Anaesthetic block, alone or in combination with other treatments, represents a therapeutic resource for treating different types of headaches. However, there is significant heterogeneity in patterns of use among different professionals.
DevelopmentThis consensus document has been drafted after a thorough review and analysis of the existing literature and our own clinical experience. The aim of this document is to serve as guidelines for professionals applying anaesthetic blocks. Recommendations are based on the levels of evidence of published studies on migraine, trigeminal autonomic cephalalgias, cervicogenic headache, and pericranial neuralgias. We describe the main technical and formal considerations of the different procedures, the potential adverse reactions, and the recommended approach.
ConclusionAnaesthetic block in patients with headache should always be individualised and based on a thorough medical history, a complete neurological examination, and expert technical execution.
Los bloqueos anestésicos constituyen un recurso terapéutico para el manejo de distintos dolores de cabeza, de forma aislada o combinado con otros tratamientos. Sin embargo, existe una importante heterogeneidad en los patrones de uso entre los distintos profesionales.
DesarrolloA partir de una exhaustiva revisión y análisis de la bibliografía existente y de nuestra experiencia clínica se ha elaborado este documento de consenso cuyo objetivo es servir como guía para aquellos profesionales que quieran aplicar estas técnicas. Se establecen recomendaciones basadas en los niveles de evidencia que ofrecen los estudios revisados en migraña, cefaleas trigémino-autonómicas, cefalea cervicogénica y neuralgias pericraneales. Se describen los principales aspectos técnicos y formales de los diferentes procedimientos, así como las posibles reacciones adversas que pueden surgir y la actitud recomendada.
ConclusionesEl tratamiento con bloqueos anestésicos del paciente con cefalea debe ser siempre individualizado y basarse en una correcta anamnesis, exploración neurológica y ejecución técnica.
Anaesthetic block (AB), a treatment used to manage different types of headache, may be used in monotherapy or combined with other treatments. Several decades have now passed since doctors began to perform AB on different cranial or cervical nerves in patients with various types of headache and craniofacial neuralgia, and experience has continued to increase in recent years. Local anaesthetics act as reversible inhibitors of the production and conduction of stimuli in any type of excitable membranes, especially those in nervous tissue. When these anaesthetics come into contact with a nerve fibre, they cross the myelin sheath and the neural membrane in a non-ionised form. Once within the cell, they become partially ionised, and the ionised fraction binds to the inner pore of the voltage-gated sodium channel, thus keeping it from opening and preventing firing and propagation of the action potential. This is the mechanism by which local anaesthetics are able to inhibit nerve impulse conduction.1
The technique most commonly used in clinical practice is blockade of the greater occipital nerve (GON). A growing body of scientific evidence supports the modulating effect of the occipital nerves on the nociceptive afferents carried by the trigeminal nerve. At the highest segments of the cervical spinal cord, sensory neurons corresponding to the occipital region are interwoven with neurons from the spinal trigeminal nucleus. From a functional standpoint, there is also a convergence of cervical and trigeminal fibres over the same second-order neurons. By blocking the arrival of nociceptive impulses via the first cervical nerves, AB may act on this trigeminal-cervical complex by inhibiting transmission from trigeminal afferents as well.2,3
Medical literature now contains numerous descriptions of patients treated with AB. However, current usage patterns vary considerably among different professionals. In 2012, the Annual Meeting of the Spanish Society of Neurology (SEN) displayed results from a survey sent to the members of the SEN study group for headaches (GECSEN) inquiring about use of AB.4 The survey revealed frank disparities in the technical and formal considerations, which is what motivated us to draw up a consensus statement.
Based on an exhaustive literature review and analysis, as well as on our clinical experience, we prepared this consensus document to provide guidelines for doctors interested in applying these techniques. Levels of evidence and grades of recommendation are defined according to the classification proposed by the Centre for Evidence Based Medicine at the University of Oxford.5 Recommendations for each headache type and the most important considerations for each of the procedures appear summarised in table form for ease of use.
Use of anaesthetic block for different headache typesMigraineThe first placebo-controlled double blind trial to examine migraine prevention by means of GON blockade was carried out by Piovesan et al.6 in a sample of 37 patients with episodic migraines and a relatively low to moderate frequency of attacks (mean: 3.6 per month; range: 1-8). Using a cross-over design, this group scheduled patient visits spaced 30 days apart. One of the groups (n=20) was treated with bupivacaine 0.5% in the first session and placebo (sterile saline) in the second; the other group (n=17) received the same 2 treatments but in reverse order. Both groups were therefore treated with the active drug, although at different times in the study. There were no significant differences in the number or duration of attacks throughout the study period, but headache intensity decreased in the first group during the second observation period (measured at 60 days following AB).
In 2015, Ruiz Piñero et al.7 published results from a prospective, open non-controlled study in 60 patients with migraine (71.7% with chronic migraine [CM]); all had tried prophylactic treatment (at least one beta-blocker or neuromodulator). To be included in the study, patients had to show sensitivity to palpation at the emergence of at least one occipital or supraorbital nerve, and have undergone AB of sensitive nerves only. At 3 months, 23 patients (38.3%) had responded completely to treatment (pain-free period of at least 2 weeks), and 24 patients (40%) showed a partial response (50% reduction in pain intensity and/or days with pain during at least 2 weeks). Thirteen patients (21.7%) did not respond to AB. The group showing a complete response comprised younger patients with shorter disease durations. There were no significant adverse events.
In the same year, Inan et al.8 assessed the efficacy of AB with bupivacaine in a double-blind randomised placebo-controlled study of 84 patients with CM. Patients were randomly assigned to receive either sterile saline (n=42) or bupivacaine (n=42) once weekly for 4 weeks. Following that step, all patients were treated with bupivacaine once a month for 2 months. A total of 72 patients completed the study. GON blockade was shown to be safe and effective; researchers observed significant reductions in pain duration and intensity, and in the number of headache days per month.
Ashkenazi et al.9 studied whether associating corticosteroids with local anaesthesia would increase the effectiveness of GON blockade and infiltrations in muscle trigger points in patients with CM. The 37 patients experiencing more than 15 days of pain per month were randomly assigned to receive injections of local anaesthetics (lidocaine 2% and bupivacaine 0.5%) with saline (n=18) or of triamcinolone (n=19). Following GON blockade and treatment of trigger points with the assigned solution, there were no significant differences between clinical parameters in these 2 groups, either at 20minutes after the intervention or during the one-month follow-up period. In their recent study with the same characteristics, Saracco et al.10 obtained similar results.
Also in 2015, Dilli et al.11 published findings from a randomised double-blind placebo-controlled study in patients with chronic and episodic migraine (more than one attack per week). They were treated with either 2.5mL bupivacaine 0.5% plus 20mg methylprednisolone (n=33 patients), or with placebo (2.75mL saline and 0.25mL lidocaine 1% [n=30 patients]). An evaluation 4 weeks after the procedure did not find any significant changes in the frequency of moderate to severe headache days in either group with respect to its baseline data. Nevertheless, the study had a small sample size and included patients with episodic and chronic migraine in both groups; furthermore, the procedure was only performed once, compared to the multiple times in other studies. It should be remarked that this study's placebo treatment included a small amount of anaesthetic.
Based on the studies cited here, we conclude that GON blockade may be effective as prophylaxis for CM based on reductions in the number, duration, or intensity of the attacks in the weeks or months following the intervention (level II of evidence, grade B of recommendation).12 On the other hand, the addition of corticosteroids has not been shown to increase the efficacy of AB for preventing migraines.
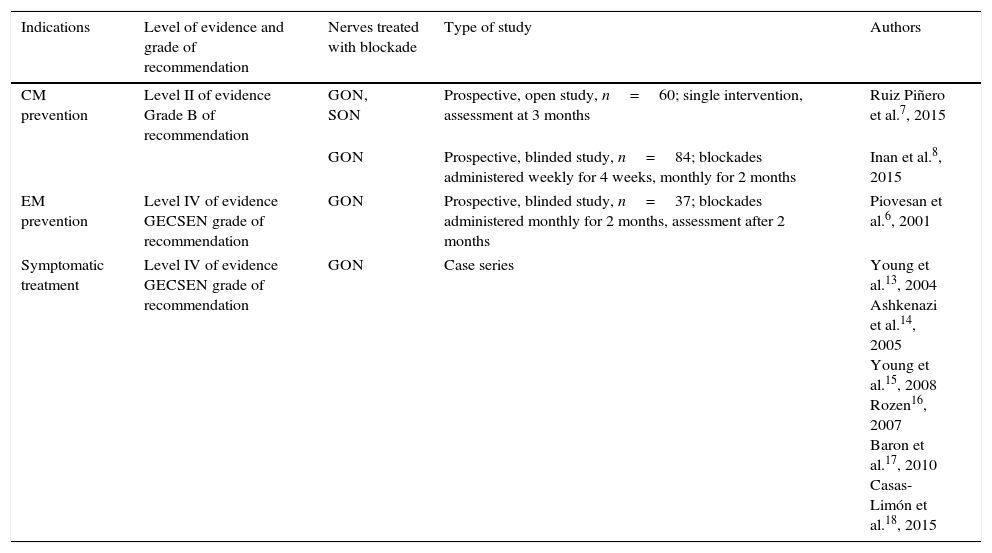
GON blockades also seem to have an immediate effect on symptoms when administered during a migraine episode. Researchers have reported decreases in pain intensity, allodynia, and photophobia in a significant number of patients.13–15 There are also reports of 3 cases of hemiplegic migraine and a single case of basilar-type migraine in which aura quickly subsided following GON blockade.16–18 GON blockade may be a treatment alternative for refractory episodes (level IV of evidence, GECSEN grade of recommendation).12Table 1 lists studies on migraine and the levels of evidence for different indications.
Anaesthetic block for migraine.
| Indications | Level of evidence and grade of recommendation | Nerves treated with blockade | Type of study | Authors |
|---|---|---|---|---|
| CM prevention | Level II of evidence Grade B of recommendation | GON, SON | Prospective, open study, n=60; single intervention, assessment at 3 months | Ruiz Piñero et al.7, 2015 |
| GON | Prospective, blinded study, n=84; blockades administered weekly for 4 weeks, monthly for 2 months | Inan et al.8, 2015 | ||
| EM prevention | Level IV of evidence GECSEN grade of recommendation | GON | Prospective, blinded study, n=37; blockades administered monthly for 2 months, assessment after 2 months | Piovesan et al.6, 2001 |
| Symptomatic treatment | Level IV of evidence GECSEN grade of recommendation | GON | Case series | Young et al.13, 2004 Ashkenazi et al.14, 2005 Young et al.15, 2008 Rozen16, 2007 Baron et al.17, 2010 Casas-Limón et al.18, 2015 |
Levels of evidence and grades of recommendation.
