The effect of immunomodulatory therapy with tocilizumab for coronavirus disease 2019 (COVID-19) in real-life clinical practice remains controversial.
MethodsSingle-center retrospective matched cohort analysis including 47 consecutive patients treated with intravenous tocilizumab for severe COVID-19 pneumonia (“TCZ group”), matched by age, comorbidities, time from symptoms onset and baseline SpO2/FiO2 ratio with 47 patients receiving standard of care alone (“SoC group”).
ResultsThere were no significant differences between the TCZ and SoC groups in the rate of clinical improvement (hospital discharge and/or a decrease of ≥2 points on a six-point ordinal scale) by day 7 (51.1% [24/47] versus 48.9% [23/47]; P-value=1.000). No differences were observed at day 14 in terms of clinical improvement (72.3% versus 76.6%; P-value=0.791), all-cause mortality (10.6% versus 12.8%; P-value=1.000), and the composite of invasive mechanical ventilation and/or death (25.5% versus 23.4%; P-value=1.000) either. Patients in the TCZ group had a more rapid normalization of C-reactive protein levels.
ConclusionsNo apparent benefit was observed in patients with severe COVID-19 treated with tocilizumab as compared to a matched retrospective cohort.
El efecto del tratamiento inmunomodulador con tocilizumab en la COVID-19 sigue siendo controvertido.
MétodosEstudio unicéntrico de cohortes retrospectivas pareadas que incluyó a 47 pacientes con COVID-19 grave tratados con tocilizumab intravenoso («grupo TCZ»), emparejados por edad, comorbilidades mayores, evolución de síntomas y cociente SpO2/FiO2 basal con 47 pacientes que recibieron tratamiento estándar únicamente («grupo SoC»).
ResultadosNo observamos diferencias significativas entre los grupos de TCZ y SoC en la tasa de mejoría clínica (alta hospitalaria y/o descenso de ≥ 2 puntos en una escala ordinal de 6 puntos) al día 7 (51,1% [24/47] vs. 48,9% [23/47]; P=1,000). Tampoco hubo diferencias al día 14 en las tasas de mejoría clínica (72,3% vs. 76,6%; P=0,791), mortalidad (10,6% vs. 12,8%; P=1,000) o en el compuesto de ventilación mecánica invasiva y/o muerte (25,5% vs. 23,4%; P=1,000). Los pacientes en el grupo de TCZ presentaron una normalización más rápida de la proteína C reactiva.
ConclusionesRespecto a una cohorte retrospectiva pareada, no detectamos un beneficio asociado al tratamiento con tocilizumab en pacientes con neumonía por COVID-19.
Therapeutic immunomodulation has emerged as a potentially life-saving option in severe coronavirus disease 2019 (COVID-19). The abrogation of the pro-inflammatory cytokine interleukin (IL)-6 has raised particular interest, since elevated IL-6 levels mediate systemic inflammatory responses triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Various cohort studies have reported outcomes in patients treated off-label with intravenous (IV) or subcutaneous tocilizumab (TCZ), a humanized monoclonal antibody targeting the IL-6 receptor (IL-6R), with conflicting results.2–5 Most of these studies analyzed death or requirement for invasive mechanical ventilation (IMV) as outcome variables, but lacked details on the evolution of clinical status or laboratory parameters. More recently, the publication of randomized clinical trials (RCTs) yielding negative results have contributed to increase the uncertainties regarding the benefit derived from TCZ therapy.6,7 The lack of clinical evidence during the very early pandemic phase implied that patients with severe COVID-19 pneumonia during the first two weeks of March that would have potentially benefited from TCZ did not receive this agent at our center, offering a suitable comparison group. Our research was aimed at elucidating the effect of IV TCZ in the achievement of clinical improvement by using a matched cohort that received standard of care (SoC) alone.
Materials and methodsStudy population and designThis retrospective single-center matched cohort study was performed in accordance with the STROBE guidelines. The Clinical Research Ethics Committee approved the study protocol (number 20/117). Consecutive patients admitted due to COVID-19 pneumonia that received one or more doses of IV TCZ from March 16 to March 22, 2020, were included (“TCZ group”). In addition, one matched patient also diagnosed with COVID-19 pneumonia between March 8 and March 16 and fulfilling the criteria for TCZ therapy but not receiving this agent was selected (“SoC group”), as detailed in Supplementary Methods. Clinical variables, laboratory parameters and outcomes were retrospectively collected using a standardized case report form. The respiratory status was assessed by the pulse oximetry oxygen saturation to fraction of inspired oxygen (SpO2/FiO2) ratio. A six-point ordinal scale was applied to analyze clinical status: 1. – not hospitalized; 2. – hospitalized, not requiring supplemental oxygen; 3. – hospitalized, requiring low-flow supplemental oxygen (FiO2 <40%); 4. – hospitalized, requiring high-flow supplemental oxygen (FiO2 ≥40%) or non-invasive mechanical ventilation; 5. – hospitalized, requiring IMV, extracorporeal membrane oxygenation, or both; and 6. – death.
