Analyse the clinical profile, associated tumour types, and response to treatment of paraneoplastic neurological syndromes associated with antibodies against Ma proteins.
MethodsA retrospective study of patients with antibodies against Ma proteins identified in a neuroimmunology laboratory of reference.
ResultsOf the 32 patients identified, 20 showed reactivity against Ma2 only (anti-Ma2 antibodies), 11 against Ma1 and Ma2 (anti-Ma antibodies), and 1 with reactivity against Ma1 only (anti-Ma1 antibodies). The most common clinical presentations were limbic encephalopathy, diencephalic dysfunction, or brainstem encephalopathy, frequently appearing as a combination of these features. Three patients had isolated cerebellar dysfunction with anti-Ma antibodies, and 2 exhibited peripheral nervous system syndrome with anti-Ma2 antibodies. Testicular tumours were the most common neoplasms (40%) in the anti-Ma2 cases. In the group associated with anti-Ma1 antibodies, the most common were lung tumours (36%), followed by testicular tumours. All idiopathic cases were reactive to Ma2. The clinical outcome was significantly better in the anti-Ma2 group. The patient with anti-Ma1 presented with limbic encephalitis and brainstem dysfunction associated with lymphoepithelioma of the bladder.
ConclusionsSpecifically determining the different reactivities of anti-Ma protein antibodies in order to differentiate between Ma1 and Ma2 antibodies is important because anti-Ma2-associated paraneoplastic syndromes have a better outcome. Lastly, this study is the first to confirm that there may be cases that react exclusively to antibodies against Ma1.
Analizar el perfil clínico, los tipos de tumour asociado y la respuesta al tratamiento de los síndromes neurológicos paraneoplásicos asociados a anticuerpos contra proteínas Ma.
MétodosEstudio retrospectivo de los pacientes con anticuerpos contra proteínas Ma identificados en un laboratorio de referencia en neuroinmunología.
ResultadosSe diagnosticó a 32 pacientes, 20 con reactividad frente a Ma2 aislada (anticuerpos anti-Ma2), 11 con reactividad frente a Ma1 y Ma2 (anticuerpos anti-Ma) y uno con reactividad frente a Ma1 aislada (anticuerpos anti-Ma1). La presentación clínica más frecuente fue un cuadro neurológico que de forma aislada o en combinación afectó al sistema límbico, diencéfalo y mesencéfalo. Tres pacientes presentaron un cuadro cerebeloso aislado con anti-Ma y 2 un síndrome periférico con anti-Ma2. Los tumores testiculares fueron los más frecuentes (40%) en los casos anti-Ma2. En el grupo asociado a anti-Ma1, los más frecuentes fueron los tumores de pulmón (36%), seguidos de los testiculares. Todos los casos idiopáticos fueron reactivos frente a Ma2. La evolución clínica fue significativamente mejor en el grupo anti-Ma2. El paciente con anti-Ma1 presentó un cuadro de encefalitis límbica y mesodiencefálica asociado a un cáncer linfoepitelial de vejiga.
ConclusionesLa determinación específica de las diferentes reactividades de las proteínas Ma, diferenciando los anticuerpos frente a Ma1 y Ma2, es importante pues los síndromes neurológicos asociados a anticuerpos anti-Ma2 responden mejor al tratamiento. Finalmente, se confirma por primera vez que puede haber casos con anticuerpos que solo reaccionan contra Ma1.
Paraneoplastic syndromes are clinical profiles that can favour early diagnosis of the associated tumour, which makes recognising them very important.1,2 Anti-Ma antibodies are included in the group known as ‘well characterised onconeuronal antibodies’. As such, they allow doctors to diagnose ‘definite paraneoplastic syndrome’, even when no tumour has been detected.3 The anti-Ma2 antibody is present in men with testicular tumours and isolated or combined limbic encephalitis (LE), diencephalic encephalitis (DE), or brainstem encephalitis (BE). Before the discovery of anti-Ma2 antibodies, anti-Ma antibodies had been reported in 4 patients with associated tumours of diverse aetiology and location (parotid gland, breast, lung [non-small-cell], and colon), and with pancerebellar syndromes or brainstem involvement.4 Both types of antibody recognise Ma family proteins, which are exclusively found in neurons and testicular germ cells, 2 locations considered immunologically privileged. While anti-Ma2 antibodies recognise Ma2 protein (PNMA2), anti-Ma antibodies recognise Ma1 and Ma2 (PNMA1 and PNMA2).5
The interest sparked by the identification of a link between anti-Ma2, LE/DE/BE, and testicular cancer6 led to classifying patients with anti-Ma antibodies exclusively as having Ma2, or to analysing only Ma2 reactivity in other cases. In this context, it was not clear whether the patient presented anti-Ma2 or anti-Ma antibodies. Differentiating between the 2 antibodies is important, since neurological symptoms seem to be associated with different tumours. In order to clarify this topic, we have reviewed our series of patients with anti-Ma and anti-Ma2 antibodies to better define associated neurological symptoms and tumour types, as well as responses to treatment.
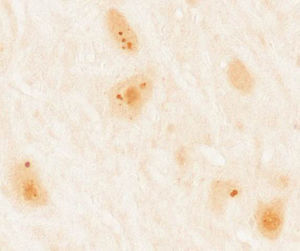
Patients and methodsWe gathered a total of 36 patients aged 18 years and older diagnosed with paraneoplastic syndrome associated with antibodies against Ma proteins. Diagnosis was performed at the neuroimmunological research laboratory at Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Hospital Clínic de Barcelona. We excluded 4 patients due to lack of clinical information, resulting in a final sample of 32 patients. In all cases, we initially performed immunohistochemical screening on frozen sections of rat brain perfusion-fixed with 2% paraformaldehyde. Subsequently, results indicating characteristic immunoreactivity (Fig. 1) were confirmed by commercial immunoblot (Ravo Diagnostika, Friburgo, Germany) (Fig. 2). Patients’ clinical data were gathered from the medical histories and examinations at that hospital, or else provided by neurologists responsible for their care at other hospitals. Additionally, we conducted a literature review of new cases dating back to 2008, beginning immediately after the last published review on this topic,7 using a PubMed database search of the following keywords: “anti-Ma”, “anti-Ta”, “anti-Ma2”, “Ma1”, “Ma2”, and “paraneoplastic neurological syndromes”.