GECSEN: study group for headaches, Spanish Society of Neurology (SEN); EM: episodic migraine; CM: chronic migraine; GON: greater occipital nerve; SON: supraorbital nerve.
GON blockade has also been proved effective against cluster headache (CH).18–24 In 2002, Peres et al.19 treated 14 patients (5 with chronic CH and 9 with episodic CH) by delivering 3mL lidocaine 1% and 40mg de triamcinolone to the GON ipsilateral to pain. Four patients (28.5%) responded well (pain-free period lasting longer than 2 weeks); 5 (35.7%) showed a moderate response (pain-free period of less than 2 weeks); and another 5 patients (35.7%) did not respond. However, the latter group did display decreases in the number, duration, and intensity of episodes. Overall, the mean number of pain-free days after the procedure was 13.1±23.6. There were no differences with regard to whether headaches were chronic or episodic.
In their double-blind clinical trial, Ambrosini et al.20 randomly selected patients to receive a mixture of 0.5mL lidocaine 2% and 2mL betamethasone (13 patients) or a mixture of 0.5mL lidocaine 2% and 2mL saline (10 patients). Episodes ceased in 11 of the patients receiving corticosteroids (61%) and this remission continued for at least 4 weeks. In contrast, patients treated with saline did not show any improvements. At a later date, Busch et al.21 injected local anaesthetics only (5mL prilocaine 1%) in 15 patients with CH, also ipsilaterally to pain. From a neurophysiological standpoint, they were able to confirm a decrease in the nociception-specific blink reflex response; clinically, however, only 9 patients experienced partial pain relief. In 2011, Leroux et al.22 performed a randomised, double-blind, placebo-controlled study of 43 patients with CH (15 with chronic CH and 18 with episodic CH) experiencing at least 2 attacks daily in the 72hours prior to being included. Patients underwent 3 suboccipital infiltrations with 3.75mg cortivazol or placebo separated by 48 to 72 hour intervals. The treatment group showed a significant decrease in the number of attacks, regardless of whether headaches were chronic or episodic.
Gantenbein et al.23 completed a retrospective 4-year study of the efficacy and safety of 121 GON blocks using local anaesthetic and depot corticosteroid in a sample of 60 patients with episodic or chronic CH. Eighty per cent of the infiltrations resulted in at least a partial response (decreased number, duration, and intensity of episodes); response to 45% of the infiltrations was complete. These improvements persisted over a mean of 3.5 weeks in patients with chronic forms with more prolonged relief in patients with episodic headache.
Lastly, Lambru et al.24 completed a prospective, open study of the efficacy of AB of the GON ipsilateral to pain in 83 patients with chronic CH (mean of 4 attacks daily [range, 1-12] and a 9-year mean history of the disease [range, 3-69]). Patients were injected with a mixture of 2mL lidocaine 2% and 80mg methylprednisolone every 3 months. The first block resulted in complete relief (pain-free period of at least 7 days) in 42% of the patients, with 15% experiencing partial relief (decrease ≥50% in pain intensity/frequency during at least 7 days). Mean duration of pain relief was 21 days (range, 7-504 days). Immediately following the infiltration, 6% of the patients felt worse. Of the 37 patients reporting complete resolution, 26 remained pain-free on day 15, 12 remained pain-free on day 30, and 2 remained pain-free on day 90. These results resemble those recorded after the second, third, and fourth AB. No significant adverse reactions were recorded.
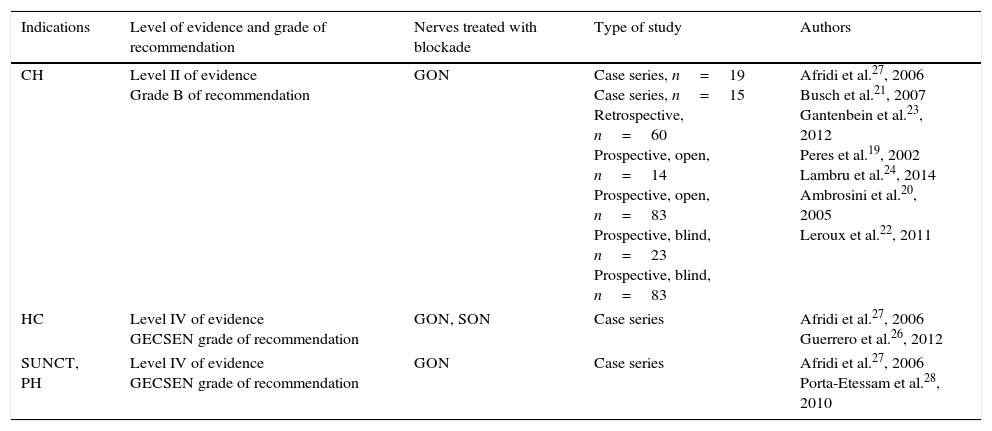
Based on the reviewed literature (Table 2), we can conclude that AB of the GON is an effective treatment for CH (level II of evidence, grade B of recommendation). Most of the studies published to date have featured a combination of a corticosteroid (triamcinolone, betamethasone, methylprednisolone) and a local anaesthetic.25
Anaesthetic block for trigeminal autonomic cephalalgias.
| Indications | Level of evidence and grade of recommendation | Nerves treated with blockade | Type of study | Authors |
|---|---|---|---|---|
| CH | Level II of evidence Grade B of recommendation | GON | Case series, n=19 Case series, n=15 Retrospective, n=60 Prospective, open, n=14 Prospective, open, n=83 Prospective, blind, n=23 Prospective, blind, n=83 | Afridi et al.27, 2006 Busch et al.21, 2007 Gantenbein et al.23, 2012 Peres et al.19, 2002 Lambru et al.24, 2014 Ambrosini et al.20, 2005 Leroux et al.22, 2011 |
| HC | Level IV of evidence GECSEN grade of recommendation | GON, SON | Case series | Afridi et al.27, 2006 Guerrero et al.26, 2012 |
| SUNCT, PH | Level IV of evidence GECSEN grade of recommendation | GON | Case series | Afridi et al.27, 2006 Porta-Etessam et al.28, 2010 |
Levels of evidence and grades of recommendation.
CH: cluster headache; GECSEN: study group for headaches, Spanish Society of Neurology; HC: hemicrania continua; PH: paroxysmal hemicrania; GON: greater occipital nerve; SON: supraorbital nerve; SUNCT: short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing.
Regarding other types of trigeminal autonomic cephalalgia, Guerrero et al.26 completed a prospective, open study of the efficacy of AB of the GON (with 2mL bupivacaine 0.5% and mepivacaine 2%, 1:1 ratio) and the supraorbital nerve (SON) (0.5mL of the same active solution). The study included patients undergoing AB of one or both nerves; in some patients, the anaesthetic was associated or combined with 4mg triamcinolone delivered to the trochlear nerve (9 patients with hemicrania continua and indometacin intolerance). All patients displayed sensitivity to palpation in at least one of the target territories. Pain resolved completely in 5 patients, with the other 4 patients showing a partial response. Pain relief began immediately after the procedure, and response to treatment continued for a mean of 2 to 10 months. Positive responses have also been reported in cases of paroxysmal hemicrania27 and short-lasting unilateral neuralgiform headache.27,28
Cervicogenic headacheThe convergence of nociceptive stimuli arriving from interspinal muscles, facet joints, and intervertebral discs and those from the trigeminal nerve in the trigeminal cervical complex is the biological substrate underlying cervicogenic headache (CGH). AB of the occipital nerves is another common technique for managing this type of headache. Multiple observational studies of AB performed with or without an associated corticosteroid have recorded favourable responses in more than 70% of the cases.29–31 Headache relief was already apparent a few minutes after the procedure; this response supports the diagnosis of CGH. Naja et al.32 demonstrated the efficacy of the technique in a randomised, controlled double-blind study with a 2-week follow-up period. In an open study, the same authors observed that repeated injections with the active drug were able to deliver longer remission periods.33 Based on results from a series of 28 patients with CGH, Inan et al.34 showed that the effect obtained using AB of the GON was similar to that resulting from direct blockade of the C2 and C3 nerves.
More recently, Lauretti et al.35 compared the classic GON block technique (40mg lidocaine and 10mg dexamethasone) to a subcompartimental block technique (40mg lidocaine, 10mg dexamethasone, with non-ionic iodinated contrast agent and saline) using a fluoroscope. This series contained 30 patients, randomly assigned to 3 groups (n=10) receiving 5, 10, or 15mL of final volume. The pain-free period was longer for subcompartimental block (24 weeks) than for the classic block (2 weeks). There were no significant intergroup differences associated with the volume injected.
From these findings, we deduce that occipital AB results in immediate cessation of pain in patients with CGH, and that the procedure may be followed by a more or less prolonged remission period36 (level II of evidence, grade B of recommendation).
Occipital neuralgia and other pericranial neuralgiasAccording to the 2013 diagnostic criteria established by the Headache Classification Subcommittee of the International Headache Society,37 occipital neuralgia should respond, at least temporarily, to GON block; in these cases, the technique is both diagnostic and therapeutic. Two retrospective studies recorded response rates of about 85% with a mean response duration of 1 to 2 months.38,39 The previous International Headache Society classification (2004)40 recognised supraorbital nerve, nasociliary nerve, and other trigeminal terminal branch neuralgias as nosological entities; their diagnostic criteria include pain that subsides with AB. Despite their controversial absence from the latest version of the classification, they are very much in evidence in normal clinical practice. AB of the supraorbital and nasociliary nerves41,42 and other pericranial nerves (supratrochlear43, lacrimal44,45, infratrochlear46,47, infraorbital48, and auriculotemporal49) has been proved effective, but the technique requires careful examination of the nerve exit points and thorough knowledge of their anatomical distribution.
Other headachesAlthough cases are less numerous, responses to AB have been favourable for primary headache associated with sexual activity, post-dural-puncture headache (PDPH), and post-traumatic headache (PTH). Selekler et al.50 have published a case of orgasmic headache that responded to occipital blockade with 20mg prilocaine and 125mg methylprednisolone. Matute et al.51 described 2 patients with PDPH who responded immediately to bilateral infiltration of both GON with 4mL bupivacaine 0.25% and 20mg triamcinolone. Two years later, Takmaz et al.52 published an observation of pain remission 2minutes after the patient had undergone infiltration of both occipital nerves with 2mL bupivacaine 0.5%. Naja et al.53 completed a prospective, single-blind study in 50 patients with PDPH to compare conventional treatment (bedrest, hydration, and analgesics) to bilateral blockade of the greater and lesser occipital nerves using a 10mL mixture of lidocaine 2%, lidocaine 2% with epinephrine (1:200000), bupivacaine 0.5%, fentanyl, and clonidine. After undergoing 1 or 2 blockades, 68.4% of the patients experienced complete pain relief. Overall, patients in this group also reported less pain on the analogue visual scale (AVS), reduced their consumption of analgesics, and spent fewer days in hospital.