Study outcomesThe primary outcome was the proportion of patients that achieved clinical improvement (defined as hospital discharge and/or a decrease of ≥2 points from day 0 on the six-point ordinal scale) by day 7. Secondary outcomes included the rates of clinical improvement, all-cause mortality, and the composite of IMV and/or death by day 14.
Criteria for TCZ therapyThe off-label use of TCZ was only considered in patients deemed eligible for intensive care unit (ICU) admission and IMV, with bilateral (or rapidly progressive) interstitial or alveolar infiltrates in chest X-ray or computerized tomography scan, and fulfilling at least one of the following: (a) respiratory frequency >30 breaths per minute and/or SpO2 <92% on room air; (b) C-reactive protein (CRP) levels >10mg/dL; (c) IL-6 levels >40pg/mL; and/or (d) D-dimer level >1500ng/mL. Exclusion criteria included the presence of significant liver function abnormalities, uncontrolled bacterial or fungal superinfection, or acute diverticulitis or bowel perforation. An initial IV 400mg (if body weight <75kg) or 600mg (if body weight ≥75kg) TCZ dose was administered as one-hour infusion, with a second 400mg dose 12h later. A third dose could be given after 24h to selected patients exhibiting a partial response. Further methodological details (including statistical analysis) are available as Supplementary Methods.
ResultsWe included 47 pairs of patients within the TCZ and SoC groups (Table S1 in Supplementary Results). In the TCZ group, 40.4% (19/47), 51.1% (24/47) and 8.5% (4/47) of patients received one, two and three IV doses, respectively. Within the SoC group the median variation in the SpO2/FiO2 ratio from day −1 to day 0 was −22.5% (interquartile range [IQR]: −8.7% to −35.7%). There were no differences in vital signs at day 0 between both groups. Laboratory values were also similar, with the exception of higher neutrophil-to-lymphocyte ratio and serum CRP level in the TCZ group. No differences were observed in the use of corticosteroids, either before or after day 0 (Table S2).
