Comparison of the clinical information and laboratory results in patients with COVID-19 depending on classification of ferritin level.
| Median (IQR) | ||||
|---|---|---|---|---|
| Characteristics | Group A (ferritin≤500μg/L)n=29 | Group B (500μg/L<ferritin<1500μg/L)n=35 | Group C (ferritin≥1500μg/L)n=36 | p value |
| Basic information on admission | ||||
| Temperature (°C) | 36.4 (36.2–36.9) | 38.0 (36.7–38.5) | 38.3 (37.9–38.5) | <0.001 |
| Gender (Male %) | 10 (34.5) | 21 (60.0) | 27 (75.0) | 0.004 |
| Time from symptom onset to hospital (days) | 19 (15–24) | 19 (11–32) | 11 (6–23) | 0.016 |
| Age (year) | 59 (48–73) | 68 (57–76) | 67 (58–76) | 0.108 |
| Blood cell count and coagulation function | ||||
| WBC (×109/L) | 5.41 (4.43–9.91) | 7.84 (5.51–10.96) | 9.35 (5.77–13.32) | 0.065 |
| Neutrophils (×109/L) | 4.19 (2.79–7.51) | 6.39 (4.26–9.45) | 8.70 (4.78–12.43) | 0.005 |
| Lymphocytes (×109/L) | 1.06 (0.85–1.41) | 0.61 (0.46–0.93) | 0.47 (0.33–0.78) | <0.001 |
| PT (s) | 14.1 (13.3–14.6) | 15.1 (14.3–17.1) | 15.3 (14.2–17.2) | 0.001 |
| PTA (%) | 85.5 (75.3–97.0) | 78.5 (57.5–86.3) | 74.0 (60.0–84.0) | 0.088 |
| INR (s) | 1.08(1.00–1.13) | 1.17 (1.10–1.37) | 1.20 (1.09–1.40) | 0.001 |
| Fibrinogen (g/L) | 3.91 (3.44–5.00) | 4.07 (3.10–4.96) | 6.33 (3.35–7.26) | 0.008 |
| D dimer (μg/ml) | 1.05 (0.39–2.22) | 3.88 (1.06–21.00) | 3.28 (1.22–21.00) | <0.001 |
| Distribution of others | ||||
| Hs-TnI (pg/ml) | 8.55 (2.00–20.55) | 14.15 (6.05–152.25) | 37.10 (13.10–367.05) | <0.001 |
| Myoglobin (ng/ml) | 32.00 (18.68–94.33) | 52.45 (33.65–101.20) | 183.75 (98.33–1020.40) | <0.001 |
| CK-MB (ng/ml) | 0.70 (0.20–1.75) | 0.90 (0.60–2.10) | 2.10 (1.00–6.00) | 0.012 |
| NT-proBNP (pg/ml) | 141.00 (36.25–1583.50) | 487.00 (159.50–4345.00) | 1430.50 (263.00–3931.50) | 0.007 |
| PCT (ng/ml) | 0.08 (0.02–0.41) | 0.13 (0.06–0.42) | 0.22 (0.18–0.92) | 0.001 |
| IL-6 (pg/ml) | 9.58 (3.91–35.82) | 27.73 (7.91–45.61) | 69.47 (20.03–252.35) | <0.001 |
| IL-8 (pg/ml) | 11.45 (6.63–19.15) | 18.60 (10.70–28.38) | 30.50 (18.15–83.20) | <0.001 |
| IL-10 (pg/ml) | 5.00 (5.00–5.53) | 5.65 (5.00–10.70) | 12.70 (5.55–17.95) | <0.001 |
| TNF-α (pg/ml) | 8.85 (6.48–10.80) | 7.35 (6.10–10.20) | 10.30 (7.85–20.15) | 0.010 |
| ALT (U/L) | 18.00 (11.00–28.00) | 35.00(20.00–50.00) | 30.00 (21.00–46.00) | <0.001 |
| BUN (mmol/L) | 5.00 (2.85–9.05) | 5.70 (4.00–7.80) | 9.25 (5.78–12.55) | 0.001 |
| Creatinine (μmol/L) | 63.00 (50.00–88.00) | 70.00 (61.00--85.00) | 91.50 (73.00–110.75) | 0.001 |
| Uric acid (μmol/L) | 249.00 (185.50–277.00) | 209.00(180.00–341.50) | 253.00 (183.50–364.50) | 0.301 |
| CRP (mg/L) | 17.10 (4.55–82.10) | 83.70 (73.30–96.50) | 71.60 (47.45–89.40) | <0.001 |
| MV (n, %) | 3 (10.3) | 7 (20.0) | 9 (25.0) | 0.320 |
| In-hospital death,n(%) | 4 (13.8) | 17 (48.6) | 29 (80.6) | <0.001 |
Data are expressed as median and interquartile range for non-normally distributed data, or n (%). P values denoted the comparison among three groups depending on classification of ferritin level of patients with COVID-19.