In the study by Niraj et al.54 including 24 patients with PDPH and no response to conservative treatment, 66% of those treated with bilateral occipital infiltration with dexamethasone responded completely, with partial responses in 33%. In the most recent study, Akyol et al.55 included 21 patients with PDPH and no response to conventional analgesic treatment in a 48-hour period. All patients underwent bilateral blockade of both occipital nerves with 4mL levobupivacaine 0.25%. Twenty-four hours after the procedure, all patients with a prior AVS score of 4 to 6 (n=12) and only one of the patients with a prior score of 7 to 9 (n=9) reported an AVS score of 1. In the second arm, the mean score on the AVS scale had dropped to 5.56.
Although Saadah and Taylor56 obtained a response rate of only 9% in a group of patients with PTH, some authors have reported good results following GON blockade for this type of headache. Gawel and Rothbart57 reviewed their series of 87 patients with PTH and migraine features and determined that 72% felt significantly better after undergoing one or more GON blockades with lidocaine and methylprednisolone. Hecht58 completed a retrospective analysis of response to AB with bupivacaine 0.5% in 10 patients with PTH in whom GON pressure reproduced headache pain. Eight of these patients (80%) responded well, while the other 2 (20%) showed a partial response. Tobin and Flitman39 performed blocks on both GON in 12 patients with PTH using a mixture of 1.5mL bupivacaine and 60mg methylprednisolone. All patients noticed improvement after the injections; overall decrease in pain was 86% and the mean duration of relief was 4.4 weeks.
Anaesthetic block techniquesGeneral remarksLocal anaesthetics are a type of drug able to interrupt conduction of the nerve impulse by blocking voltage-gated sodium channels; their effect is reversible. The lipid-soluble non-ionised fraction is the one that penetrates the axon and determines latency time. Nevertheless, the drug's action mechanism depends on its ionised fraction. Associating these drugs with a vasoconstrictor (epinephrine) reduces systemic absorption of the anaesthetic; however, this combination is not recommended for our patients due to the risk of tissue necrosis.
The choice of one anaesthetic or another is arbitrary. The following amide-type anaesthetics are often injected: lidocaine 1% and 2% (10-20mg/mL), mepivacaine 2% (20mg/mL), and bupivacaine 0.25% and 0.5% (2.5-6mg/mL) as short, medium, and long-term treatments respectively. They may be administered as monotherapy; when combined, the recommended lidocaine/bupivacaine ratio is between 1:1 and 1:3.59 AB has been shown to be an effective treatment option in pregnancy,60 and the recommended treatment for pregnant women consists of lidocaine (FDA [Food and Drug Administration] category B) rather than bupivacaine (FDA category C); corticosteroids are not recommended since they may accelerate foetal lung development. Adverse effects may be minimised by respecting a maximum dose per session of 300mg for lidocaine, 400mg for mepivacaine, or 175mg for bupivacaine.59,61
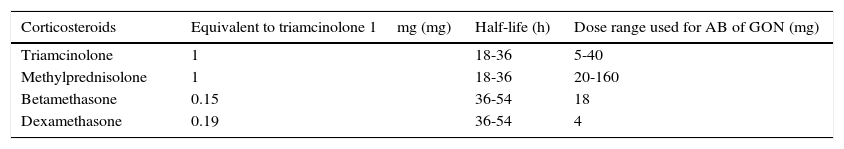
Adding corticosteroids may be considered in patients with CH and CGH, or other headaches that do not respond to infiltration with local anaesthetic only. In any case, corticosteroids should not be used in blockades of the trigeminal branches because of the risk of local trophic changes. The most widely used corticosteroids are triamcinolone, methylprednisolone, betamethasone, and dexamethasone; of these, only methylprednisolone exerts a mineral corticoid effect. Table 3 lists their main characteristics.
Characteristics of corticosteroids used for anaesthetic block.