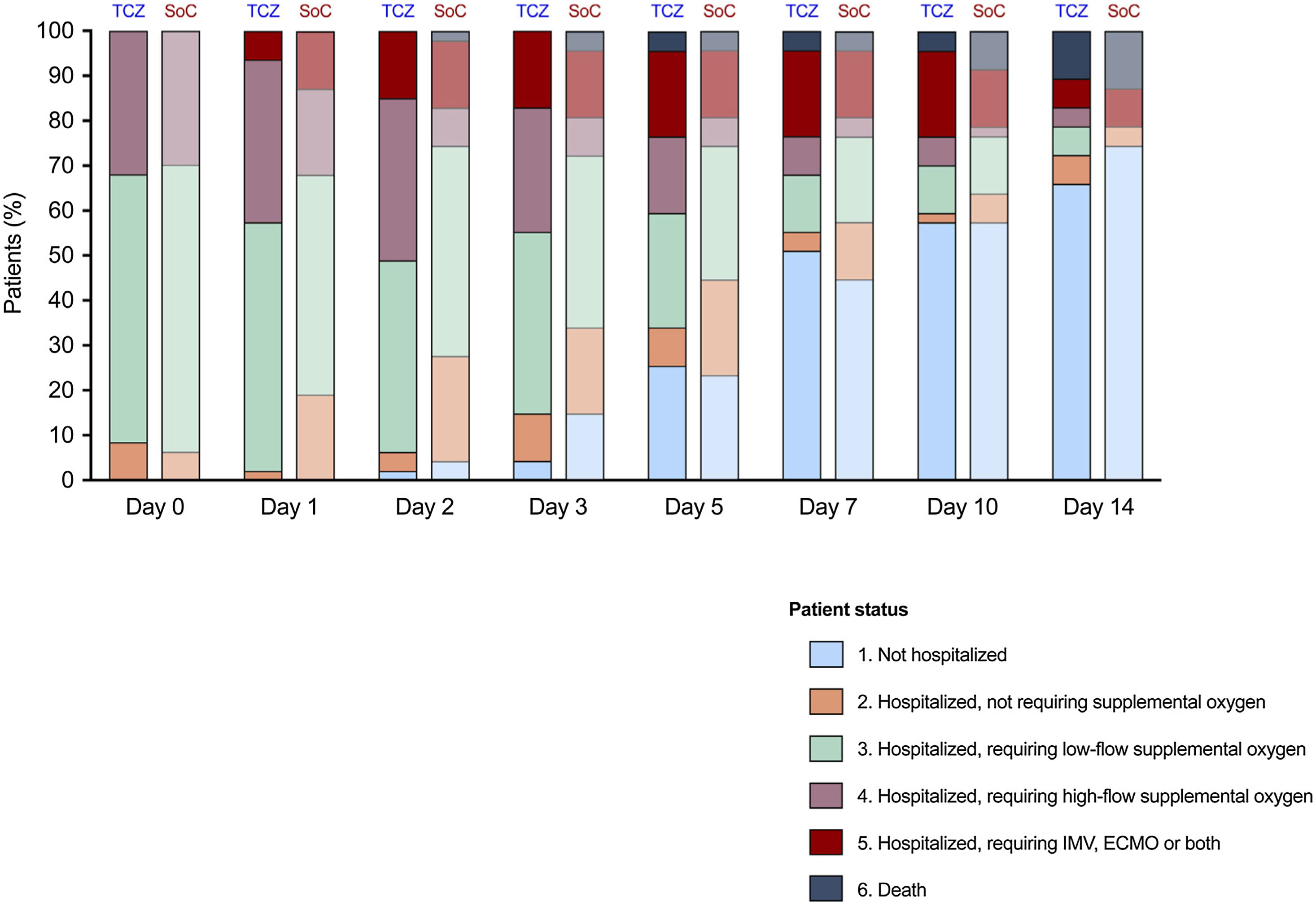
There were no differences in the proportion of patients that achieved the primary outcome of clinical improvement by day 7 (51.1% [24/47] in the TCZ group versus 48.9% [23/47] in the SoC group; P-value=1.000; absolute difference: 2.1; 95% confidence interval [CI]: −18.0–22.3). Similar median scores in the six-point scale were also obtained by day 7 (1 [IQR: 1–4] versus 2 [IQR: 1–3], respectively; P-value=0.870) (Table 1). The evolution of the patient's status in both groups is shown in Fig. 1.
Comparison of primary and secondary study outcomes in the TCZ and SoC groups.
| Variable | TCZ group (n=47) | SoC group (n=47) | P-value | Absolute risk difference (95% CI) |
|---|---|---|---|---|
| Clinical improvement at day 7 [n (%)] | 24 (51.1) | 23 (48.9) | 1.000 | 2.1 (−18.0–22.3) |
| Six-point ordinal scale score at day 7 [median (IQR)] | 1 (1–4) | 2 (1–3) | 0.870 | |
| Difference in six-point ordinal scale scores between day 0 and day 7 [median (IQR)] | −1 (−2–0) | −1 (−2–0) | 0.096 | |
| Clinical improvement at day 14 [n (%)] | 34 (72.3) | 36 (76.6) | 0.791 | −4.3 (−21.9–13.3) |
| Six-point ordinal scale score at day 14 [median (IQR)] | 1 (1–3) | 1 (1–2) | 0.871 | |
| Difference in six-point ordinal scale scores between day 0 and day 14 [median (IQR)] | −2 (−2–0) | −2 (−2 to −1) | 0.564 | |
| All-cause mortality at day 14 [n (%)] | 5 (10.6) | 6 (12.8) | 1.000 | −2.1 (−15.1–10.9) |
| ICU admission at day 14 [n (%)] | 10 (21.3) | 7 (14.9) | 0.549 | 6.38 (−9.12–21.9) |
| ICU admission and/or death at day 14 [n (%)] | 12 (25.5) | 11 (23.4) | 1.000 | 2.1 (−15.2–19.5) |
| Microbiologically documented bacterial superinfection at day 28 [n (%)] | 2 (4.3) | 1 (2.1) | 1.000 | 2.2 (−4.9–9.3) |
CI: confidence of interval; ICU: intensive care unit; IQR: interquartile range; SoC: standard of care; TCZ: tocilizumab.