WBC indicates white blood cells; PT prothrombin time; PTA prothrombin activity; INR international normalized ratio; APTT activated partial thromboplastin time; TT plasma thrombin time; Hs-TnI high sensitive troponin I; CK-MB creatine kinase-MB; NT-proBNP N-terminal pro-B-type natriuretic peptide; PCT procaicltonin; IL-6/8/10 interleukin-6/8/10; TNF-α tumor necrosis factor-α; ALT alanine aminotransferase; BUN urea nitrogen; CRP C reactive protein; MV mechanical ventilation; ns not significant.
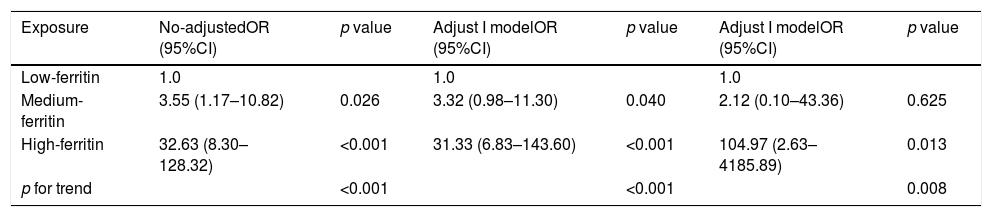
The logistic regression model was performed in the non-adjusted model, and the adjusted models I and II (Table 4). Compared with that in the low-ferritin group, the risks of death in the medium (OR=3.55, 95% CI 1.17–10.82, p<0.001) and the high-ferritin groups (OR=32.63, 95% CI 8.30–128.32, p<0.001) increased by 3.55 and 32.63 times, respectively. After adjusting the potential factors in model I and II, the OR and 95% CI were also calculated respectively. These results revealed that high serum ferritin was an independent risk factor of death in patients with COVID-19.
Logistic regression for the relationship between ferritin and in-hospital mortality in patients with COVID-19.
| Exposure | No-adjustedOR (95%CI) | p value | Adjust I modelOR (95%CI) | p value | Adjust I modelOR (95%CI) | p value |
|---|---|---|---|---|---|---|
| Low-ferritin | 1.0 | 1.0 | 1.0 | |||
| Medium-ferritin | 3.55 (1.17–10.82) | 0.026 | 3.32 (0.98–11.30) | 0.040 | 2.12 (0.10–43.36) | 0.625 |
| High-ferritin | 32.63 (8.30–128.32) | <0.001 | 31.33 (6.83–143.60) | <0.001 | 104.97 (2.63–4185.89) | 0.013 |
| p for trend | <0.001 | <0.001 | 0.008 |
Non-adjusted model adjusts for: None.
Adjusted I model adjust for: Gender, Age, MV, HBP, DM, CHD.
Adjusted I model adjust for: Gender, Age, MV, HBP, DM, CHD, WBC, D dimer, Hs-TnI, NT-proBNP, PCT, IL-6, Creatinine, eGFR, CRP.
OR odd ratio; CI confidence index; MV mechanical ventilation; HBP high blood pressure; DM diabetic mellitus; CHD coronary heart disease; WBC white blood cells; Hs-TnI high sensitive troponin I; NT-proBNP N-terminal pro-B-type natriuretic peptide; PCT procaicltonin; IL-6 interleukin-6; eGFR ovulated glomerular filtration rate; CRP C reactive protein.