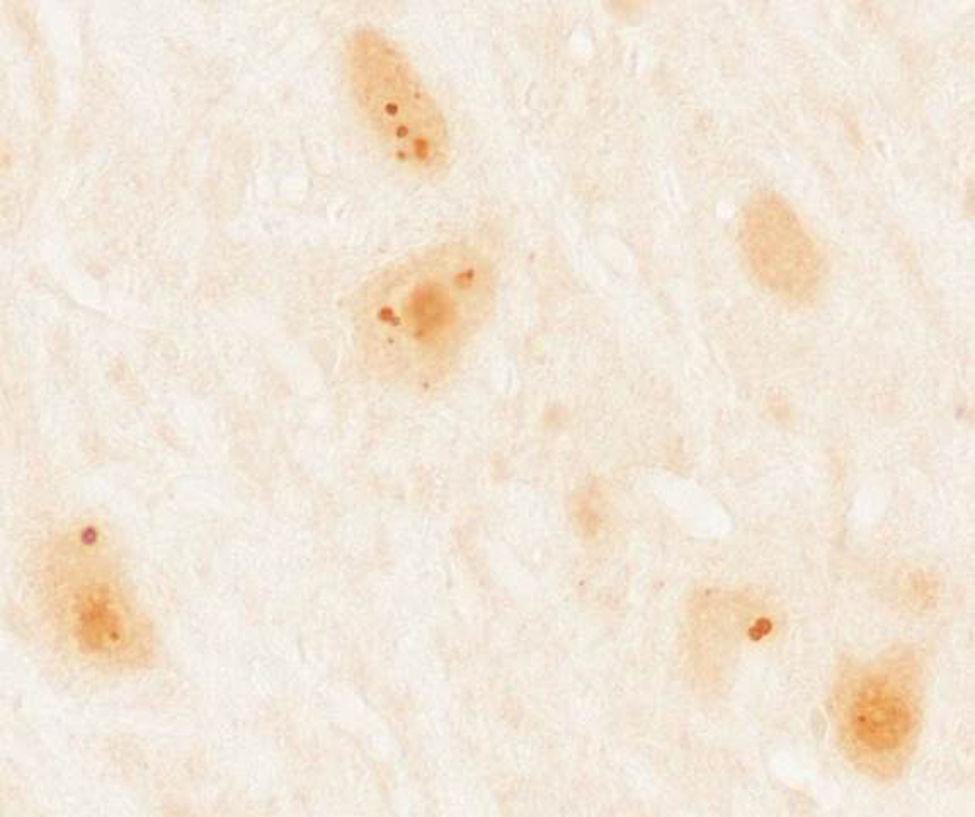
Immunohistochemical study of rat cerebellum incubated with serum from a patient with anti-Ma2 antibodies using the immunoperoxidase technique with avidin-biotin-peroxidase complex. We observed reactivity in the shape of large intracytoplasmic dots in neurons of the dentate nucleus.
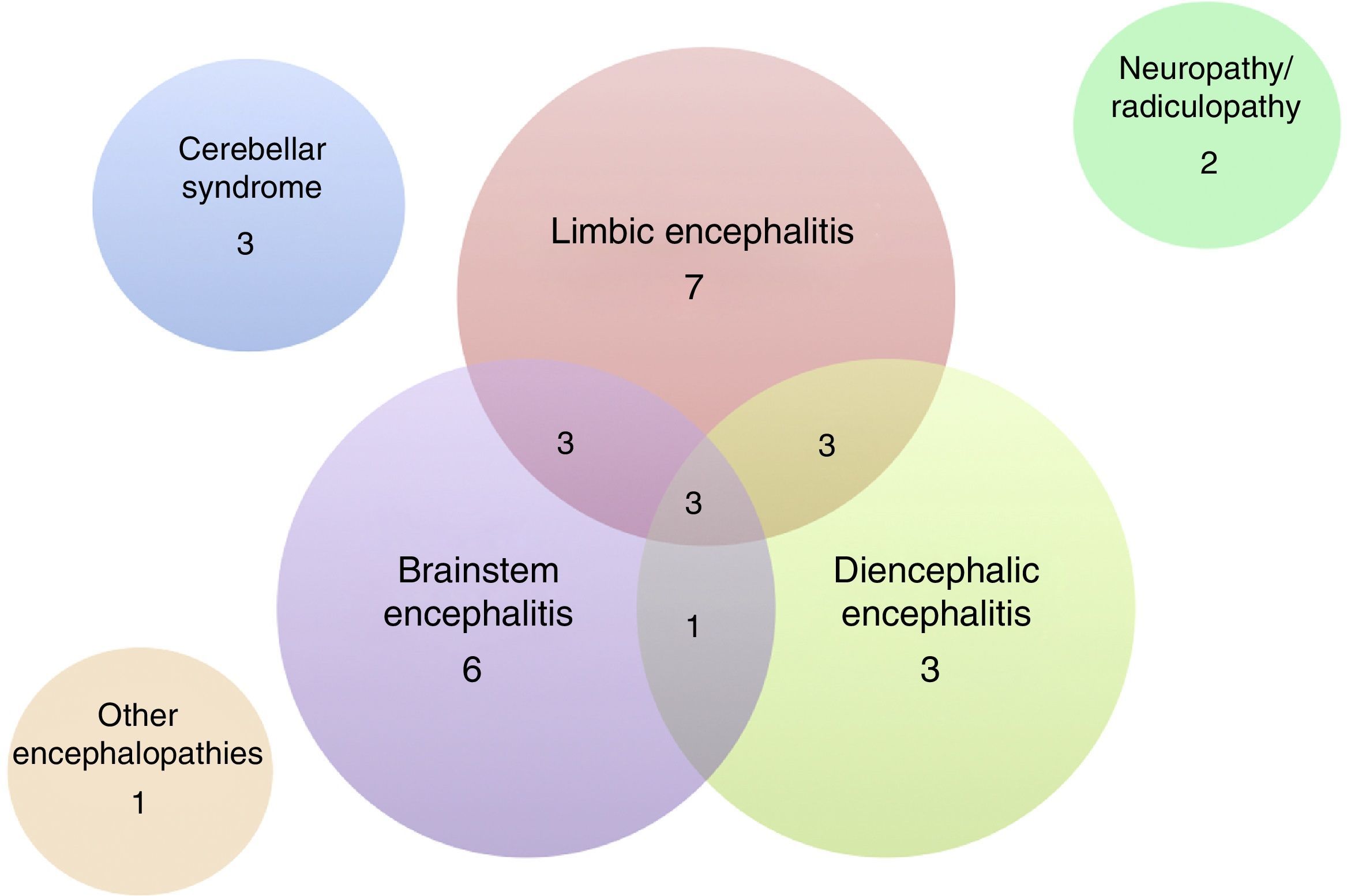
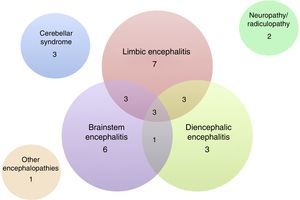
Of the 32 patients included, 24 (75%) were men. The median age was 60 years (range, 18–81). The patients’ clinical characteristics are listed in Tables 1 and 2. The most frequent presentation was LE in 7 patients, followed by BE and DE or any of several combinations of these 3 entities (Fig. 3). Five patients presented difficult-to-control seizures