Patient status according to the six-point ordinal scale at different times in the TCZ and SoC groups. The median scores were similar in both groups, either by day 7 (1 [IQR: 1–4] versus 2 [IQR: 1–3]; P-value=0.870) or day 14 (1 [IQR: 1–3] versus 1 [IQR: 1–2], respectively; P-value=0.871). ECMO: extracorporeal membrane oxygenation; IMV: invasive mechanical ventilation; SoC: standard of care; TCZ: tocilizumab.
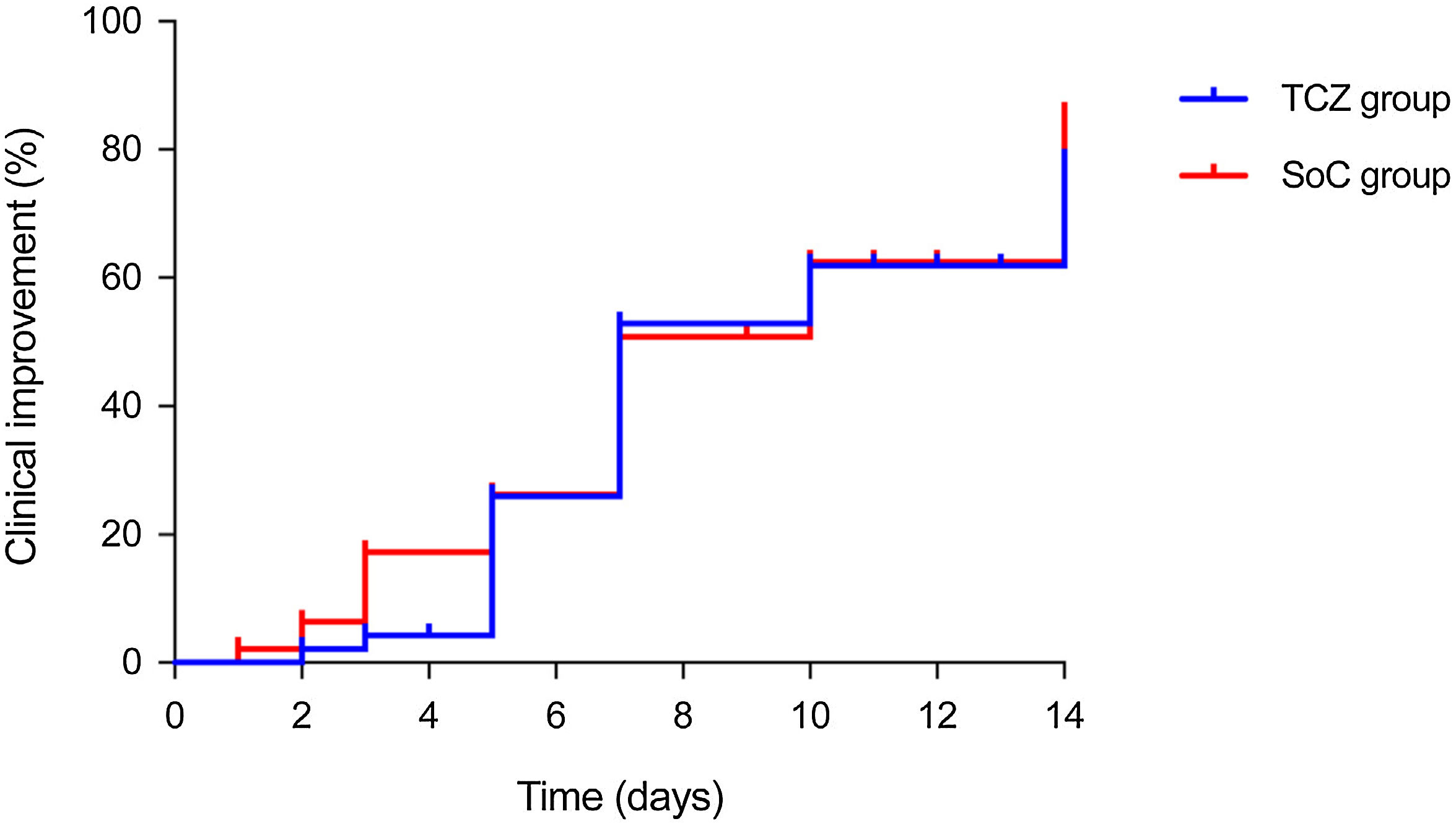
Regarding secondary outcomes, the proportion of patients with clinical improvement by day 14 was similar between the TCZ and SoC groups (72.3% [34/47] versus 76.6% [36/47]; P-value=0.791; absolute difference: −4.3; 95% CI: −21.9–13.3) (Table 1). Kaplan–Meier incidence curves for clinical improvement through day 0 to day 14 are depicted in Fig. 2. No significant differences were observed for all-cause mortality (10.6% [5/47] versus 12.8% [6/48]; P-value=1.000; absolute difference: −2.1; 95% CI: −15.1–10.9) or the composite of IMV and/or death by day 14 (25.5% [12/47] versus 23.4% [11/47]; P-value=1.000; absolute difference: 2.1; 95% CI: −15.2–19.5) either (Figure S1).