| Corticosteroids | Equivalent to triamcinolone 1mg (mg) | Half-life (h) | Dose range used for AB of GON (mg) |
|---|---|---|---|
| Triamcinolone | 1 | 18-36 | 5-40 |
| Methylprednisolone | 1 | 18-36 | 20-160 |
| Betamethasone | 0.15 | 36-54 | 18 |
| Dexamethasone | 0.19 | 36-54 | 4 |
Modified from Blumenfeld et al.59
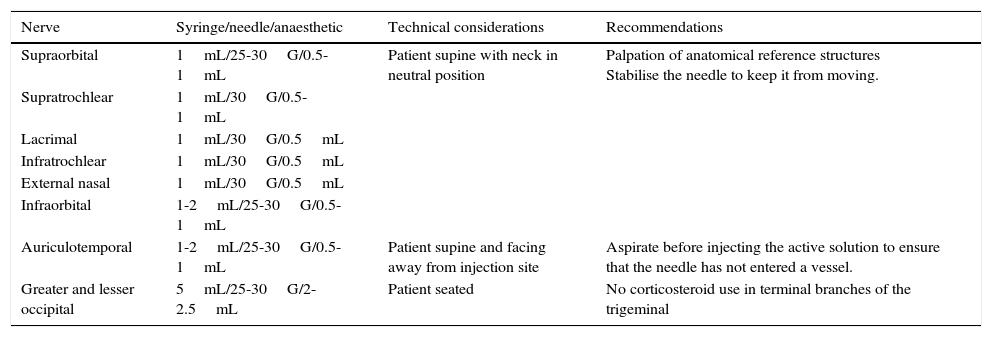
The most relevant considerations for using the AB technique in each territory are summarised in Table 4.
Main characteristics of different anaesthetic block techniques.
| Nerve | Syringe/needle/anaesthetic | Technical considerations | Recommendations |
|---|---|---|---|
| Supraorbital | 1mL/25-30G/0.5-1mL | Patient supine with neck in neutral position | Palpation of anatomical reference structures Stabilise the needle to keep it from moving. |
| Supratrochlear | 1mL/30G/0.5-1mL | ||
| Lacrimal | 1mL/30G/0.5mL | ||
| Infratrochlear | 1mL/30G/0.5mL | ||
| External nasal | 1mL/30G/0.5mL | ||
| Infraorbital | 1-2mL/25-30G/0.5-1mL | ||
| Auriculotemporal | 1-2mL/25-30G/0.5-1mL | Patient supine and facing away from injection site | Aspirate before injecting the active solution to ensure that the needle has not entered a vessel. |
| Greater and lesser occipital | 5mL/25-30G/2-2.5mL | Patient seated | No corticosteroid use in terminal branches of the trigeminal |
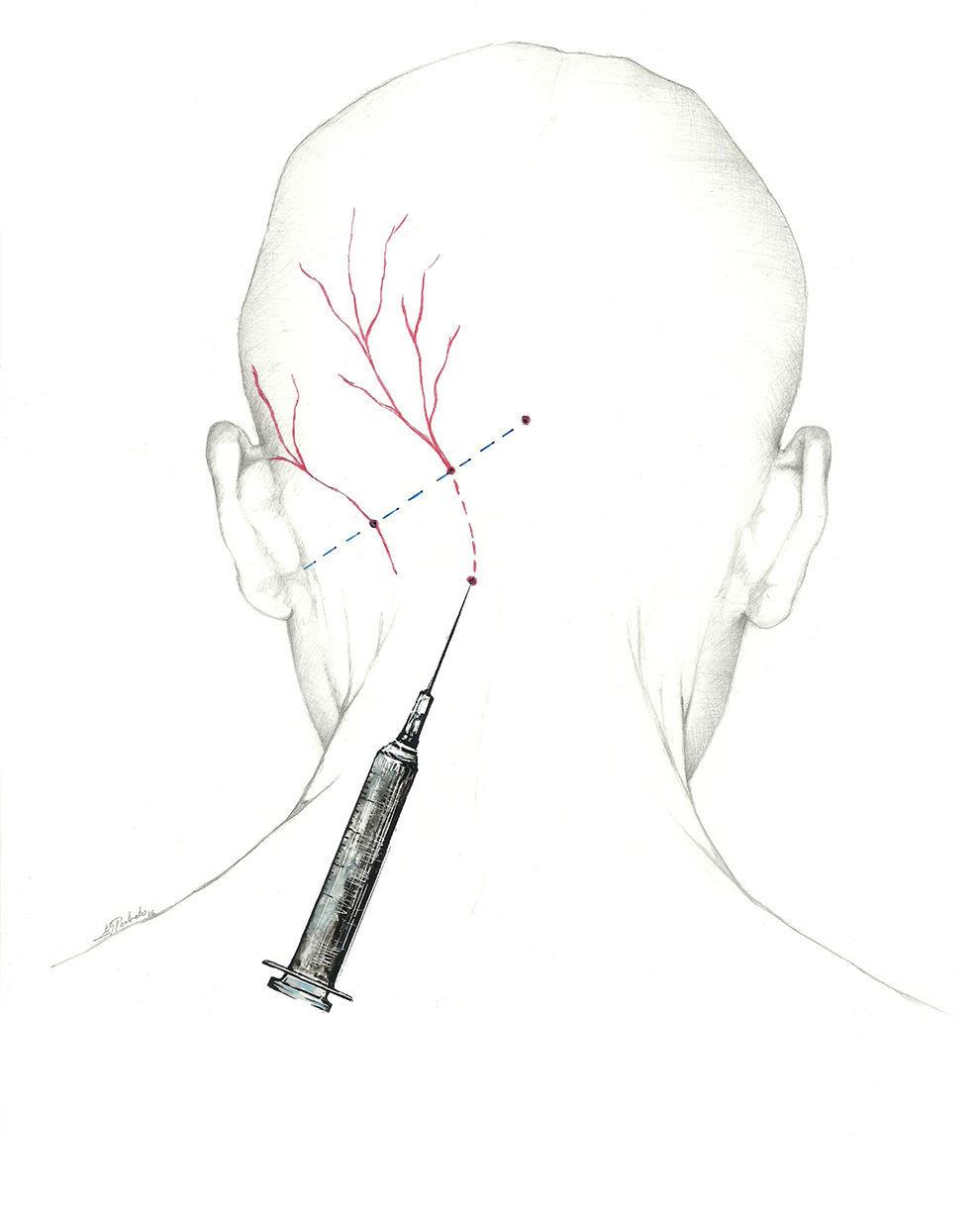
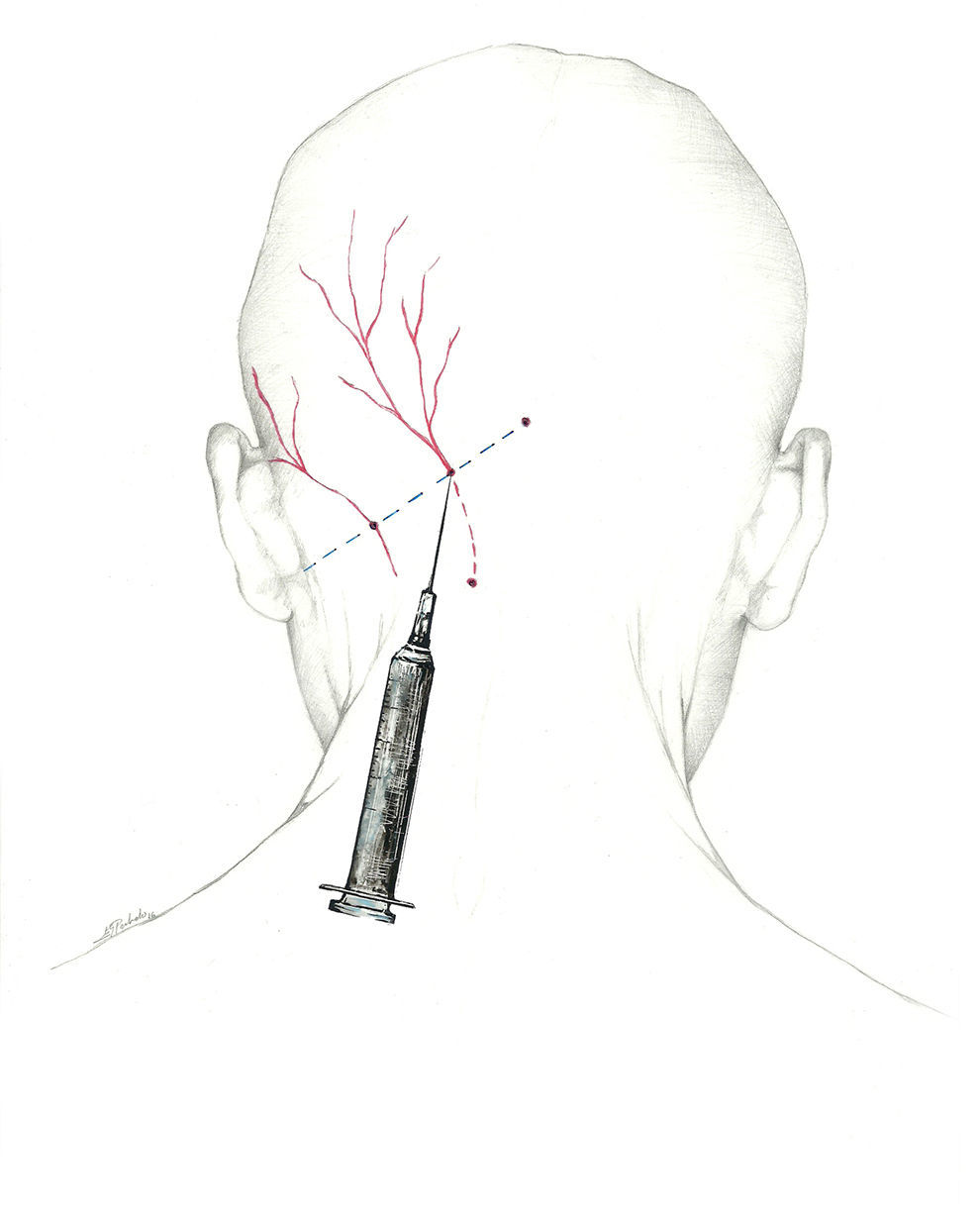
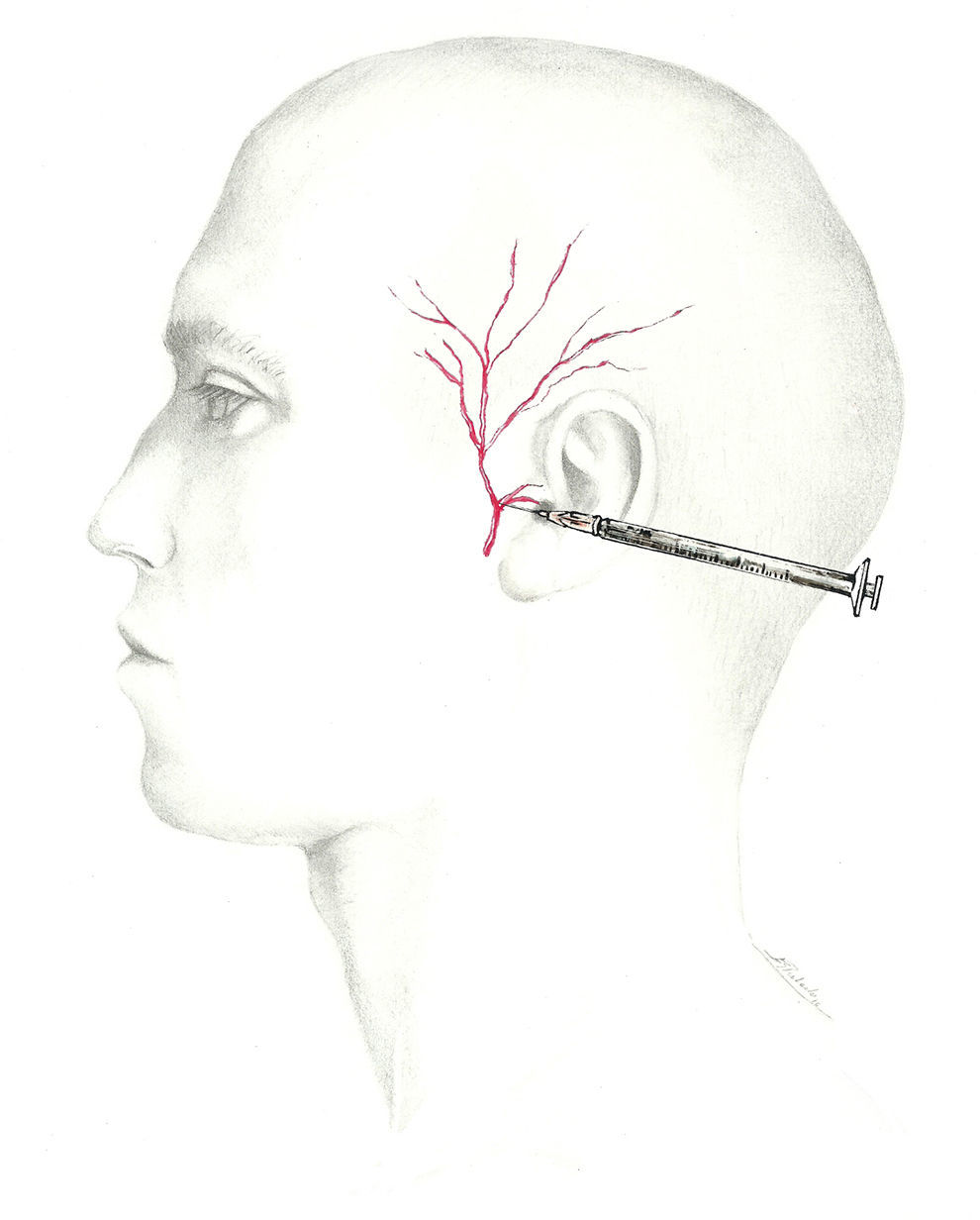
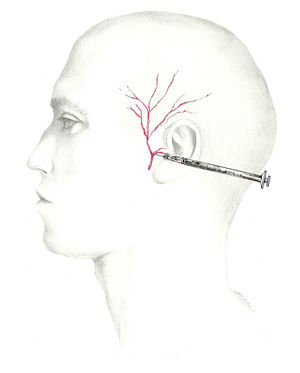
A more fitting term for the network providing sensory information from the occipital region would be ‘occipital nerve complex’. The first component is the GON, which is the dorsal ramus of the second cervical nerve.62–64 It exits through the axis and atlas and extends between the obliquus capitis inferior and semispinalis capitis. From that point, it perforates the aponeurosis of the trapezius to reach the surface65 at the muscle insertion point medial to the occipital artery. This is the target zone for the GON, and it is located on the imaginary line connecting the mastoid process with the inion at the point between the middle and medial thirds of that line.66
The lesser occipital nerve is a branch of the superficial cervical plexus arising from the rami of C2 and C3. It emerges along the posterior aspect of the sternocleidomastoid and continues in a superior and posterior direction along the line between the middle and lateral thirds of the same imaginary mastoid-inion line.62–64 Occipital artery-nerve interaction is present in more than half of all individuals.67 The third and last of the occipital nerves is the dorsal ramus of the third cervical nerve. It innervates the skin of the nape of the neck along its midline.62–64
Of these options, the most likely to receive an injection is the GON. There is no standard technique for a nerve block at this site. Two locations are commonly used as injection sites:
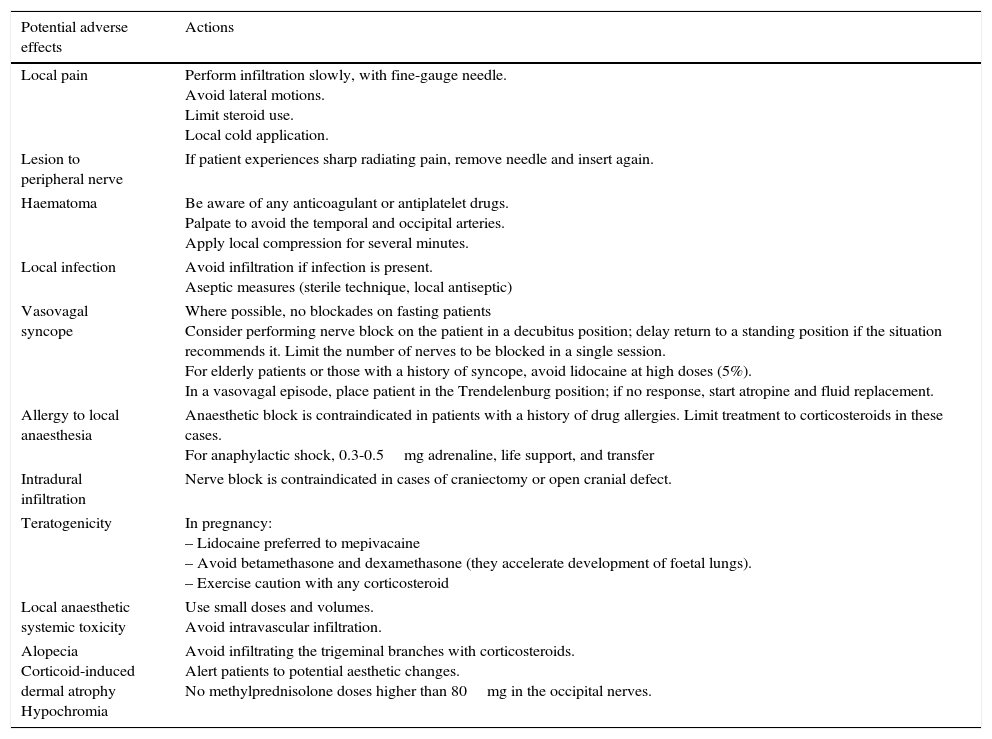
- •
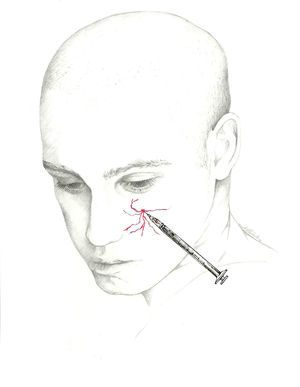
Using the proximal approach, we locate where the occipital nerve emerges from muscle approximately 3cm below and 1.5cm lateral to the inion (Fig. 1). This technique produces an additional response in that it also infiltrates the paraspinal muscles and enables delivery of a higher volume of anaesthetic solution.68
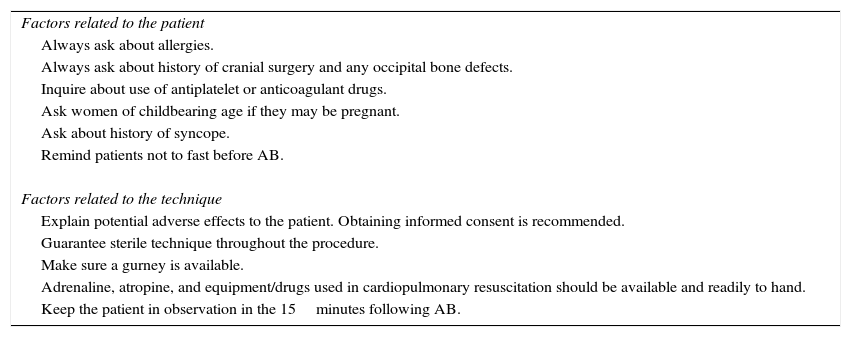
- •
Additionally, the injection can be delivered distally, near the muscle insertion point medial to the occipital artery described above, once the nerve is clear of muscle tissue. This location is recommended by the interventional procedures special interest section of the American Headache Society1 (Fig. 2).
