Analysing drug consumption in large population groups lets us observe consumption trends and compare them between different settings.
ObjectiveTo analyse the time trends for consumption and costs of specific drugs used to treat dementia in the region of Madrid (Spain) and compare trends by sex and age cohort.
MethodsDescriptive study of cholinesterase inhibitors (N06DA) and memantine (N06DX01) dispensed in Madrid between 2002 and 2012 and covered by the Spain's national health system. Consumption was calculated by analysing changes in DDD (defined daily doses) to find total and yearly increases. The cost was estimated based on DDD price. To compare consumption rates by age and sex, we calculated DDD per 100 inhabitants/day.
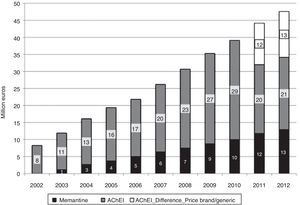
ResultsBetween 2002 and 2012, consumption of drugs used to treat dementia increased sixfold. During this period, cholinesterase inhibitors accounted for 76.70% of the drugs consumed and memantine, 23.30%. The estimated cost rose by a factor of 5.7 over 11 years (or by a factor of 4 taking into account the use of generic drugs).
In 2012, 2.42% of the patients aged 65 or over consumed cholinesterase inhibitors (women 2.82%, men 1.83%) and 0.90% consumed memantine (women 1.10%, men 0.61%). Consumption increased in age cohorts up to 86 to 90 (5.84% for cholinesterase inhibitors and 2.33% for memantine) and declined thereafter.
ConclusionsConsumption of cholinesterase inhibitors and memantine gradually increased, but consumption in 2012 did not reach levels equivalent to dementia prevalence figures. Pharmaceutical expenditure restraint measures may temporarily slow the cost increase temporarily but if the same trend of consumption persists, costs will rise.
El análisis del consumo de fármacos en grandes grupos poblacionales permite ver tendencias de consumo y comparar diferentes ámbitos.
ObjetivoAnalizar la tendencia temporal de consumo y costes de fármacos específicos para la demencia en la comunidad autónoma de Madrid (CAM) y comparar por grupos de edad y sexo.
MétodosEstudio descriptivo, seleccionando anticolinesterásicos (N06DA) y memantina (N06DX01) dispensados en la CAM del 2002 al 2012 con cargo al Sistema Nacional de Salud. El consumo se calculó analizando la evolución de las dosis diarias definidas (DDD), con incrementos totales y anuales. El coste se estimó por precio de DDD. Para comparar el consumo por edad y sexo, se calcularon las DDD por 100 habitantes-día.
ResultadosDel 2002 al 2012 se multiplicó por 6 el consumo de fármacos para la demencia. El 76,70% de los fármacos consumidos en este periodo fueron anticolinesterásicos y el 23,30% memantina. La evolución del coste estimado se multiplicó por 5,7 en 11 años (por 4 considerando utilización de fármacos genéricos).
En 2012, el 2,42% de los mayores de 65 años consumió anticolinesterásicos (2,82% mujeres, 1,83% hombres) y el 0,90% memantina (1,10% mujeres, 0,61% hombres). El consumo aumentó hasta los 86-90 años (5,84% en anticolinesterásicos; 2,33% en memantina), disminuyendo posteriormente.
ConclusionesEl consumo de anticolinesterásicos y memantina aumentó progresivamente, sin alcanzar en 2012 cifras equivalentes a la prevalencia poblacional de demencia. Las medidas de contención de gasto farmacéutico pueden frenar el aumento del coste, aunque este volverá a crecer si persiste la misma tendencia temporal de consumo.