In a set of sensitivity analyses, no significant differences in the primary outcome were found when the comparison was restricted to patients ≥60 years, less than 9 days since symptom onset, or a SpO2/FiO2 ratio at day 0<316 (data not shown).
Finally, the kinetics of laboratory values were compared. The SpO2/FiO2 ratio was lower in the TCZ group from days 1 through 3 (Figure S2). Despite CRP at day 0 being higher among patients treated with TCZ, serum levels decreased rapidly thereafter to become significantly lower than the SoC group at days 3, 5 and 7 (Figure S3). No significant differences were found in the absolute lymphocyte count (Figure S4), whereas lactate dehydrogenase levels were higher in the TCZ group at day 5 (Figure S5).
DiscussionThe role of TCZ as immunomodulatory therapy for COVID-19 remains controversial since many observational studies lacked a comparative group. In addition, patient eligibility criteria for RCTs may compromise the generalizability of results to real-life practice, particularly in a pandemic setting with overwhelmed healthcare resources.1,6,8 We were not able to demonstrate an apparent benefit from the use of IV TCZ in terms of clinical improvement, requirement of IMV or all-cause mortality. Consecutive patients receiving TCZ were carefully matched with non-TCZ-treated patients for a number of variables that have been shown to be critical determinants of outcome. These matching criteria were chosen to ensure that patients in each groups were at comparable clinical phases and exhibited similar disease severity.
Previous retrospective comparative studies with a design similar to ours have also failed to confirm the impact of TCZ on the outcome of COVID-19 patients.2,5 The lack of clinical benefit derived from the IL-6 blockade in the present and previous studies is in contrast with results reported by other authors.3,4,9 A plausible explanation may lie in the low baseline risk of poor outcomes among patients selected to receive TCZ, which would negatively impact the capacity to reveal the benefit associated to therapy. Indeed, the median age for both groups in our series was notably lower than that of other observational cohorts.2,4,8 None of the patients in the TCZ or SoC groups were receiving IMV at day 0, in contrast to the cohort of Somers et al.3 Various RCTs also failed to demonstrate a benefit in the occurrence of various composite outcomes including death or requirement for IMV.6–8 Nevertheless, a recently published adaptive trial reported improved outcomes for critically-ill patients receiving organ support treated with TCZ or the IL-6-targeted monoclonal antibody sarilumab.10 Due to drug shortages over the first weeks of the pandemic and initial concerns about the safety of immunomodulatory therapies, the use of TCZ had to be prioritized for patients deemed suitable for ICU admission, which likely implied that younger individuals with lower comorbidity burden were positively selected. In addition, our institutional policy assumed that the highest benefit from IL-6 blockade would be obtained at relatively early stages of the disease, before the development of acute respiratory distress syndrome. Although the optimal timing for immunomodulation in COVID-19 has not been yet established, it may be hypothesized that the effect of TCZ could have been more evident among patients at more advanced phases of infection.
Our study has some limitations. Despite efforts to select matched pairs of patients with similar characteristics and at comparable stages of COVID-19, we cannot rule out the presence of unmeasured imbalances. Patients in the TCZ group showed lower SpO2/FiO2 ratios from days 1 to 3, which would suggest a poorer baseline status. Nevertheless, a transient early deterioration of respiratory function has been also described with the use of TCZ.5 Our study may have been underpowered to detect clinically relevant differences in study outcomes. Although the difference did not reach statistical significance, the proportion of TCZ-treated patients that had previously received corticosteroids was numerically higher as compared to the SoC group. This baseline between-group imbalance, however, would have skewed results in favor of TCZ, thus overestimating the actual benefit (if any) of this therapy. The lack of measurements of serum IL-6 levels in the SoC group prevented us from comparing the evolution of this parameter according to the treatment administered. Finally, the single-center design hampers the generalizability of our findings.