It may occasionally be necessary to inject the area corresponding to the lower occipital nerve, especially in neuralgia cases in which sensitivity to palpation is fundamentally present at that point.69
The most comfortable way of administering a blockade is with the patient seated with the doctor at his/her back. A 5mL syringe is used (between 2 and 2.5mL of anaesthetic solution is to be injected into each nerve) with a 25 to 30-gauge needle angled cranially. Since the occipital artery extends parallel to the GON laterally, it must be located to prevent accidental puncture. Although ultrasound can be used to locate the nerve, there is also a simpler method of ensuring a proper nerve block. Once the needle has been placed, we aspirate to confirm that it has not hit a vessel. After injecting a third of the total volume of solution with the needle inserted vertically, we retract the needle to the subcutaneous level before changing its angle by 30°, first to one side and then the other, to deliver the 2 remaining thirds of the solution to each of these locations. By following these steps, we guarantee distribution of the anaesthetic in a 60° arc.
Supraorbital nerveThe frontal nerve is one of the 3 terminal branches of the ophthalmic nerve (V1), along with the lacrimal and nasociliary nerves. It passes through the orbit at the superior orbital fissure, laterally to the anulus of Zinn, and continues forward between the levator palpebræ superioris and the orbit. Upon reaching the superior orbital edge, it divides into 2 branches, the supratrochlear (internal frontal) and the supraorbital (external frontal). The supraorbital nerve emerges from the supraorbital notch and flexes upward to innervate the medial area of the forehead and the anterior scalp.62–64
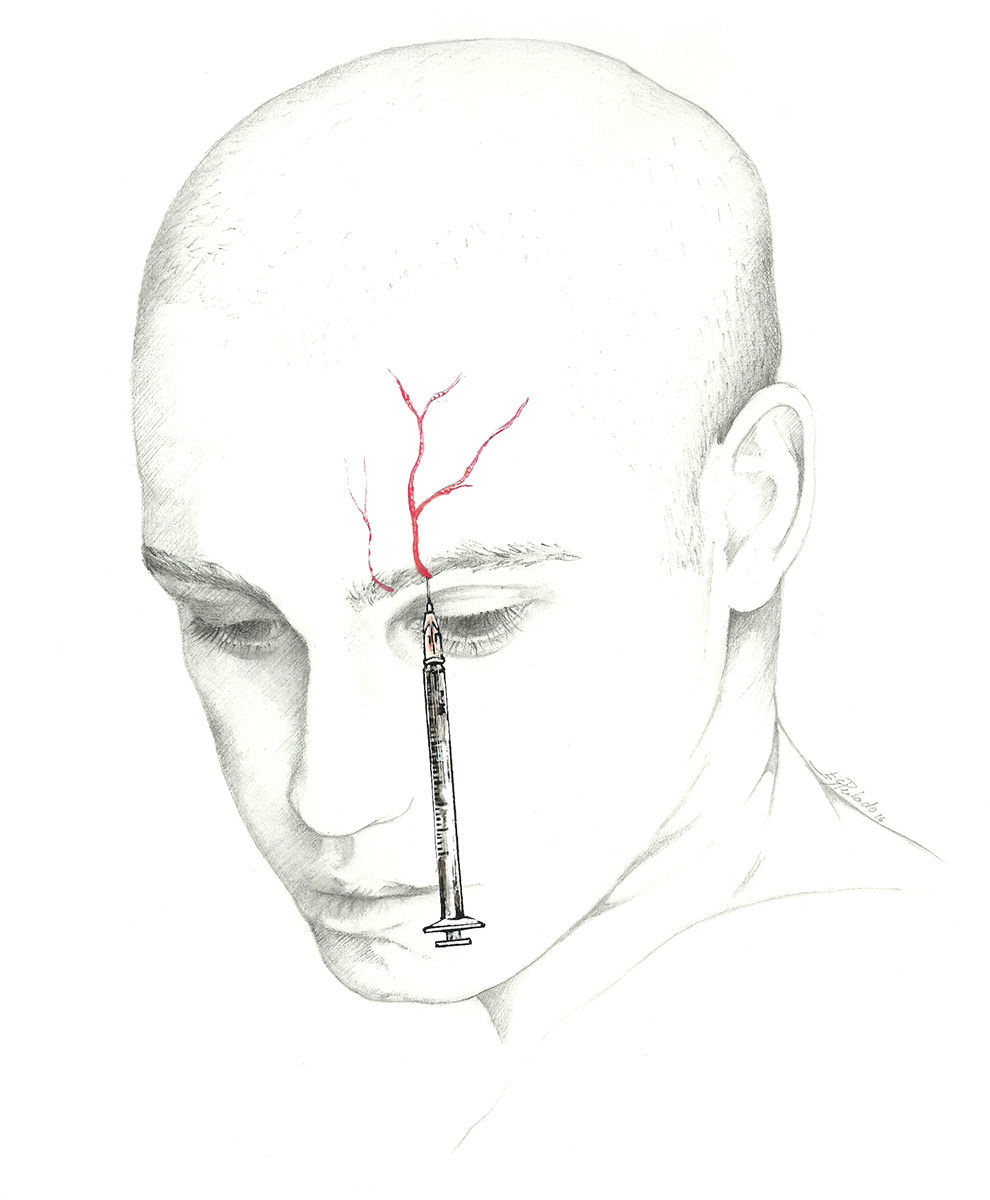
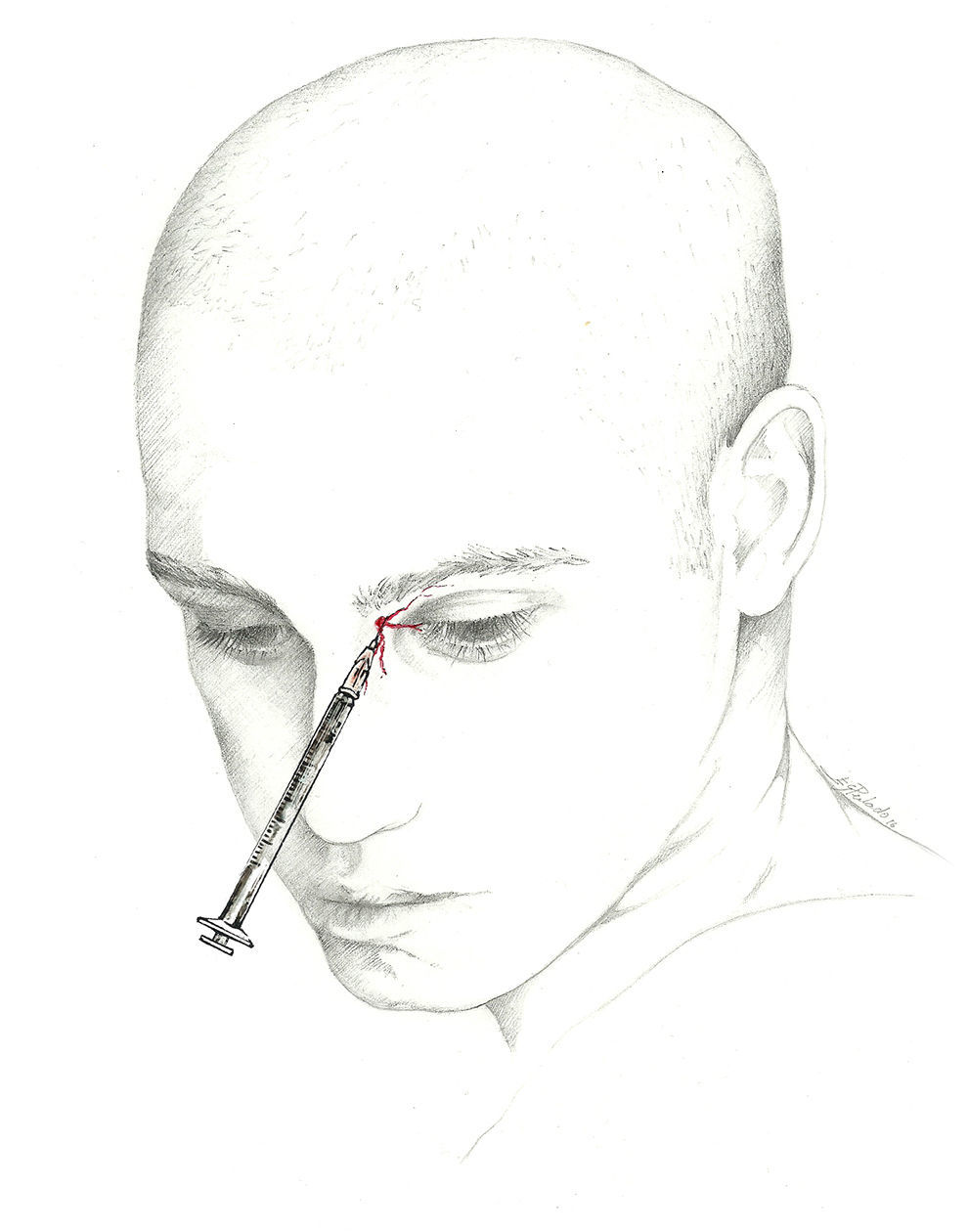
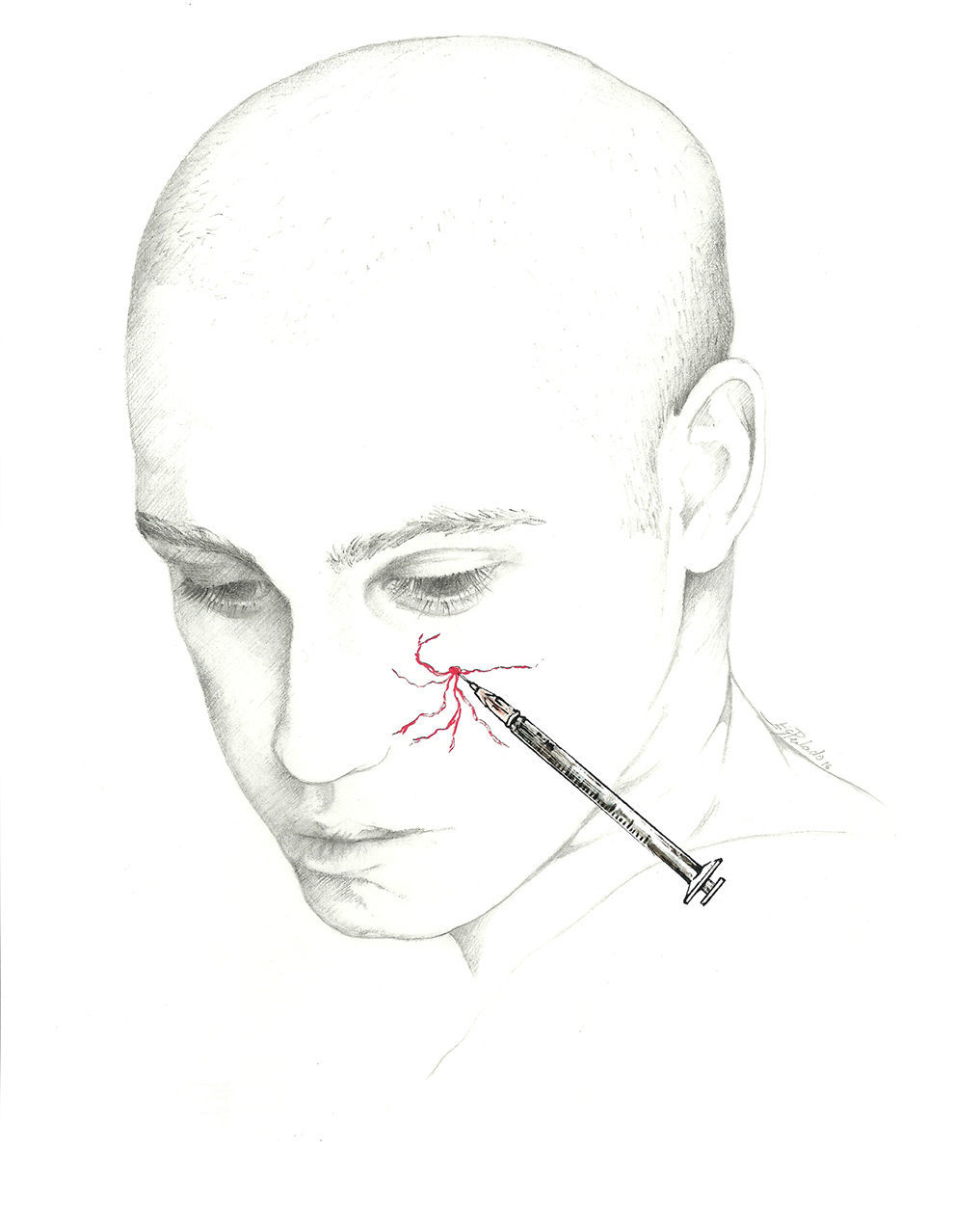
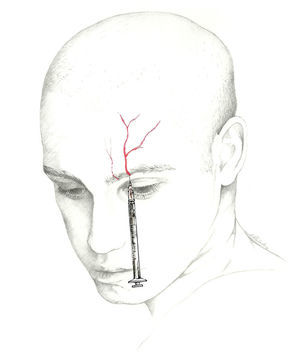
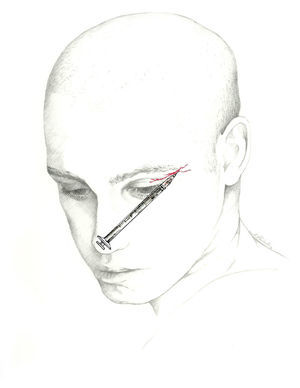