suggestive of temporal lobe involvement; only one of them presented epilepsy as the sole clinical manifestation. In addition to the encephalic variants, 3 patients manifested a pure cerebellar syndrome (2 with pancerebellar syndrome and the other, vermis involvement and opsoclonus), 2 patients showed affectation of the peripheral nervous system only, manifesting as sensory ganglionitis and bilateral cervical radiculopathy (severe in both cases), and one patient had encephalopathy syndrome and subacute cognitive impairment suggestive of frontal involvement, associated with gait disturbances.
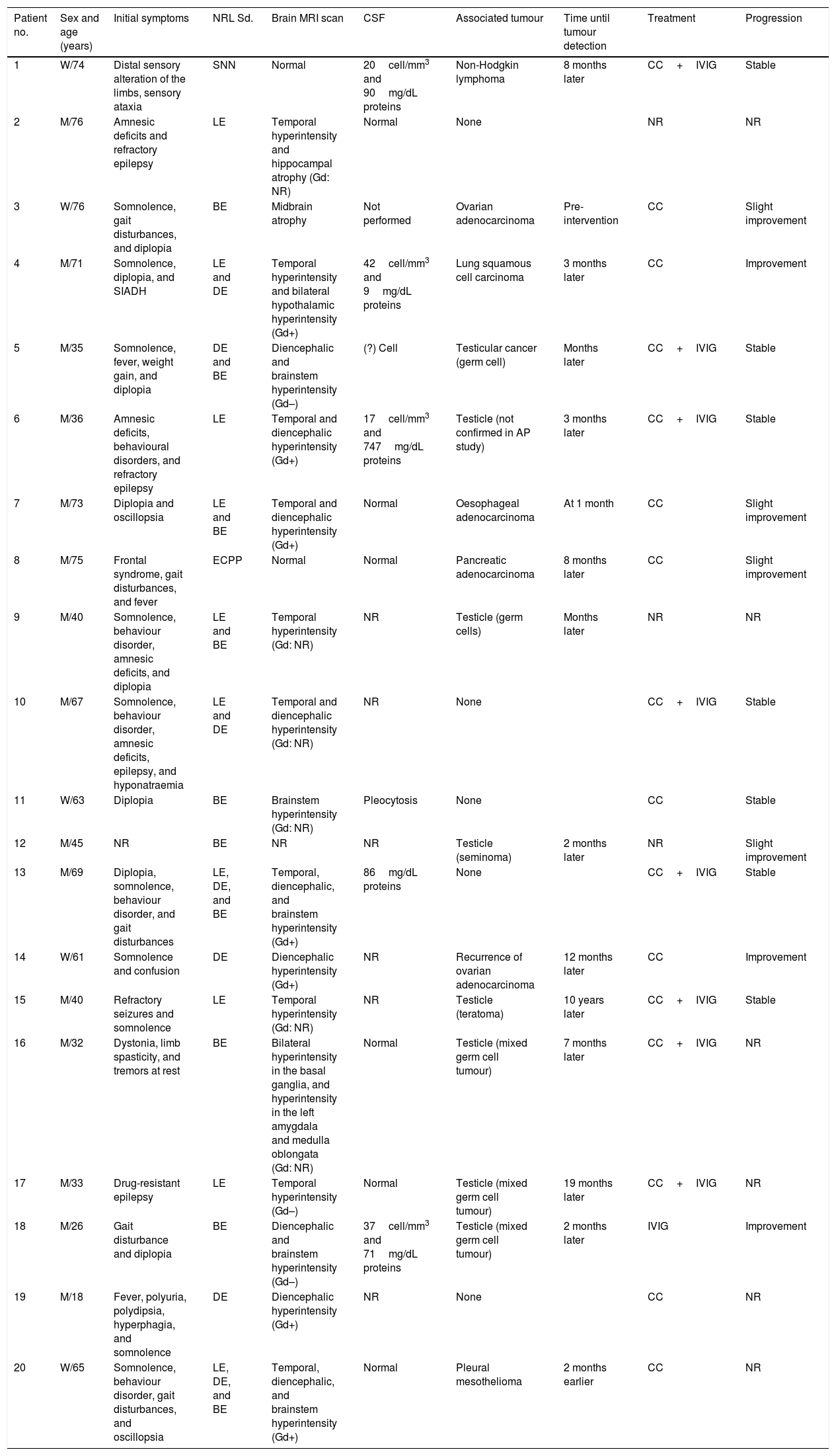
Clinical characteristics of the 20 patients with paraneoplastic syndromes associated with anti-Ma2 antibodies.