Matched cohort studies may have the ability to minimize inherent confounding by indication bias in the assignment of treatment options, thus contributing to validate in real-life patients the findings derived from trial populations. We have not demonstrated significant differences in the rates of clinical improvement between patients with severe COVID-19 consecutively treated with TCZ and a group of matched patients that only received SoC. This lack of apparent benefit is in line with results reported from some6–8 but not all RCTs.10 Whether the expected benefit from TCZ-based therapy may be more evident for older patients at more severe stages of disease remains to be investigated.
Authors’ contributionsMFR, FLM, OC and JMA designed research; MFR, FLM and OC analyzed data; MFR, FLM and JMA were involved in the clinical management of patients and acquisition of data; MFR, FLM, OC, PP and TMR collected clinical and laboratory data; MFR and FLM drafted the original version of the manuscript; and JMA critically revised the definitive version of the manuscript.
Funding sourcesThis work was supported by Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation (COVID-19 Research Call COV20/00181) — co-financed by European Development Regional Fund “A way to achieve Europe”. M.F.R. holds a research contract “Miguel Servet” (CP18/00073) from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation.
Conflict of interestsAll the authors declare no potential conflict of interest regarding this study.
Unit of Infectious Diseases: Rafael San Juan, Manuel Lizasoain, Jose T. Silva, Isabel Rodríguez-Goncer, Pilar Hernández-Jiménez, Laura Corbella, María Ruiz-Ruigómez; Department of Internal Medicine: María Asunción Pérez-Jacoiste Asín, Cristina de la Calle, Borja de Miguel, Raquel Díaz Simón, Antonio Lalueza, Guillermo Maestro de la Calle, Mar Ripoll, Carlos Lumbreras; Department of Pharmacy: José Manuel Caro-Teller, José Miguel Ferrari; Department of Pneumology: Eva Arias Arias, Javier Sayas Catalán, Rocío García-García; Department of Nephrology: Hernando Trujillo, Fernando Caravaca, Eduardo Gutiérrez, Ángel Sevillano, Amado Andrés, Manuel Praga; Department of Rheumatology: María Martín-López, José L. Pablos; Department of Hematology: Joaquín Martínez-López, María Liz Paciello, Denis Zafra, Cristina García Sánchez; Department of Medical Oncology: Carlos Gómez, Carmen Díaz-Pedroche, Flora López, Luis Paz-Ares; Department of Intensive Care Medicine: Jesús Abelardo Barea Mendoza, Paula Burgüeño Laguia, Mercedes Catalán, Helena Domínguez Aguado, Amanda Lesmes González de Aledo, Juan Carlos Montejo; Department of Emergency Medicine: Antonio Blanco Portillo, Laura Castro Reyes, Manuel Gil Mosquera, José Luis Montesinos Díaz, Isabel Fernández Marín, Julia Origüen; Department of Immunology: Rocío Laguna-Goya, Óscar Cabrera-Marante, Antonio Serrano-Hernández, Daniel Pleguezuelo, Édgar Rodríguez de Frías, Paloma Talayero, Laura Naranjo-Rondán, Ángel Ramírez-Fernández, María Lasa-Lázaro, Daniel Arroyo-Sánchez, Estela Paz-Artal; Department of Microbiology: Rafael Delgado, María Dolores Folgueira.






![Patient status according to the six-point ordinal scale at different times in the TCZ and SoC groups. The median scores were similar in both groups, either by day 7 (1 [IQR: 1–4] versus 2 [IQR: 1–3]; P-value=0.870) or day 14 (1 [IQR: 1–3] versus 1 [IQR: 1–2], respectively; P-value=0.871). ECMO: extracorporeal membrane oxygenation; IMV: invasive mechanical ventilation; SoC: standard of care; TCZ: tocilizumab. Patient status according to the six-point ordinal scale at different times in the TCZ and SoC groups. The median scores were similar in both groups, either by day 7 (1 [IQR: 1–4] versus 2 [IQR: 1–3]; P-value=0.870) or day 14 (1 [IQR: 1–3] versus 1 [IQR: 1–2], respectively; P-value=0.871). ECMO: extracorporeal membrane oxygenation; IMV: invasive mechanical ventilation; SoC: standard of care; TCZ: tocilizumab.](https://static.elsevier.es/multimedia/23870206/0000015800000012/v1_202206230718/S2387020622002741/v1_202206230718/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