Dementia prevalence is approximately 6.4% in patients older than 65 years, and it increases with age, from 0.8% in patients aged 65 to 69, 10% in patients older than 70 years, and 25% to 54% in the group of patients aged 90 or older.1–3 Alzheimer disease (AD) is the most frequent type of dementia and the main cause of disability among elderly patients. It places a significant burden on caregivers and generates social costs which increase as the disease progresses.4,5 Researchers estimate that some 600,000 people have dementia and 400,000 people have AD in Spain.2 According to the ECO study,6 the average monthly cost per patient is 1425 euro. Almost 88% of the cost of illness corresponds to informal care, which is borne by the patient's own family. This percentage is higher than the European average, which is about 56%.7 Healthcare costs include pharmacological treatments, as well as diagnostic tests, office visits, hospitalisation, and institutionalisation.
Since there is no curative treatment for dementia, treatment approach is focused on treating cognitive impairment symptoms, as well as behavioural and psychological symptoms. Its target is also to prevent complications and functional disability, and manage comorbidities. Acetylcholinesterase inhibitors (AChEI) are indicated as specific treatment for mild to moderate AD.8–10 These include donepezil (marketed in Spain since 1998), rivastigmine (since 2000), and galantamine (since 2001). Memantine, a non-competitive N-methyl-d-aspartate (NMDA)-receptor antagonist, was authorised in 2003 for use in mild to severe AD.8–10 AChEI and memantine can be administered in combination, but studies yield contradictory results.8,10–12 In addition to AD, both drugs can be used to treat vascular dementia, and diseases with Lewy bodies (dementia with Lewy bodies and Parkinson's disease), but they are not indicated to treat frontotemporal dementia.8,9
Their effects are modest and temporary and not all patients respond to treatment.13 However, according to clinical practice guidelines,8 treatment with these drugs should be started as soon as AD is diagnosed, considering that previous identification of patients who will respond to treatment is not possible.14 Spanish regulations establish that every first or maintenance prescription should be authorised by the medical inspection service after a neurologist, gerontologist, or psychiatrist issues the initial report confirming the disease and the treatment indication. There is no consensus on when to suspend treatment with these drugs,15–17 but some Spanish regions have established rules regarding use and continuity.18 The cost-benefit ratio of these treatments is currently being debated. Although several studies indicate that starting drug treatments, especially in mild-moderate stages, can delay disease progression, institutionalisation, and associated costs, results remain uncertain.19–21 Therefore, they should be interpreted considering those differences between public health systems that would influence cost distribution.14
Consumption of AChEI and memantine has increased progressively since they were introduced to the market,22–26 and they are listed among the 35 most expensive active ingredients for the Spanish Health System.27 This increase, which may be partly due to the increased prevalence of dementia caused by population ageing, also depends on the higher percentage of dementia patients treated since these drugs began to be indicated habitually. Thus, a study performed in a primary care setting simultaneously with our own showed that the percentage of dementia patients treated with these drugs had increased from 23% in 2002 to 41% in 2011.28
Regarding pharmacoepidemiological studies, quantitative studies of the consumption of drugs using prescription invoicing data and/or sales turnover figures show the actual consumption trends of the drugs in large populations. They also provide an overview of the actual costs of the disease and permit comparisons between different settings or populations.29
The aim of our study is to analyse time trends of consumption and costs of cholinesterase inhibitors (AChEI) and memantine (specific drugs used to treat dementia) in the region of Madrid between 2002 and 2012. Consumption profiles by age and sex were also compared to one another.
Material and methodsA population-based descriptive study of the consumption of specific drugs used to treat dementia was performed between 1 January 2002 and 31 December 2012 in the region of Madrid. Our data source was the system for information and analysis on prescriptions and pharmaceutical services of the region of Madrid (Farm@drid), which serves as the database of official medical prescriptions dispensed by pharmacies in the region of Madrid and invoiced to the Spanish Health System.
We selected cholinesterase inhibitors belonging to the chemical subgroup N06DA and identified the active ingredients donepezil (N06DA02), rivastigmine (N06DA03), and galantamine (N06DA04). We selected memantine (N06DX01) from the N06DX subgroup but excluded ginkgo biloba (N06DX02) as it is not used specifically to treat dementia.