| Patient no. | Sex and age (years) | Initial symptoms | NRL Sd. | Brain MRI scan | CSF | Associated tumour | Time until tumour detection | Treatment | Progression |
|---|---|---|---|---|---|---|---|---|---|
| 1 | W/74 | Distal sensory alteration of the limbs, sensory ataxia | SNN | Normal | 20cell/mm3 and 90mg/dL proteins | Non-Hodgkin lymphoma | 8 months later | CC+IVIG | Stable |
| 2 | M/76 | Amnesic deficits and refractory epilepsy | LE | Temporal hyperintensity and hippocampal atrophy (Gd: NR) | Normal | None | NR | NR | |
| 3 | W/76 | Somnolence, gait disturbances, and diplopia | BE | Midbrain atrophy | Not performed | Ovarian adenocarcinoma | Pre-intervention | CC | Slight improvement |
| 4 | M/71 | Somnolence, diplopia, and SIADH | LE and DE | Temporal hyperintensity and bilateral hypothalamic hyperintensity (Gd+) | 42cell/mm3 and 9mg/dL proteins | Lung squamous cell carcinoma | 3 months later | CC | Improvement |
| 5 | M/35 | Somnolence, fever, weight gain, and diplopia | DE and BE | Diencephalic and brainstem hyperintensity (Gd–) | (?) Cell | Testicular cancer (germ cell) | Months later | CC+IVIG | Stable |
| 6 | M/36 | Amnesic deficits, behavioural disorders, and refractory epilepsy | LE | Temporal and diencephalic hyperintensity (Gd+) | 17cell/mm3 and 747mg/dL proteins | Testicle (not confirmed in AP study) | 3 months later | CC+IVIG | Stable |
| 7 | M/73 | Diplopia and oscillopsia | LE and BE | Temporal and diencephalic hyperintensity (Gd+) | Normal | Oesophageal adenocarcinoma | At 1 month | CC | Slight improvement |
| 8 | M/75 | Frontal syndrome, gait disturbances, and fever | ECPP | Normal | Normal | Pancreatic adenocarcinoma | 8 months later | CC | Slight improvement |
| 9 | M/40 | Somnolence, behaviour disorder, amnesic deficits, and diplopia | LE and BE | Temporal hyperintensity (Gd: NR) | NR | Testicle (germ cells) | Months later | NR | NR |
| 10 | M/67 | Somnolence, behaviour disorder, amnesic deficits, epilepsy, and hyponatraemia | LE and DE | Temporal and diencephalic hyperintensity (Gd: NR) | NR | None | CC+IVIG | Stable | |
| 11 | W/63 | Diplopia | BE | Brainstem hyperintensity (Gd: NR) | Pleocytosis | None | CC | Stable | |
| 12 | M/45 | NR | BE | NR | NR | Testicle (seminoma) | 2 months later | NR | Slight improvement |
| 13 | M/69 | Diplopia, somnolence, behaviour disorder, and gait disturbances | LE, DE, and BE | Temporal, diencephalic, and brainstem hyperintensity (Gd+) | 86mg/dL proteins | None | CC+IVIG | Stable | |
| 14 | W/61 | Somnolence and confusion | DE | Diencephalic hyperintensity (Gd+) | NR | Recurrence of ovarian adenocarcinoma | 12 months later | CC | Improvement |
| 15 | M/40 | Refractory seizures and somnolence | LE | Temporal hyperintensity (Gd: NR) | NR | Testicle (teratoma) | 10 years later | CC+IVIG | Stable |
| 16 | M/32 | Dystonia, limb spasticity, and tremors at rest | BE | Bilateral hyperintensity in the basal ganglia, and hyperintensity in the left amygdala and medulla oblongata (Gd: NR) | Normal | Testicle (mixed germ cell tumour) | 7 months later | CC+IVIG | NR |
| 17 | M/33 | Drug-resistant epilepsy | LE | Temporal hyperintensity (Gd–) | Normal | Testicle (mixed germ cell tumour) | 19 months later | CC+IVIG | NR |
| 18 | M/26 | Gait disturbance and diplopia | BE | Diencephalic and brainstem hyperintensity (Gd–) | 37cell/mm3 and 71mg/dL proteins | Testicle (mixed germ cell tumour) | 2 months later | IVIG | Improvement |
| 19 | M/18 | Fever, polyuria, polydipsia, hyperphagia, and somnolence | DE | Diencephalic hyperintensity (Gd+) | NR | None | CC | NR | |
| 20 | W/65 | Somnolence, behaviour disorder, gait disturbances, and oscillopsia | LE, DE, and BE | Temporal, diencephalic, and brainstem hyperintensity (Gd+) | Normal | Pleural mesothelioma | 2 months earlier | CC | NR |
AP: anatomical pathology; CC: corticosteroids; ECPP: encephalopathy; DE: diencephalic encephalitis; LE: limbic encephalitis; BE: brainstem encephalitis; GD: gadolinium enhancement; M: man; IVIG: intravenous immunoglobulins; W: woman; SNN: sensory neuronopathy; NR: not reported; NRL Sd.: neurological syndrome.