The target point for AB of this nerve is immediately above its exit site, and there are 2 techniques for this blockade.70 The first involves locating a point 0.5cm above the exit site along the pupillary midline and inserting the needle at a 45° angle with the bevelled edge pointing cranially. According to the second, the needle is inserted subcutaneously above the supraorbital notch and parallel to the bone; experts recommend performing a Z-track injection, which will involve pulling the patient's eyebrow upward (Fig. 3).
The most comfortable set-up for this procedure is with the patient supine and the head in a neutral position, and the doctor at the head of the bed or else on the same side as the target nerve. We recommend using a 1mL syringe equipped with a 25 or 30-gauge needle; 30-gauge is preferable since the site is sensitive and susceptible to bleeding.41,71 The volume to be injected is 0.5mL to 1mL per nerve. Performing a fan-shaped injection to anaesthetise this area is normally not necessary. Aspiration must be performed since the area is highly vascularised. The non-dominant hand stabilises the needle during the instillation to avoid displacement and prevent haematomas.72
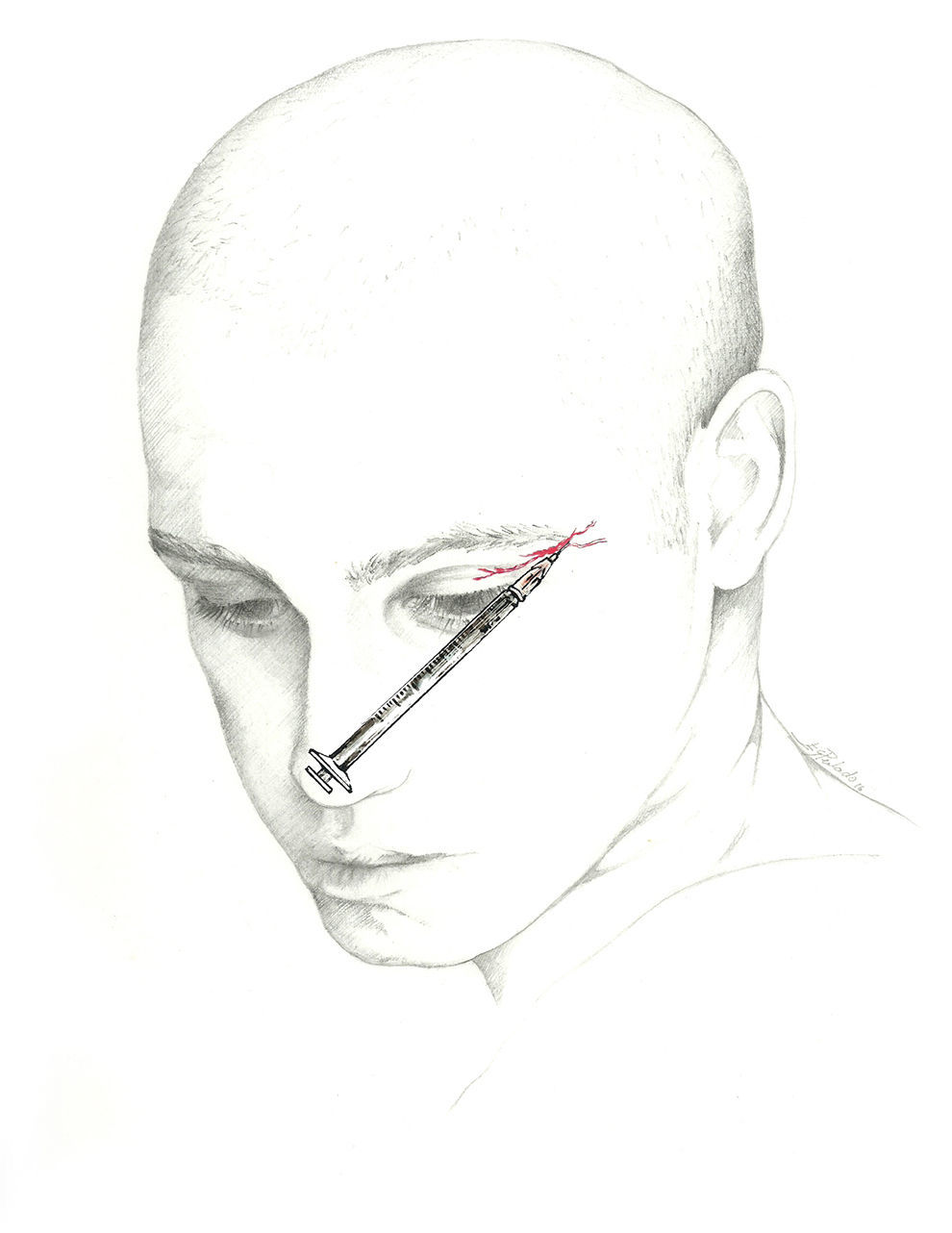
Supratrochlear nerveThe supratrochlear nerve, another branch of the frontal nerve, is smaller in diameter than the SON. It stretches above the pulley of the superior oblique muscle and gives off a branch that joins the nasociliary nerve. It exits the orbit between the pulley of the superior oblique and the supraorbital foramen. It then curves upward between the corrugator supercilii and frontalis muscles to receive sensation from the lower forehead, the skin of the eyelid, and the conjunctiva.62–64
The injection is delivered between the bridge of the nose and the supraorbital notch. The needle is inserted angled 45° in the cranial direction, preferably above the eyebrow to prevent periorbital haematoma.72 The patient is positioned similarly to the description for SON block. We recommend using a 1mL syringe with a 30-gauge needle to inject 0.5 to 1mL of local anaesthetic into each nerve (Fig. 3).