As variables associated with treatment, we analysed year of consumption, sex, and age group (in 9 categories: 51-55 years, 56-60 years, 61-65 years, 66-70 years, 71-75 years, 76-80 years, 81-85 years, 86-90 years, and older than 90 years).
To analyse drug consumption, we used the parameters ‘defined daily dose’ (DDD) and ‘daily dose per inhabitant’ (DDI) for each active ingredient.
DDD corresponds to the daily maintenance dose for a drug used for its main indication in adults and given by a specific route of administration.30 We adhered to the DDDs defined by the World Health Organization: donepezil 7.5mg, rivastigmine 9mg (9.5mg transdermally), galantamine 16mg, and memantine 20mg.31
We assessed trends in drug consumption during the study period by calculating the total number of DDD based on the total number of packages dispensed every year. To do this, we converted commercial formats in milligrams or grams to DDD. We analysed consumption trends for the DDD of each active ingredient and therapeutic chemical subgroup, the ratio between the end and the beginning of the period, and the yearly and total increase in DDD. To calculate the percentages of total increase for 2012 compared to the beginning of the study, we used the formulaΔ2012–2002=([DDD2012−DDD2002]/DDD2002)×100 for AChEI, and % Δ2012–2003=([DDD2012−DDD2003]/DDD2003)×100 for memantine (marketed since 2003).29
Annual cost increases for each active ingredient were estimated by multiplying the number of DDD consumed each year by their sales price, calculated according to figures from the Nomenclátor Digitalis drug database.32 In our cost estimation, we considered commercial formats of maintenance doses exclusively. The price per milligram for each package format was obtained to later calculate the mean price per year of the different formats. Calculated DDD prices were between 4.39 and 4.04 euro for donepezil, 3.58 and 3.24 euro for rivastagmine, 3.84 and 3.53 euro for galantamine, and 3.8 euro for memantine. We performed an additional estimation after generic forms of donepezil became available in 2011 (DDD at 1.75 euro) and rivastigmine solution in 2012 (DDD at 2.65 euro). This estimation assumed that only generic drugs were used.
Daily dose per inhabitant refers to the mean number of inhabitants receiving daily treatment with a given drug.30 DDI corresponds to the DDD typically calculated for 1000 inhabitants. In our study, we decided to use a DDI per 100 inhabitants to draw comparisons with dementia prevalence in the population, and with other prevalence studies on consumption of these drugs.23,28
We used DDI to analyse consumption by age group and sex in 2012. We calculated DDI for both sexes and each age group, and for patients older than 60, 65, or 70 years, using the following formula: number of DDI2012=(number of DDD2012/population2012)×(1/365)×100. Only DDDs consumed by patients older than 50 years were considered. Populations used in calculating this rate were obtained from Farm@drid, according to health card holder records. Data were limited to patients older than 50 years since they constitute the reference population for these drugs. We calculated confidence intervals for both sexes and each age group.
Data extracted from the Farm@drid digital record did not include variables that could identify patients; therefore, our study preserves data confidentiality.
Data were analysed with the SPSS statistical software, version 19.0; Excel® version 12.3.0 (110427); and Epidat 3.0.