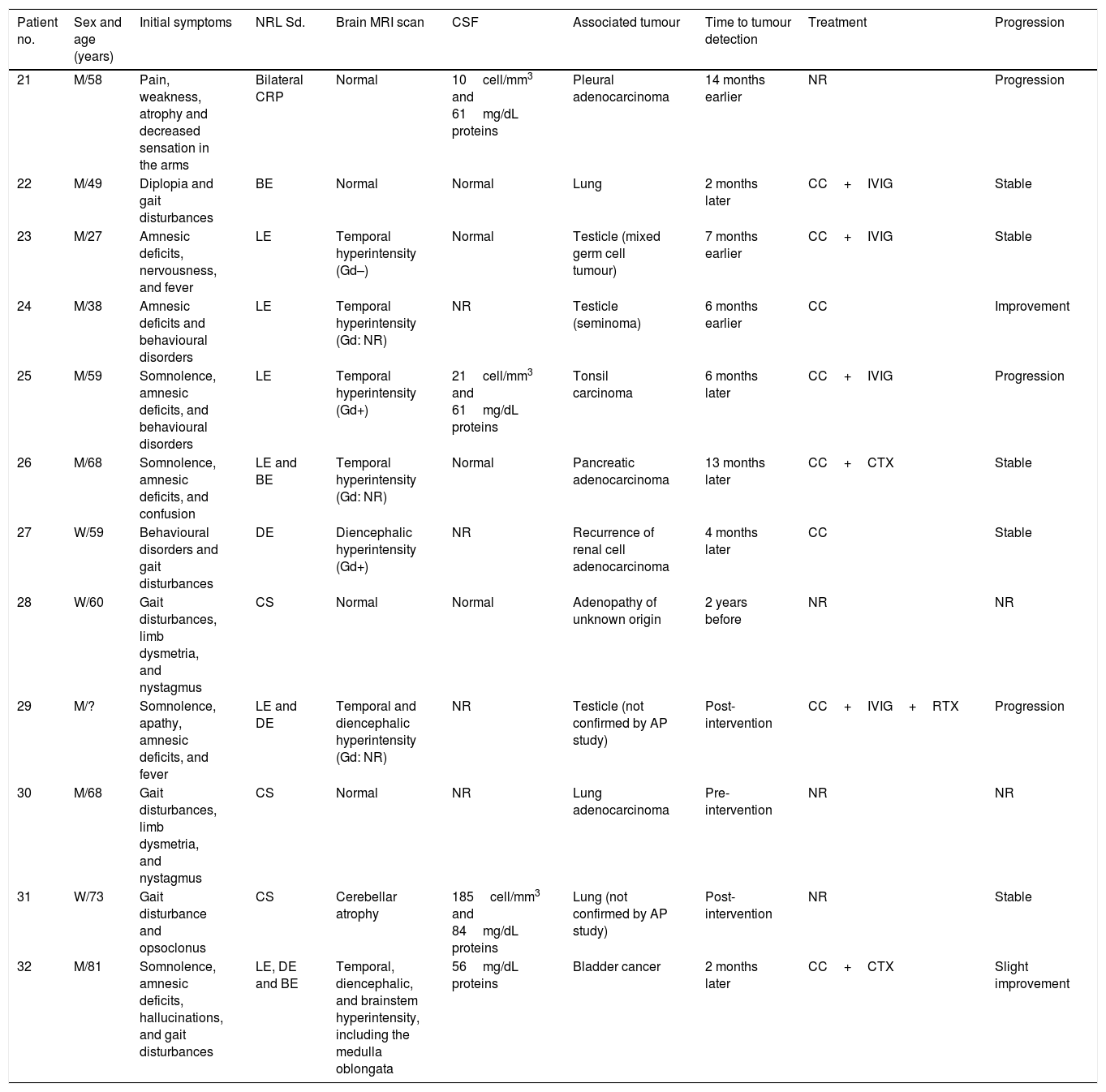
Clinical characteristics of the 11 patients with paraneoplastic syndromes associated with anti-Ma1 antibodies.
| Patient no. | Sex and age (years) | Initial symptoms | NRL Sd. | Brain MRI scan | CSF | Associated tumour | Time to tumour detection | Treatment | Progression |
|---|---|---|---|---|---|---|---|---|---|
| 21 | M/58 | Pain, weakness, atrophy and decreased sensation in the arms | Bilateral CRP | Normal | 10cell/mm3 and 61mg/dL proteins | Pleural adenocarcinoma | 14 months earlier | NR | Progression |
| 22 | M/49 | Diplopia and gait disturbances | BE | Normal | Normal | Lung | 2 months later | CC+IVIG | Stable |
| 23 | M/27 | Amnesic deficits, nervousness, and fever | LE | Temporal hyperintensity (Gd–) | Normal | Testicle (mixed germ cell tumour) | 7 months earlier | CC+IVIG | Stable |
| 24 | M/38 | Amnesic deficits and behavioural disorders | LE | Temporal hyperintensity (Gd: NR) | NR | Testicle (seminoma) | 6 months earlier | CC | Improvement |
| 25 | M/59 | Somnolence, amnesic deficits, and behavioural disorders | LE | Temporal hyperintensity (Gd+) | 21cell/mm3 and 61mg/dL proteins | Tonsil carcinoma | 6 months later | CC+IVIG | Progression |
| 26 | M/68 | Somnolence, amnesic deficits, and confusion | LE and BE | Temporal hyperintensity (Gd: NR) | Normal | Pancreatic adenocarcinoma | 13 months later | CC+CTX | Stable |
| 27 | W/59 | Behavioural disorders and gait disturbances | DE | Diencephalic hyperintensity (Gd+) | NR | Recurrence of renal cell adenocarcinoma | 4 months later | CC | Stable |
| 28 | W/60 | Gait disturbances, limb dysmetria, and nystagmus | CS | Normal | Normal | Adenopathy of unknown origin | 2 years before | NR | NR |
| 29 | M/? | Somnolence, apathy, amnesic deficits, and fever | LE and DE | Temporal and diencephalic hyperintensity (Gd: NR) | NR | Testicle (not confirmed by AP study) | Post-intervention | CC+IVIG+RTX | Progression |
| 30 | M/68 | Gait disturbances, limb dysmetria, and nystagmus | CS | Normal | NR | Lung adenocarcinoma | Pre-intervention | NR | NR |
| 31 | W/73 | Gait disturbance and opsoclonus | CS | Cerebellar atrophy | 185cell/mm3 and 84mg/dL proteins | Lung (not confirmed by AP study) | Post-intervention | NR | Stable |
| 32 | M/81 | Somnolence, amnesic deficits, hallucinations, and gait disturbances | LE, DE and BE | Temporal, diencephalic, and brainstem hyperintensity, including the medulla oblongata | 56mg/dL proteins | Bladder cancer | 2 months later | CC+CTX | Slight improvement |
Patient 32 is the one associated with anti-Ma1.