Lacrimal nerveThe lacrimal nerve is one of the terminal branches of the ophthalmic nerve.62–64 In the orbit, this nerve runs along the upper border of the lateral rectus and divides into 2 branches. The lateral branch innervates the lacrimal gland, and the medial branch forms several filaments that receive sensory information from the lateral part of the upper eyelid and adjacent area of the temple.
The lacrimal nerve can be blocked at the surface without difficulty. The patient is positioned in dorsal decubitus, and the doctor uses a 1mL syringe fitted with a fine (30-gauge) needle. The needle is inserted at the lateral end of the eyebrow and pointed upwards and laterally towards the temple before injecting 0.5mL of local anaesthetic (Fig. 4).44,73 A lacrimal nerve block can also be performed from a point within the orbit; a 25-gauge needle is inserted near the lateral wall of the orbit, above the external canthus, to a depth of approximately 2.5cm.45 However, this nerve block presents greater technical challenges.
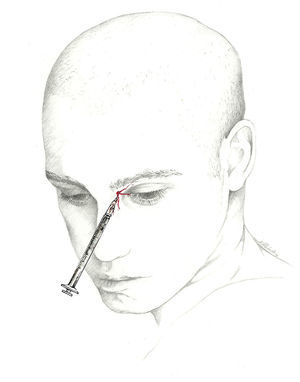
Infratrochlear nerveThe infratrochlear nerve is one of the 2 terminal branches of the nasociliary nerve that continues in the same direction along the lower edge of the superior oblique muscle.62–64 Upon reaching the lower part of the trochlea, the infratrochlear nerve gives off branches to the medial half of the upper eyelid, the lateral aspect of the bridge of the nose, and the tear ducts (lacrimal sac and caruncle).
The infratrochlear nerve is blocked using a 1mL syringe and a 30-gauge needle. With the patient in dorsal decubitus, the needle is inserted a few millimetres into the internal angle of the orbit, just above the caruncle. Next, the doctor injects 0.5mL of local anaesthetic (Fig. 5).46,47
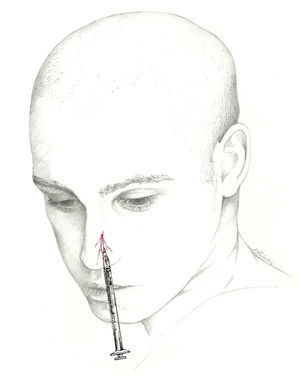
External nasal nerveThe nasociliary nerve gives off another terminal branch in addition to the infratrochlear nerve: the anterior ethmoidal nerve. This nerve enters the nasal cavity through the nasal slit and divides to give off the internal and external nasal branches. While the internal nasal branch gives off filaments to the anterior part of the nasal septum, the external nasal branch descends via a furrow on the posterior aspect of the nasal bone and borders the inferior edge of the same to innervate the distal part of the bridge, apex, and medial part of the wing of the nose.62–64
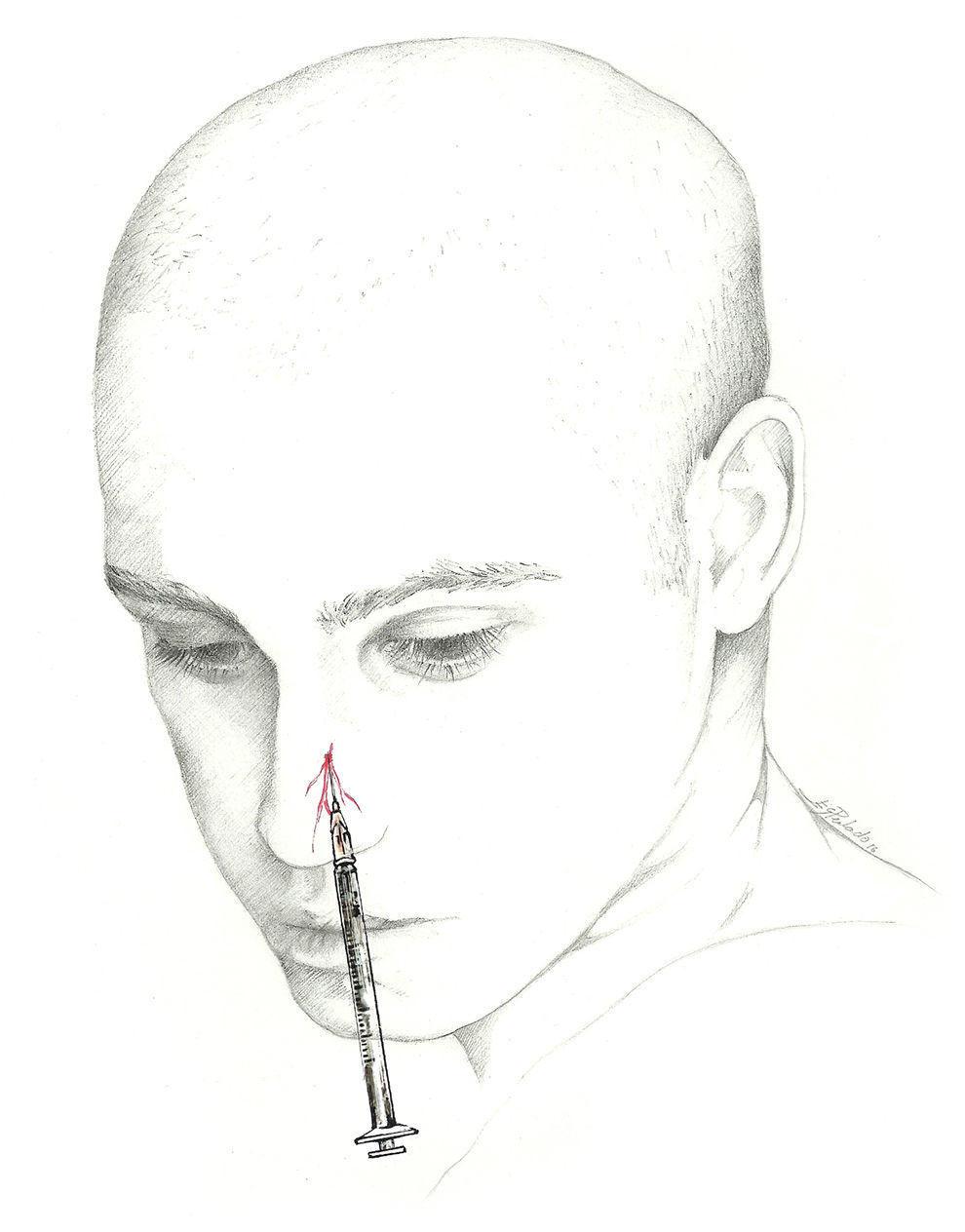
The external nasal branch may be blocked at the surface at its exit point. The block is performed with a 1mL syringe and a 30-gauge needle. With the patient in dorsal decubitus, the needle should be inserted between the nasal bone and the lateral nasal cartilage, approximately 7mm from the midline, and directed cranially through the subcutaneous tissue overlying the nasal bone. Next, the doctor injects 0.5mL of local anaesthetic (Fig. 6).42 The anterior ethmoid nerve and its branches may also be blocked using an intranasal instillation of local anaesthetic.74,75 Nevertheless, the effect of intranasal instillation may be shorter-lived than that of an anaesthetic injection.
Infraorbital nerveThe infraorbital nerve is a terminal branch of the maxillary nerve (V2). It emerges from the infraorbital foramen and provides sensory innervation to the skin of the lower eyelid, cheek, wing of the nose, and upper lip.62–64
This nerve is blocked at its exit point from the infraorbital foramen. This foramen may be identified 6mm below the infraorbital rim and 25mm from the midline.76 With the patient supine, the doctor remains on the same side as the nerve targeted for the injection. We recommend using a 1 to 2mL syringe and a 25 to 30-gauge needle. The needle is angled medially 45° and slightly upward77 (Fig. 7); next, 0.5 to 1mL of anaesthetic solution is injected.61 Injecting excessive volumes of solution or angling the needle too sharply may result in orbital diffusion of the anaesthetic, a situation best avoided.
Auriculotemporal nerveThe auriculotemporal nerve is the posterior branch of the mandibular nerve (V3). It arises as 1 to 4 roots (typically 2), that encircle the middle meningeal artery. It runs between the neck of the mandible and the sphenomandibular ligament, superior to the maxillary vessels. Turning at the condylar head, it stretches vertically to emerge at the superior part of the parotid gland between the tragus and the temporomandibular joint.49 It ascends to the temporal region and supplies parotid and articular branches, both anterior auricular nerves, and branches communicating with the facial nerve and sympathetic plexus. This nerve provides sensory innervation to the temporomandibular joint, middle ear mucosa, tympanic membrane, ligaments of malleus, anterior cochlear surface, external auditory canal and skin in the temporal region, and the anterior part of the pinna.
This nerve is blocked just anterior to the tragus with the bevelled edge of the needle pointing towards the zygomatic arch72 (Fig. 8). Since the temporal artery is located immediately anterior to the nerve, the doctor must palpate the area thoroughly and aspirate to ensure that the needle has not entered the artery.72
We recommend having the patient in the supine position and the doctor on the side receiving the injection, with the patient's head turned towards the contralateral side. This block is best performed with a 1 to 2mL syringe, a 25- to 30-gauge needle, and 0.5 to 1mL of anaesthetic solution.