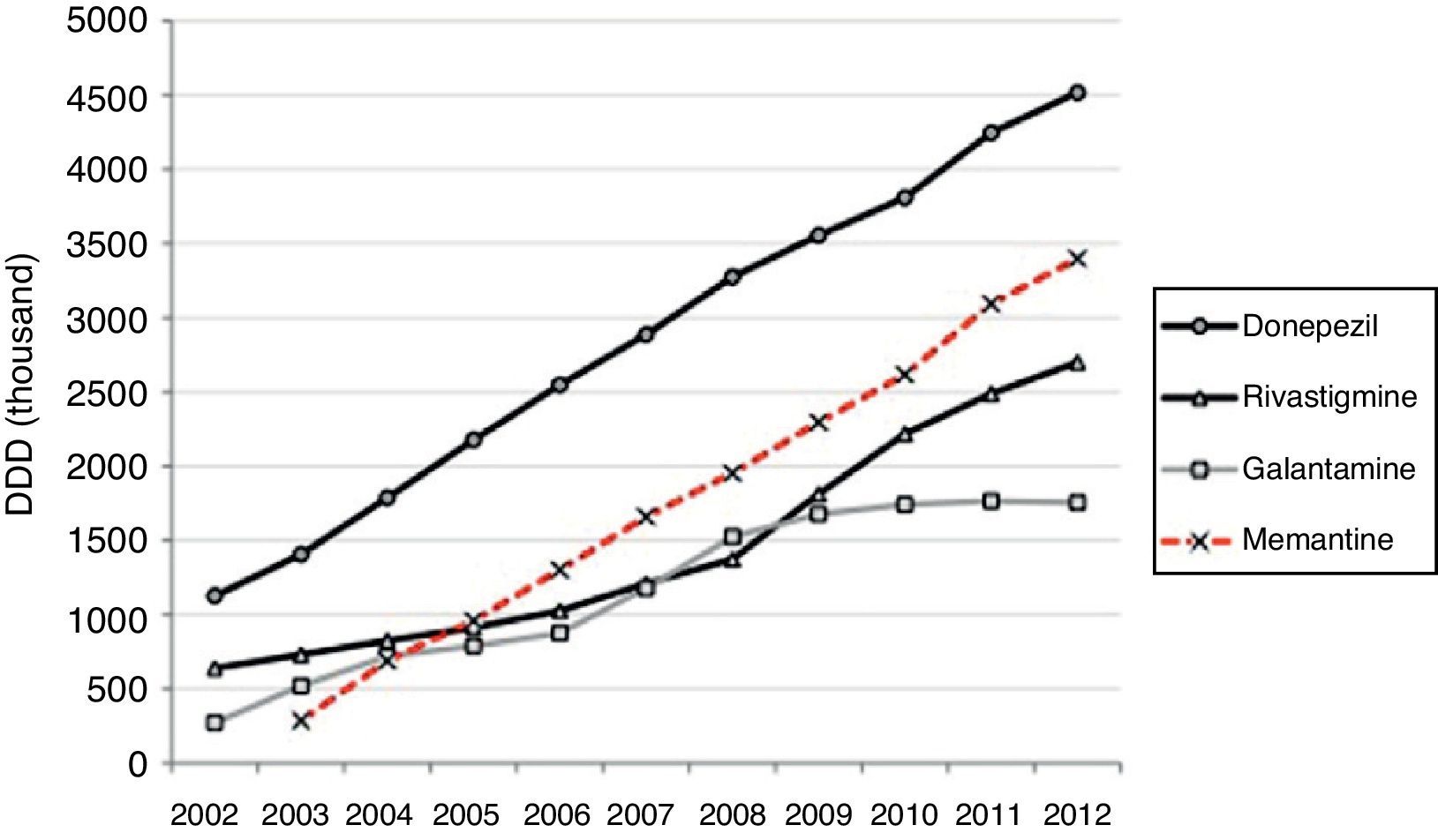
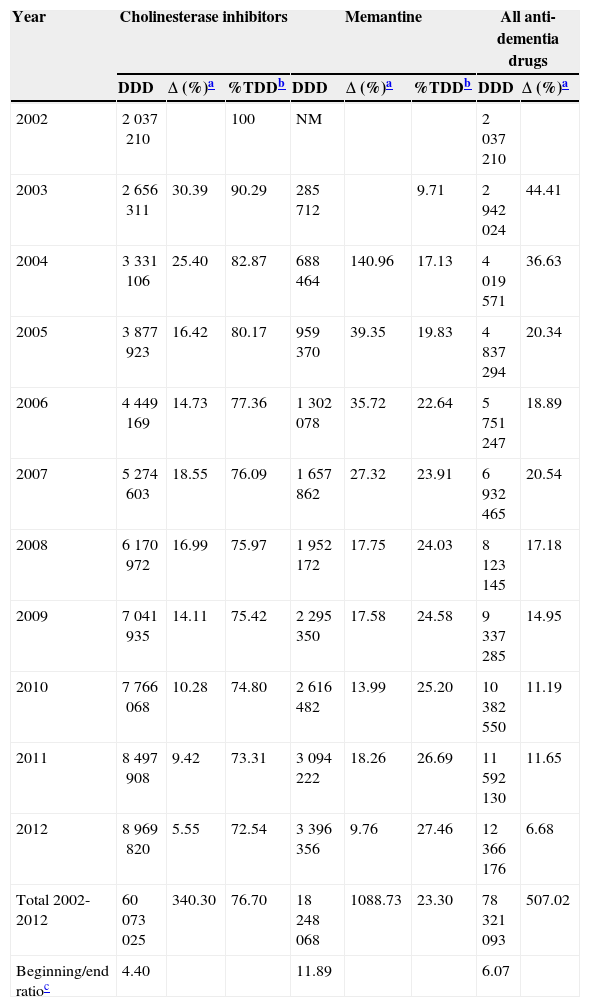
ResultsConsumption of the specific drugs used to treat dementia, cholinesterase inhibitors (AChEI) and memantine, increased by a factor of 6 between 2002 and 2012 in the region of Madrid. This meant a total increase in DDD of 507%. In this period, 76.7% of the drugs consumed to treat dementia were AChEI, with 23.3% corresponding to memantine (Table 1).
Changes in consumption of specific drugs to treat dementia (cholinesterase inhibitors and memantine) in the region of Madrid between 2002 and 2012, in defined daily doses.
| Year | Cholinesterase inhibitors | Memantine | All anti-dementia drugs | |||||
|---|---|---|---|---|---|---|---|---|
| DDD | Δ (%)a | %TDDb | DDD | Δ (%)a | %TDDb | DDD | Δ (%)a | |
| 2002 | 2037210 | 100 | NM | 2037210 | ||||
| 2003 | 2656311 | 30.39 | 90.29 | 285712 | 9.71 | 2942024 | 44.41 | |
| 2004 | 3331106 | 25.40 | 82.87 | 688464 | 140.96 | 17.13 | 4019571 | 36.63 |
| 2005 | 3877923 | 16.42 | 80.17 | 959370 | 39.35 | 19.83 | 4837294 | 20.34 |
| 2006 | 4449169 | 14.73 | 77.36 | 1302078 | 35.72 | 22.64 | 5751247 | 18.89 |