AP: anatomical pathology; CC: corticosteroids; CTX: cyclophosphamide; DE: diencephalic encephalitis; LE: limbic encephalitis; BE: brainstem encephalitis; GD: gadolinium enhancement; M: man; IVIG: intravenous immunoglobulins; W: woman; NR: not reported; R: rituximab; CRP: cervical radiculopathy; CS: cerebellar syndrome; NRL Sd.: neurological syndrome.
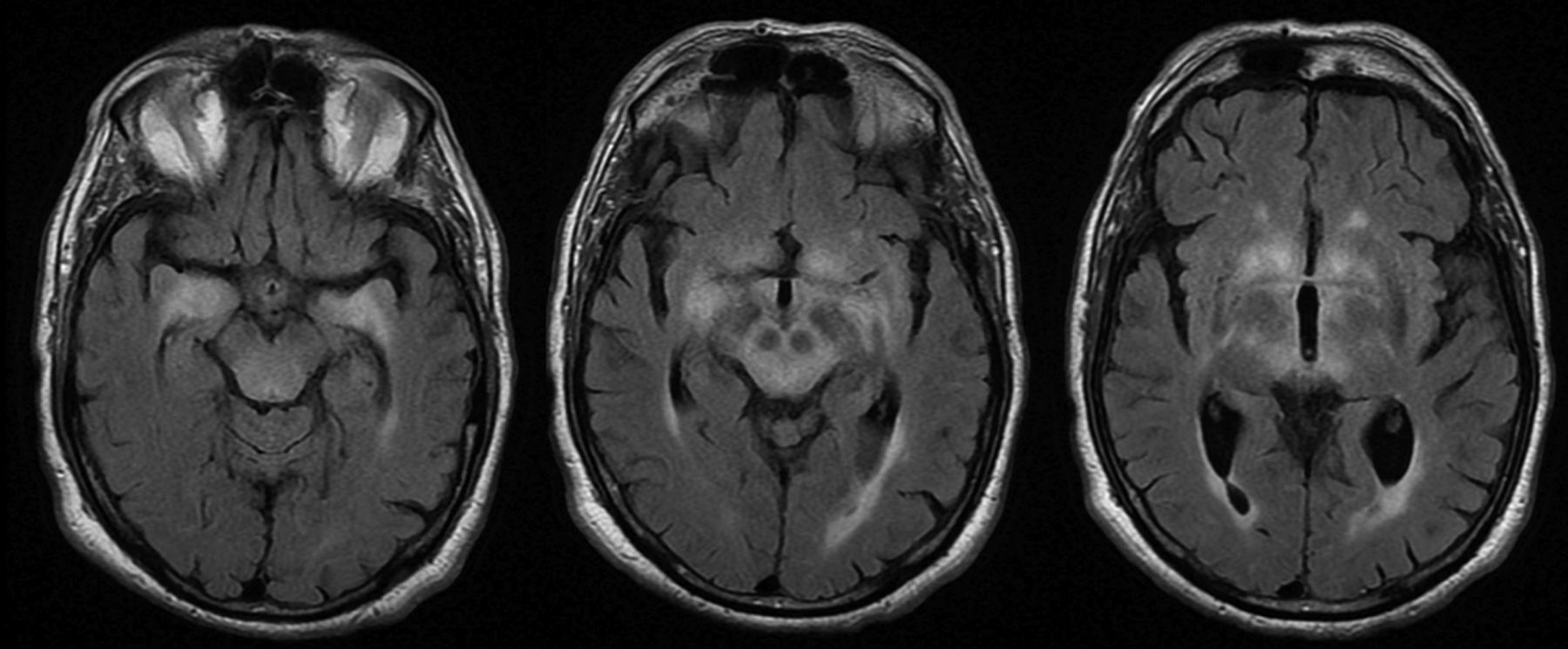
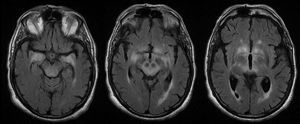
MRI findings were pathological in 25 (78%) patients. The most frequent radiological finding was unilateral or bilateral hyperintensity in T2-weighted and FLAIR sequences of the temporal lobes (essentially the hippocampus and amygdala), diencephalon (thalamus and hypothalamus), and/or midbrain (Fig. 4). Hyperintensity was found in 23 patients. In BE cases, clinical and radiological findings were identified exclusively in the midbrain, except for 2 patients who also presented bulbar involvement. In 9 of the 23 patients with hyperintense lesions in T2-weighted sequences, we observed contrast enhancement (data not available in 8 cases). Atrophy was detected in the initial MRI scans of 3 patients: one with LE and hippocampal sclerosis, one with isolated midbrain atrophy, and another with cerebellar atrophy.