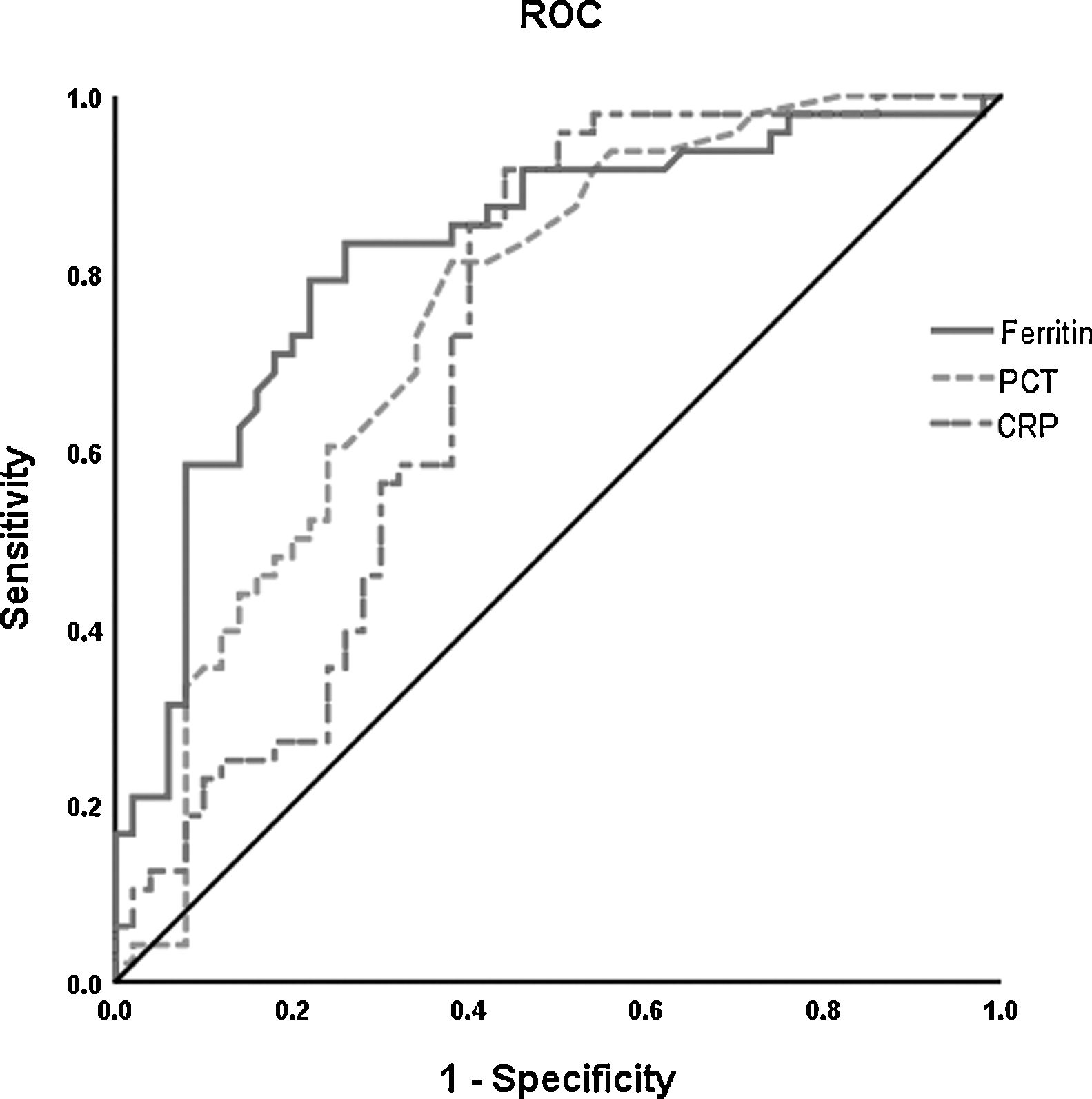
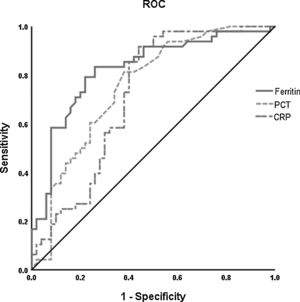
The ROC curve analysis was performed to investigate the predicting value of ferritin, PCT, and CRP in the survival and death groups. Higher AUC was found for ferritin (0.822, 95% CI 0.737–0.907) than PCT (0.751, 95% CI 0.654–0.848) and CRP (0.714, 95% CI 0.609–0.819) to differentiate death group from survival group (Fig. 2).
Receiver operating characteristic (ROC) curve analysis was performed for prediction of mortality by ferritin, PCT and CRP levels in patients with COVID-19 during hospitalization. The PCT (- - -) had AUC of 0.75 and 95% CI (0.65–0.85); CRP (— -) had AUC of 0.71 and 95% CI (0.61–0.82). The level of ferritin (——) has a better prognosis value (AUC=0.82, 95% CI 0.74–0.91). *The cut-off points were selected by maximum Youden index with the sum of sensitivity and specificity. PCT indicates procaicltonin; CRP indicates C reactive protein.
In this study, we found that the concentration of ferritin in severe or critical groups was 2.3–4.6 times higher compared with moderate group. Moreover, the median ferritin level in death group was 3.4 times higher than that in survival group. The in-hospital mortality rate was also elevated in high-ferritin group. In addition, the logistic regression analysis and ROC curve showed that ferritin was an independent risk factor and with higher AUC. Therefore, we recommend that the serum ferritin may serve as a good biomarker for the prediction of in-hospital mortality in clinical practice, especially in ICU.
Once evading the respiratory system, SARS-Cov-2 induces an immune and inflammation reaction, which is due to the infiltration of many immune cells, such as Th1 cells, CD14+CD16+ monocytes, macrophages, and neutrophils, resulting in the manifestation of a cytokine storm, including the highly expression of IL-6, IL-8, IL-10, TNF-α, monocyte chemoattractant protein-1, CRP, and ferritin.10 Among them, the cytokine storm is similar to that in SARS-CoV and MERS-CoV infections,11,12 but more severe and plays a crucial role in the pathogenesis of ARDS and MOF.