| 2007 | 5274603 | 18.55 | 76.09 | 1657862 | 27.32 | 23.91 | 6932465 | 20.54 |
| 2008 | 6170972 | 16.99 | 75.97 | 1952172 | 17.75 | 24.03 | 8123145 | 17.18 |
| 2009 | 7041935 | 14.11 | 75.42 | 2295350 | 17.58 | 24.58 | 9337285 | 14.95 |
| 2010 | 7766068 | 10.28 | 74.80 | 2616482 | 13.99 | 25.20 | 10382550 | 11.19 |
| 2011 | 8497908 | 9.42 | 73.31 | 3094222 | 18.26 | 26.69 | 11592130 | 11.65 |
| 2012 | 8969820 | 5.55 | 72.54 | 3396356 | 9.76 | 27.46 | 12366176 | 6.68 |
| Total 2002-2012 | 60073025 | 340.30 | 76.70 | 18248068 | 1088.73 | 23.30 | 78321093 | 507.02 |
| Beginning/end ratioc | 4.40 | 11.89 | 6.07 | |||||
DDD, defined daily dose; NM, not on the market.
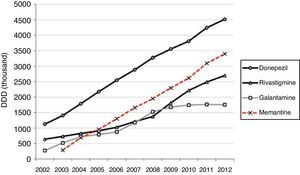
Half of the AChEI prescriptions filled during the study period were for donepezil. Galantamine consumption increased progressively up to 2008, when platformed off. Beginning in 2009, rivastigmine consumption increased until it made up 30% of all AChEI prescriptions in 2012. The increasing trend in consumption was more homogeneous for donezepil and memantine throughout the 11-year study period (Fig. 1).