Data from the CSF analysis were available for 21 patients. In 52% of these cases, CSF analysis results were pathological, with slightly to moderately high protein levels in half of the samples and mononuclear pleocytosis in 35%.
Tumour association and chronology with respect to the neurological syndromeAn associated tumour was detected in 27 patients (84%). The most frequent tumour types were testicular tumours (n=11), followed by lung and pleural adenocarcinoma (n=6), and digestive tract cancer (n=4, including 2 pancreatic cancers, oesophageal cancer, and tonsil tumour). The remaining tumours were ovarian adenocarcinoma (n=2), non-Hodgkin lymphoma (n=1), renal cell adenocarcinoma (n=1), bladder carcinoma (n=1), and one case of metastatic cervical adenopathy of unknown origin in which no primary tumour was identified during follow-up.
Nineteen patients (70%) manifested the paraneoplastic syndrome before tumour diagnosis or recurrence; the mean time was 12.8 months (median of 4 months). In 8 of the patients (30%), neurological symptoms presented after cancer diagnosis, by a mean of 12 months (median of 10.5 months) with a maximum of 2 years. Development of the paraneoplastic neurological syndrome led to diagnosis of tumour recurrence in 2 patients who had previously responded to cancer treatment.
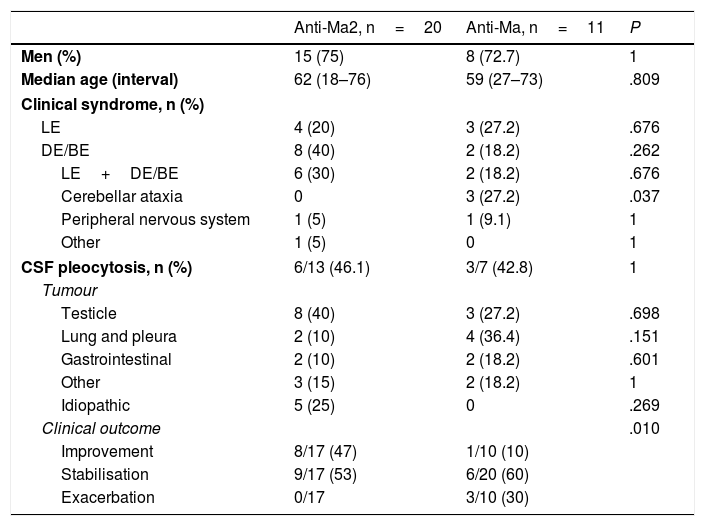
Associated antibody and characteristics for each groupTwenty patients (62.5%) tested positive for anti-Ma2 antibodies, 11 (34.3%) for anti-Ma antibodies, and one patient (3.1%) for anti-Ma1 antibodies only (Table 3). The sole statistically significant differences observed between patients with anti-Ma2 and those with anti-Ma antibodies were that all patients showing cerebellar involvement were included in the anti-Ma group (P=.037), and that patients with anti-Ma2 antibodies responded better to treatment (P=.010). We found intergroup differences in the percentages of associated tumours. Testicular tumours were the most frequent in the anti-Ma2 group (40%), while in the anti-Ma group, the most frequent types were lung and pleural tumours (36.4%), followed by testicular cancer (27.2%). Statistical analysis of the different associated tumours revealed no statistically significant differences. All of the 5 patients in whom no tumours were detected were positive for anti-Ma2 antibodies.
Patient characteristics broken down by type of antibody.
| Anti-Ma2, n=20 | Anti-Ma, n=11 | P | |
|---|---|---|---|
| Men (%) | 15 (75) | 8 (72.7) | 1 |
| Median age (interval) | 62 (18–76) | 59 (27–73) | .809 |
| Clinical syndrome, n (%) | |||
| LE | 4 (20) | 3 (27.2) | .676 |
| DE/BE | 8 (40) | 2 (18.2) | .262 |
| LE+DE/BE | 6 (30) | 2 (18.2) | .676 |
| Cerebellar ataxia | 0 | 3 (27.2) | .037 |
| Peripheral nervous system | 1 (5) | 1 (9.1) | 1 |
| Other | 1 (5) | 0 | 1 |
| CSF pleocytosis, n (%) | 6/13 (46.1) | 3/7 (42.8) | 1 |
| Tumour | |||
| Testicle | 8 (40) | 3 (27.2) | .698 |
| Lung and pleura | 2 (10) | 4 (36.4) | .151 |
| Gastrointestinal | 2 (10) | 2 (18.2) | .601 |
| Other | 3 (15) | 2 (18.2) | 1 |
| Idiopathic | 5 (25) | 0 | .269 |
| Clinical outcome | .010 | ||
| Improvement | 8/17 (47) | 1/10 (10) | |
| Stabilisation | 9/17 (53) | 6/20 (60) | |
| Exacerbation | 0/17 | 3/10 (30) | |
From the cases returned by the literature search, we identified 19 anti-Ma2-associated paraneoplastic syndromes8–26 and 4 anti-Ma-associated paraneoplastic syndromes.27–30 Nevertheless, presence of anti-Ma1 antibodies was only specified in 4 of the 19 anti-Ma2 cases.10,11,14,15 We therefore cannot guarantee that all of them were really anti-Ma2 cases. Lastly, we should mention the only patient to present anti-Ma1 antibodies exclusively in our series. The patient was an 81-year-old man who reported initial symptoms of intense sleep attacks present during a month and a half, followed by the appearance of gait disturbances, parkinsonian symptoms, and myoclonus. The brain MRI revealed bilateral limbic, diencephalic, and midbrain involvement with no contrast enhancement; CSF analysis showed slightly high protein levels. CSF results were positive for anti-Ma1 antibodies only. Cancer screening revealed a mass in the bladder, which was identified by biopsy as an undifferentiated high-grade lymphoepithelial carcinoma. The patient was treated with methylprednisolone and cyclophosphamide after progressive worsening of neurological symptoms, which finally resulted in hospitalisation in the ICU. His level of consciousness improved only slightly. Treating the bladder tumour was ruled out due to the patient's overall poor condition. The patient died shortly after being diagnosed following a bronchoaspiration procedure. The family did not authorise a clinical autopsy.
