Clinical manifestations of SARSCOV2 ranges from mild symptoms to respiratory failure requiring mechanical ventilation and death. Different therapeutic approaches have been tested with no solid evidence,1 or even with no placebo arm (patients without specific treatment),2,3 as there is no certain knowledge about the mechanism that lead to respiratory failure4,5 (immunological and inflammatory theories have been proposed.
In Spain, as in many other countries of the world, due to the huge number of patients hospitalized and high mortality, specific treatment for SARSCOV2 (Hydroxyclorquine alone; Hydroxyclorquine plus azithromycin; lopinavir/ritonavir) among with supportive therapies that include antibiotics and/OR corticosteroids when inflammatory markers are elevated in lab test have been used in almost all patients hospitalized, irrespective of respiratory status and time from symptoms onset.
Our Hospital is a primary care Hospital without intensive care unit and samples for SARSCOV2 polymerase-chain-reaction (PCR) have to be sent to reference hospital. Between March 14th 2020 and April 12th 2020 a total of 43 COVID+patients were discharged from our hospital and 30 COVID+patients have died due to complications directly associated with SARSCOV2 infection.
We have retrospectively analyzed clinical outcomes of COVID 19+patients admitted to hospital with pneumonia and respiratory failure in whom specific treatment for SARSCOV2 was not administered.
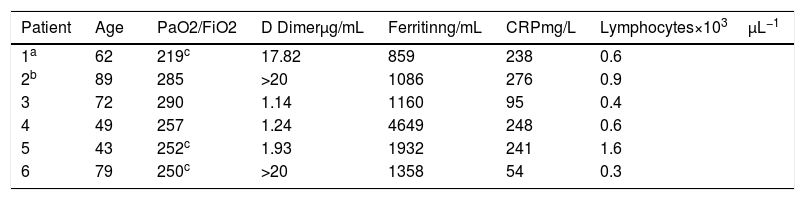
From a total of 43 patients discharged from Hospital specific treatment was not given to 6 (13.9%). In our Hospital access to specific medication is restricted to patient with positive SARSCOV2 PCR. In 4 out of 6 patients, initial negative PCR limited access to medication and all had clinical and respiratory recovery before infection diagnosis was made by PCR (3) or antibodies (1). One patient had a prolonged QT (risk of malignant arrhythmias under specific treatment) and other patient had mild infection that induced not treatment. All of them suffered mild respiratory failure evaluated by a ratio of the partial pressure of oxygen (Pao2) to the fraction of inspired oxygen (Fio2) (Pao2:Fio2) higher 200mmHg and below 300mm Hg as well as elevation of inflammatory markers in lab tests, reflected in Table 1.
Clinical characteristics of patients.
| Patient | Age | PaO2/FiO2 | D Dimerμg/mL | Ferritinng/mL | CRPmg/L | Lymphocytes×103μL−1 |
|---|---|---|---|---|---|---|
| 1a | 62 | 219c | 17.82 | 859 | 238 | 0.6 |
| 2b | 89 | 285 | >20 | 1086 | 276 | 0.9 |
| 3 | 72 | 290 | 1.14 | 1160 | 95 | 0.4 |
| 4 | 49 | 257 | 1.24 | 4649 | 248 | 0.6 |
| 5 | 43 | 252c | 1.93 | 1932 | 241 | 1.6 |
| 6 | 79 | 250c | >20 | 1358 | 54 | 0.3 |
Highest value of D Dimer, Ferritin y CRP during admission. Lowest value of lymphocytes during admission.
Among COVID+patients died, a total of 22 (73%) specific treatment was denied at the moment of admission due to bad short term prognosis. In these patient average age was 84.5 years and median time till death was 5 days.
Our data suggest that in COVID19+patients admitted to hospital with mild respiratory failure and elevated inflammatory markers at lab test, conservative treatment without specific medication and adequate respiratory support can be a valid alternative. Placebo arms should be valued in randomized trials or real world patients pool. We should be cautious interpretating series of patient without placebo arms. Now, more than ever, evidence based medicine should guide our practice.