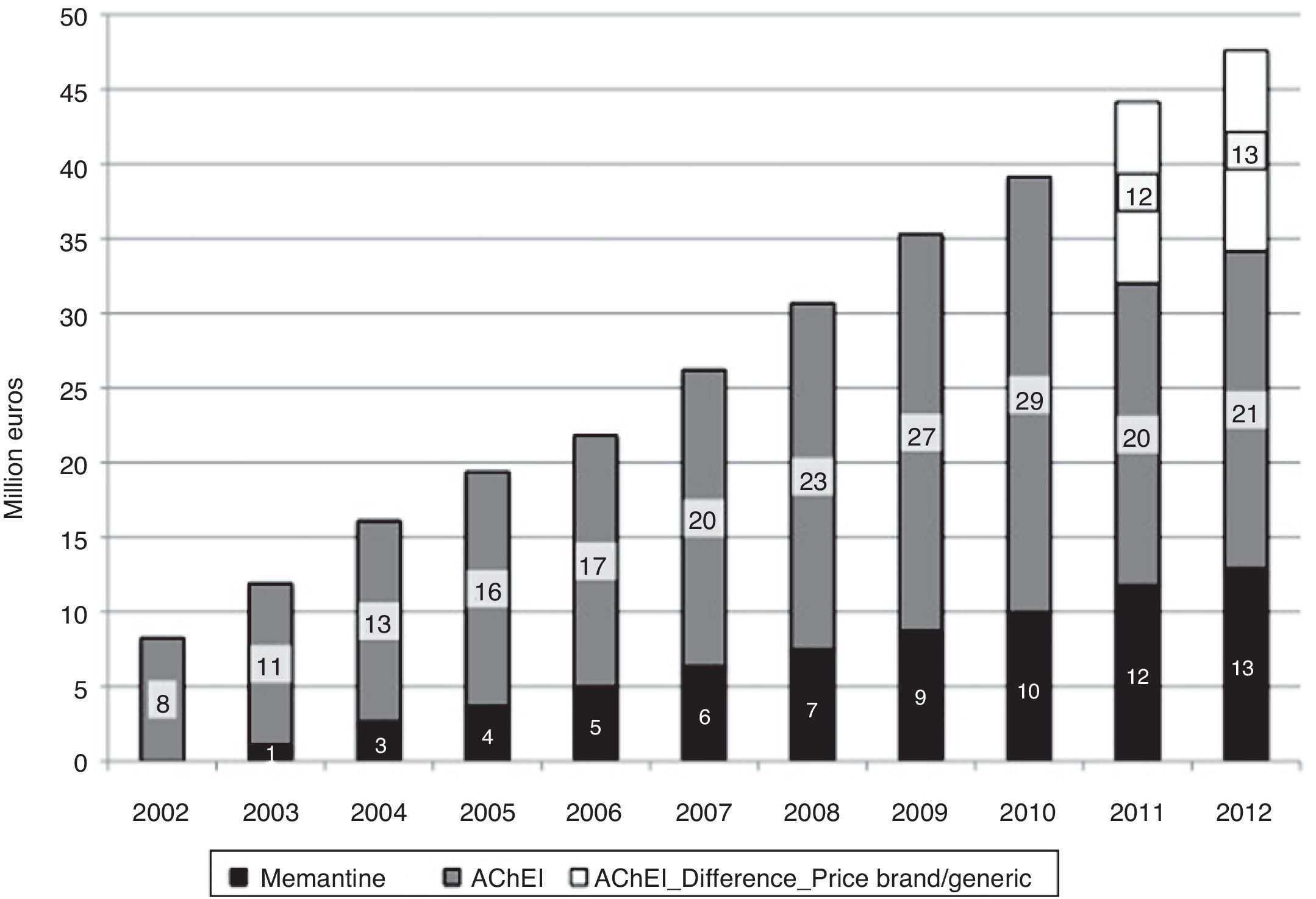
In 2012, 12.3 million DDDs of specific drugs used to treat dementia were consumed, 10.3 million more than in year 2002 (Table 1). Estimated costs increased by a factor of 5.7, from 8.2 million euro in 2002 to 47.6 million in 2012. If we consider prices for generic drugs only, the final cost amounts to 34 million, 4 times higher than at the beginning of the study period. Annual cost savings due to the use of generic drugs amounted to 12 million in 2011, and 13 million in 2013 (Fig. 2). Total costs during the 11-year period would be 300 million euro for brand-name drugs only or 275 million euro considering the generics that became available in 2011 and 2012.