Adverse reactions and high-risk situationsAdverse reactionsAB is a remarkably safe procedure; any adverse effects tend to be mild, predictable, and temporary. Table 5 lists the most common adverse reactions and recommended measures. Noteworthy potential adverse reactions include:
- •
Local symptoms of pain, ecchymosis or haematomas, and more rarely, cellulitis and lesions to nerves or adjacent structures.59 Recommended measures for minimising pain and local lesions include using fine-gauge needles and delivering the injection slowly and carefully, without multiple punctures or lateral movements. Palpating nearby occipital and temporal arteries may be helpful to avoid accidentally hitting them, especially in patients treated with antiplatelet or anticoagulant drugs. Baykal has written that puncturing the occipital artery when performing GON block is not uncommon, and this event causes a haematoma that can be treated by applying local pressure.78 Maintaining local pressure after removing the needle can help distribute the local anaesthetic, in addition to having a haemostatic effect. Intense stabbing or stinging and generally radiating pain that presents upon insertion of the needle indicates a direct nerve lesion. In this case, we recommend withdrawing the needle slightly and pointing it in another direction. Some 10% of all patients receiving a steroid injection may experience a local increase in pain during 24 to 48hours. This is less likely to occur when more soluble steroid solutions are used. Local inflammation may be reduced by applying a cold pack to the injection site.
- •
Any changes in blood pressure are generally temporary. The patient may experience pallor, sweating, nausea, bradycardia, hyperventilation, and syncope.27,59,79,80 In a series of patients undergoing GON block, Sahai-Sristava et al.79 detected the following main risk factors for vasovagal response: fasting, use of bilateral infiltrations, older age, and use of high concentrations of local anaesthetic (5% lidocaine rather than 1% or 2%).59,79 Having the patient in a decubitus position is recommended, especially where there is a history of vasovagal episodes or syncope, volume depletion due to headache-associated nausea or vomiting, and in anxious, pregnant, or elderly patients. Such patients should be returned to a standing position more slowly.59 The Trendelenburg position is recommended for patients who present a vasovagal reaction. In the absence of a response, subcutaneous or intravenous administration of 0.4 to 0.5mg atropine (1/2 vial) may be considered.81 Transient and largely inconsequential increases in arterial blood pressure have also been described, especially in elderly patients.79
- •
Headache triggered by the technique, exacerbation of existing headache,27 and neck pain.22
- •
Allergic reactions to local anaesthetic: while allergic reactions have been described, they are less likely to occur with aminoamides (lidocaine, mepivacaine, bupivacaine) than with aminoesters.1 Delayed local hypersensitivity reactions generally appear within 72hours of exposure, typically presenting as eczema-like dermatitis in the area that came in contact with the allergen. If necessary, this may be treated with topical steroids. Systemic allergic reactions (anaphylaxis) may present with urticaria, angiooedema, nausea, vomiting, pain, diarrhoea, coughing, dyspnoea, and in severe cases, complications including oedema of glottis, bronchospasm, hypotension, and shock. Anaphylaxis generally develops just minutes after exposure to the antigen, although time intervals of up to one hour have been reported. A patient presenting anaphylactic shock will require immediate delivery of 0.3 to 0.5mg adrenaline by the subcutaneous or intramuscular route. If necessary, the patient must also receive basic life support measures and transfer to the intensive care unit.81 These reactions are extremely rare; doctors and patients alike should know that such complications are highly unlikely to occur.
- •
Local anaesthetic systemic toxicity: reactions arising from overdoses are exceptional since drugs are usually used correctly and in very small doses. They sometimes occur due to an accidental and undetected intravascular injection (relative overdosing) or when other medications contribute to raising the systemic level of the local anaesthetic.1 From a technical standpoint, avoiding intravascular injections is not problematic if the doctor is careful to aspirate with the syringe and only deliver the injection if no blood appears. Nevertheless, these precautions may not be completely reliable when the needle is very small (30-gauge or finer), or if too much pressure is applied after the injection.3 False negatives may occur when the tip of the needle is obstructed by tissue or a clot that impedes the aspiration of blood.82 The prevailing belief is that the smaller the volume, the lower the risk of systemic absorption. The syndrome is caused by drug toxicity affecting the cardiovascular and central nervous systems (CNS). CNS toxicity may be mild (tinnitus, metallic taste, paraesthesias, nausea, vomiting, vertigo, restlessness, blurred vision), moderate (nystagmus, hallucinations, fasciculations, tremor, and convulsions), or severe (apnoea, coma). The first cardiovascular symptom of intoxication is hypotension; additional symptoms may include arrhythmias, syncope, shock, and cardiac arrest in asystole. These infrequent adverse reactions can generally be avoided by using low doses of the anaesthetic and a good injection technique.
- •
Local anaesthetics induce reversible myonecrosis near the injection site, but only a few clinically relevant cases of this phenomenon have been reported. Since bupivacaine is the drug most likely to cause local myotoxicity, some authors limit the frequency of injections with this drug. Nevertheless, the incidence of these cases has not been established.83 After discontinuation of the treatment, muscles typically regenerate in 3 or 4 weeks.84
- •
The onset of tissue necrosis is fundamentally related to the use of vasoconstrictors in association with local anaesthetics. Nevertheless, there are also anecdotal reports of this process occurring with use of lidocaine in the absence of vasoconstrictors, and an embolic mechanism may be involved in these cases.85
- •
Metahaemoglobinaemia, an extremely rare complication, has been described in association with prilocaine, benzocaine, tetracaine, and lidocaine.86
- •
Corticosteroid injections may be associated with local adverse reactions resulting in alopecia, pigmentation changes, and dermal atrophy around the injection site (2% of the series described by Shields et al.87); these reactions usually resolve over a few months, but they may also last more than 2 years.87–89 The risk of trophic disorders is higher for triamcinolone than for other corticosteroids, such as methylprednisolone or betamethasone.90 Corticosteroids should not be used in trigeminal branch AB due to the risk of local reactions, which may include corticosteroid-induced dermal atrophy.87 If employing methylprednisolone, doses of more than 80mg are not recommended for GON block. Systemic adverse effects include insomnia, agitation, facial flushing, Cushing syndrome, and immunosuppression, especially in the context of frequent injections and high doses. In the single published case of Cushing syndrome associated with AB, symptoms began to lessen once injections were no longer being administered.91 We therefore recommend establishing a minimum time interval of 3 months59 between infiltrations containing a corticosteroid; nevertheless, this may not be feasible in some entities, such as CH.
Potential adverse reactions to anaesthetic blocks and recommended actions.
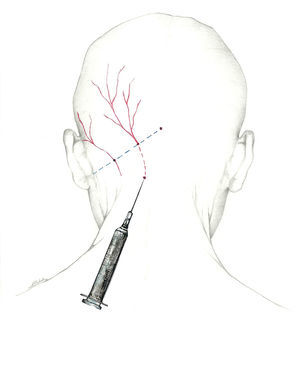
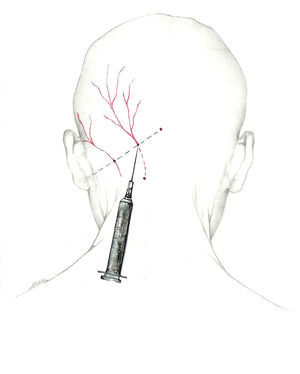
| Potential adverse effects | Actions |
|---|---|
| Local pain | Perform infiltration slowly, with fine-gauge needle. Avoid lateral motions. Limit steroid use. Local cold application. |
| Lesion to peripheral nerve | If patient experiences sharp radiating pain, remove needle and insert again. |
| Haematoma | Be aware of any anticoagulant or antiplatelet drugs. Palpate to avoid the temporal and occipital arteries. Apply local compression for several minutes. |
| Local infection | Avoid infiltration if infection is present. Aseptic measures (sterile technique, local antiseptic) |
| Vasovagal syncope | Where possible, no blockades on fasting patients Consider performing nerve block on the patient in a decubitus position; delay return to a standing position if the situation recommends it. Limit the number of nerves to be blocked in a single session. For elderly patients or those with a history of syncope, avoid lidocaine at high doses (5%). In a vasovagal episode, place patient in the Trendelenburg position; if no response, start atropine and fluid replacement. |
| Allergy to local anaesthesia | Anaesthetic block is contraindicated in patients with a history of drug allergies. Limit treatment to corticosteroids in these cases. For anaphylactic shock, 0.3-0.5mg adrenaline, life support, and transfer |
| Intradural infiltration | Nerve block is contraindicated in cases of craniectomy or open cranial defect. |
| Teratogenicity | In pregnancy: – Lidocaine preferred to mepivacaine – Avoid betamethasone and dexamethasone (they accelerate development of foetal lungs). – Exercise caution with any corticosteroid |
| Local anaesthetic systemic toxicity | Use small doses and volumes. Avoid intravascular infiltration. |
| Alopecia Corticoid-induced dermal atrophy Hypochromia | Avoid infiltrating the trigeminal branches with corticosteroids. Alert patients to potential aesthetic changes. No methylprednisolone doses higher than 80mg in the occipital nerves. |
Table 6 summarises the main points to consider before AB should be performed. It seems practical to begin by listing situations associated with potentially higher levels of risk.
- •
History of allergic reaction to local anaesthetic. Injecting corticosteroids alone is one treatment alternative for allergic patients.
- •
Craniectomy or open cranial defect in the posterior fossa entails a risk of intracranial diffusion of the anaesthetic, which may result in decreased level of consciousness and coma.92,93
- •
Doctors must be especially cautious when employing corticosteroids in patients with diabetes or glaucoma.
- •
Systemic infections and local infections at the injection site.
- •
In all cases, and especially in patients taking anticoagulants, we must palpate the area to avoid hitting neighbouring arteries, and then apply local pressure for several minutes after each injection.
Main considerations to be pondered before performing anaesthetic block.
| Factors related to the patient |
| Always ask about allergies. |
| Always ask about history of cranial surgery and any occipital bone defects. |
| Inquire about use of antiplatelet or anticoagulant drugs. |
| Ask women of childbearing age if they may be pregnant. |
| Ask about history of syncope. |
| Remind patients not to fast before AB. |
| Factors related to the technique |
| Explain potential adverse effects to the patient. Obtaining informed consent is recommended. |
| Guarantee sterile technique throughout the procedure. |
| Make sure a gurney is available. |
| Adrenaline, atropine, and equipment/drugs used in cardiopulmonary resuscitation should be available and readily to hand. |
| Keep the patient in observation in the 15minutes following AB. |
The authors have no conflicts of interest to declare.
We would like to thank Esperanza González Perlado for her commitment to this project, and for providing such masterful illustrations (Figs. 1–8).
Please cite this article as: Santos Lasaosa S, Cuadrado Pérez ML, Guerrero Peral AL, Huerta Villanueva M, Porta-Etessam J, Pozo-Rosich P, et al. Guía consenso sobre técnicas de infiltración anestésica de nervios pericraneales. Neurología. 2017;32:316–330.