Serum ferritin is used as a marker for the evaluation of iron storage and serves as a predictor of mortality in some severe diseases such as hemodialysis and sepsis.13–15 Composed of heavy subunits “H” and light subunits “L”, this 24-subunit protein plays a vital role in many diseases, such as autoimmune diseases and inflammation. Secreted by hepatocytes and macrophages, ferritin levels are correlated with macrophage activation. Like CRP or PCT, serum ferritin is an acute phase protein, which is also elevated during infection or inflammation. Serum ferritin concentration has been found elevated in viral diseases, such as influenza H5N1, hepatitis B and C, West Nile virus, and dengue fever.16–18 For dengue fever, the serum ferritin level increases during the course of illness and predicts the disease severity with sensitivity of 76.9% and specificity of 83.3%. In another study also focusing on dengue virus infection, hyperferritinemia is proved to be associated with highly active diseases, resulting in immune activation and coagulation disturbances.19
The out-of-control inflammatory state may cause widespread tissue damage in patients with COVID-19, especially in older patients with basic history diseases, such as CHD, HP, or DM, leading to high mortality rate in severe or critical patients in ICU.20–22 It has also been found that serum ferritin is elevated enormously in death patients with COVID-19. The average concentration in severe cases is over 800μg/L, which is 1.5–5.3 times higher than that in less severe cases.23 However, the ferritin level in non-survivors is 1400μg/L, which is 3–4 times higher than those in survivors.24
In this study, we showed that inflammatory cytokines, such as IL-6, IL-8, IL-10, TNF-α, CRP, and PCT, in critical patients in ICU are almost 2–10 times higher than those in non-critical patients. The concentration of ferritin is also found to be positively correlated with these inflammatory cytokines, including IL-8, IL-10, TNF-α, and CRP. Furthermore, the ferritin concentration in death group is 3.43 times higher than that in survival group. These results indicate that the hyperferritinemia may reflects disease severity and be associated with mortality rate for COVID-19.
As many other inflammatory cytokines, ferritin is secreted and released by macrophages in vivo. In cells, ferritin can stimulate pro-inflammatory pathways, resulting in the activation of the transcription factor of nuclear factor kappa-B (NF-κB), which can increase the expression of downstream pro-inflammatory mediators and other target proteins.25 Furthermore, the heavy subunit of ferritin can directly increase of gene expression of some inflammatory cytokines, such as IL-1β, IL-6, IL-12, TNF-α, and NOD-like receptor 3, showing that the pro-inflammatory properties of ferritin.26 To this extent, a vicious loop exists between ferritin and cytokines, and targeting ferritin maybe a new way to inhibit the start of pro-inflammation.
Early identification of severe patients remains a crucial topic in clinical practice when treating this emerging virus. However, the conventional biomarkers such as WBC or PCT has limited value in evaluating the severity. In this study, we showed that ferritin had higher AUC than PCT or CRP. Observation of ferritin may allow the early identification of critical patients and decrease mortality in ICU. Consideration of the sensitivity of ferritin and CRP (83.3% vs. 91.7%), we recommend that the serum ferritin itself or combined with CRP may provide better results in predicting of in-hospital mortality in clinical practice, especially in ICU wards.
The limitations of our study should not be neglected. First, this study is a retrospective study with limited sample size and performed in a single center in Wuhan, China. Second, most of the patients with COVID-19 in our hospital are severe or critical cases rather than mild or moderate cases. Third, the ferritin and other cytokine levels were only obtained at admission. More work should be done in the future to expand the sample size, to enroll different infected patients, and to observe the fluctuation of serum ferritin in multi-centers to address these limitations.
In conclusion, this study confirmed an elevated level of ferritin in patients with COVID-19. Increased ferritin level is associated with mortality rate, and ferritin is an independent factor for predicating in-hospital mortality in patients with COVID-19 in ICU.
Authors’ contributionsData collection: Fuxue Deng, Wei Jiang, Lyu Lyu, Ziwei Lu. Data analysis and manuscript draft: Fuxue Deng, Lisha Zhang, Wei Jiang. Study supervision and technical support: Dengfeng Gao, Xiaorong Ma, Yonghong Guo, Rong Wang. Final approval of the manuscript: Shouping Gong, Wei Jiang.
Ethical approvalAll activities associated with this project were approved by the Ethics Committee of the Second Affiliated Hospital of Xi’an Jiaotong University.
FundingNo funding was provided specifically for this project.
Conflict of interestsWe declare no competing interests.
We thank all the medical staffs and patients participated in this study.