Patient managementWe obtained treatment data from 25 patients (78.1%): all were treated with immunosuppressants, and 24 (96%) received corticosteroids, either intravenously or orally. Intravenous immunoglobulins were used in 13 cases (52%), always in association with corticosteroids except in one patient. Two patients with severe midbrain and diencephalic symptoms (basically decreased level of consciousness) were also treated with cyclophosphamide and a third patient received rituximab as second-line treatment; symptoms improved in one patient, stabilised in another, and progressed in the last. We found 2 patients treated surgically for refractory temporal lobe epilepsy associated with hippocampal sclerosis. The patient with seizures as the only symptom underwent surgery which did not resolve them; surgery was successful in the case of the patient with seizures and encephalopathy.
Antibody titres were monitored in some patients, but no clinical-analytical correlations were observed. Some patients showed satisfactory neurological outcomes with symptom resolution; however, antibodies persisted in serum. Furthermore, other patients showed poor outcomes despite decreasing antibody titres.
DiscussionThe clinical analysis of our series of patients with paraneoplastic neurological syndromes and anti-Ma antibodies differs from other previously published series in some areas.7,31 In our series, age of onset was not associated with antibody type and at 70%, the percentage of men was significantly higher than the previously described 43%.31 The most frequent presentation was the combination of LE, DE, and BE, but some patients presented different neurological symptoms, such as anti-Ma-associated pure cerebellar syndromes, or anti-Ma2-associated isolated involvement of the peripheral nervous system. In our literature review, we identified 5 similar cases, including 2 with cerebellar syndrome (one isolated case with anti-Ma antibodies and another case with polyneuropathy associated with anti-Ma219), one with myeloradiculopathy,11 and one case of multiple mononeuropathy23 (this patient also presented asymptomatic temporal lobe lesions in MRI scans). The latter 2 cases presented anti-Ma2 antibodies. The fifth was a possible case of primary lateral sclerosis-like phenotype and progressive spastic paraparesis associated with anti-Ma2 antibodies.22
The first series of anti-Ma2- and anti-Ma-associated paraneoplastic neurological syndromes31 included 38 patients. Thirty-four presented classical symptoms of LE, DE, and BE and only 4 had atypical syndromes: 2 with cerebellar syndrome, one with myelopathy, and the fourth patient with myelopathy and radiculo-plexopathy, although these last 2 entities were associated with LE and BE. Cerebellar involvement was detected in 11 patients (9 with anti-Ma); it was associated with classical symptoms except in 2 cases. The second series examining this entity included 22 patients (14 with anti-Ma antibodies and 8 with anti-Ma2 antibodies).7 Of the total, 19 developed typical syndromes, and only 3 showed atypical symptoms: one patient had isolated polyneuropathy, one had upper and lower motor neuron disease (both anti-Ma2), and the third patient displayed cerebellar syndrome associated with parkinsonism and anti-Ma. Cerebellar involvement, although associated with typical clinical symptoms in this series, was identified in 8 patients (7 with anti-Ma).
In the mentioned studies, the majority of patients with anti-Ma2 were young men with testicular cancer. However, in this series and in other recent ones, the percentage of testicular cancer (40%) does not account for half of the total despite being predominant in the anti-Ma2 group. The comparison between the anti-Ma2 and the anti-Ma groups did not reveal significant differences in the associations with different types of tumours (germ cell testicular cancer, lung and pleural cancer, gastrointestinal cancer, and other less frequent tumours). All idiopathic cases in our sample were associated with anti-Ma2 antibodies, and the literature review returned 4 idiopathic cases associated with anti-Ma2 (although these reports did not specify Ma1 reactivity).8,17,19,22
Clinical outcomes seem favourable for Ma2 patients, with improvement or stabilisation of all symptoms; in contrast, Ma patients showed poorer clinical outcomes.7,31 These differences may be influenced by the varying percentages of tumour types in each group, as well as by unknown but probably immunological mechanisms. Although Ma2 patients were more likely to have testicular tumours (a neoplasia with a high cure rate after orchiectomy), no significant differences were detected. In other types of tumours presenting greater infiltration or a larger volume, and in those that are more difficult to treat, such as lung cancer, persistence of cancer may contribute to a patient's severe neurological progression.
In conclusion, our series suggests that both anti-Ma2 and anti-Ma1 antibodies are linked to neurological symptoms characterised by variable degrees of association with LE, DE, and BE. However, some patients present isolated cerebellar symptoms or peripheral nervous system syndromes. Determining the reactivity of anti-Ma1 and anti-Ma2 antibodies is important since neurological syndromes associated with anti-Ma2 antibodies respond better to treatment. Finally, we can confirm the existence of cases with antibodies reacting only against Ma1. We will need to study more patients in order to characterise the clinical symptoms associated with anti-Ma1.
FundingThis study received no funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ortega Suero G, Sola-Valls N, Escudero D, Saiz A, Graus F. Síndromes neurológicos paraneoplásicos asociados a anticuerpos anti-Ma y anti-Ma2. Neurología. 2018;33:18–27.
This study has not been presented at any meeting or conference.