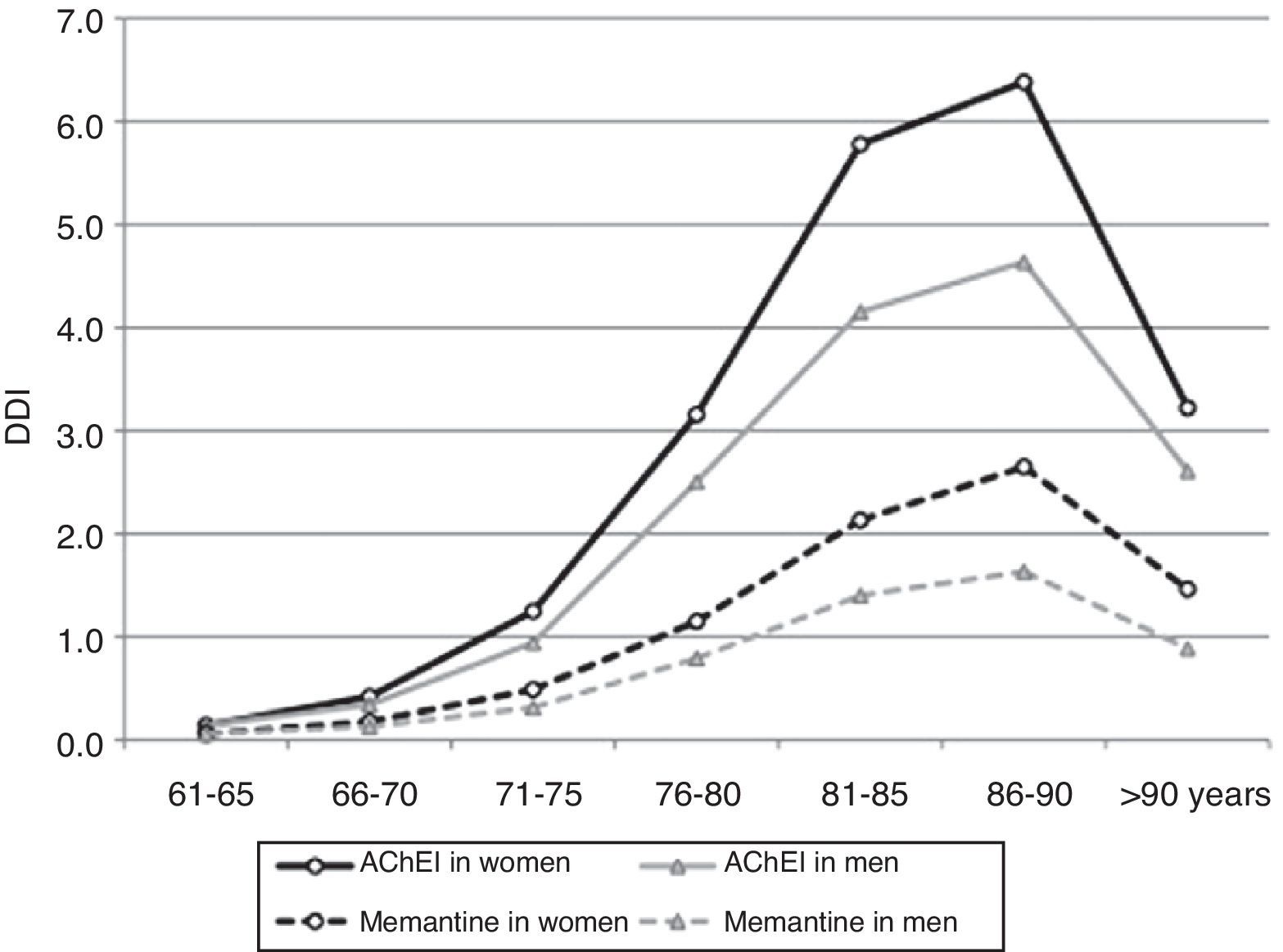
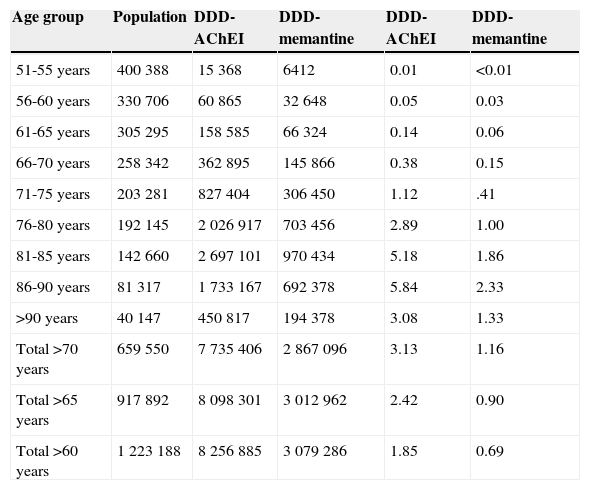
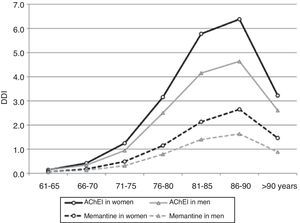
After adjusting 2012 consumption data by age and sex, 2.42% of patients older than 65 years (95% CI, 2.39-2.45) consumed AChEI, and 0.90% took memantine (95% CI, 0.88-0.92). Consumption increased for both drug groups in patients between 65 and 90 years old; 5.84% consumed AChEI (95% CI, 5.68-6.00), and 2.33% took memantine (95% CI, 2.23-2.43). Consumption decreased in patients older than 90 (Table 2 and Fig. 3).
Age distribution of consumption of cholinesterase inhibitors and memantine in the region of Madrid in 2012.
| Age group | Population | DDD-AChEI | DDD-memantine | DDD-AChEI | DDD-memantine |
|---|---|---|---|---|---|
| 51-55 years | 400388 | 15368 | 6412 | 0.01 | <0.01 |
| 56-60 years | 330706 | 60865 | 32648 | 0.05 | 0.03 |
| 61-65 years | 305295 | 158585 | 66324 | 0.14 | 0.06 |
| 66-70 years | 258342 | 362895 | 145866 | 0.38 | 0.15 |
| 71-75 years | 203281 | 827404 | 306450 | 1.12 | .41 |
| 76-80 years | 192145 | 2026917 | 703456 | 2.89 | 1.00 |
| 81-85 years | 142660 | 2697101 | 970434 | 5.18 | 1.86 |
| 86-90 years | 81317 | 1733167 | 692378 | 5.84 | 2.33 |
| >90 years | 40147 | 450817 | 194378 | 3.08 | 1.33 |
| Total >70 years | 659550 | 7735406 | 2867096 | 3.13 | 1.16 |
| Total >65 years | 917892 | 8098301 | 3012962 | 2.42 | 0.90 |
| Total >60 years | 1223188 | 8256885 | 3079286 | 1.85 | 0.69 |
DDD, defined daily dose; DDI, defined dose per 100 inhabitants per day; AChEI, cholinesterase inhibitors.
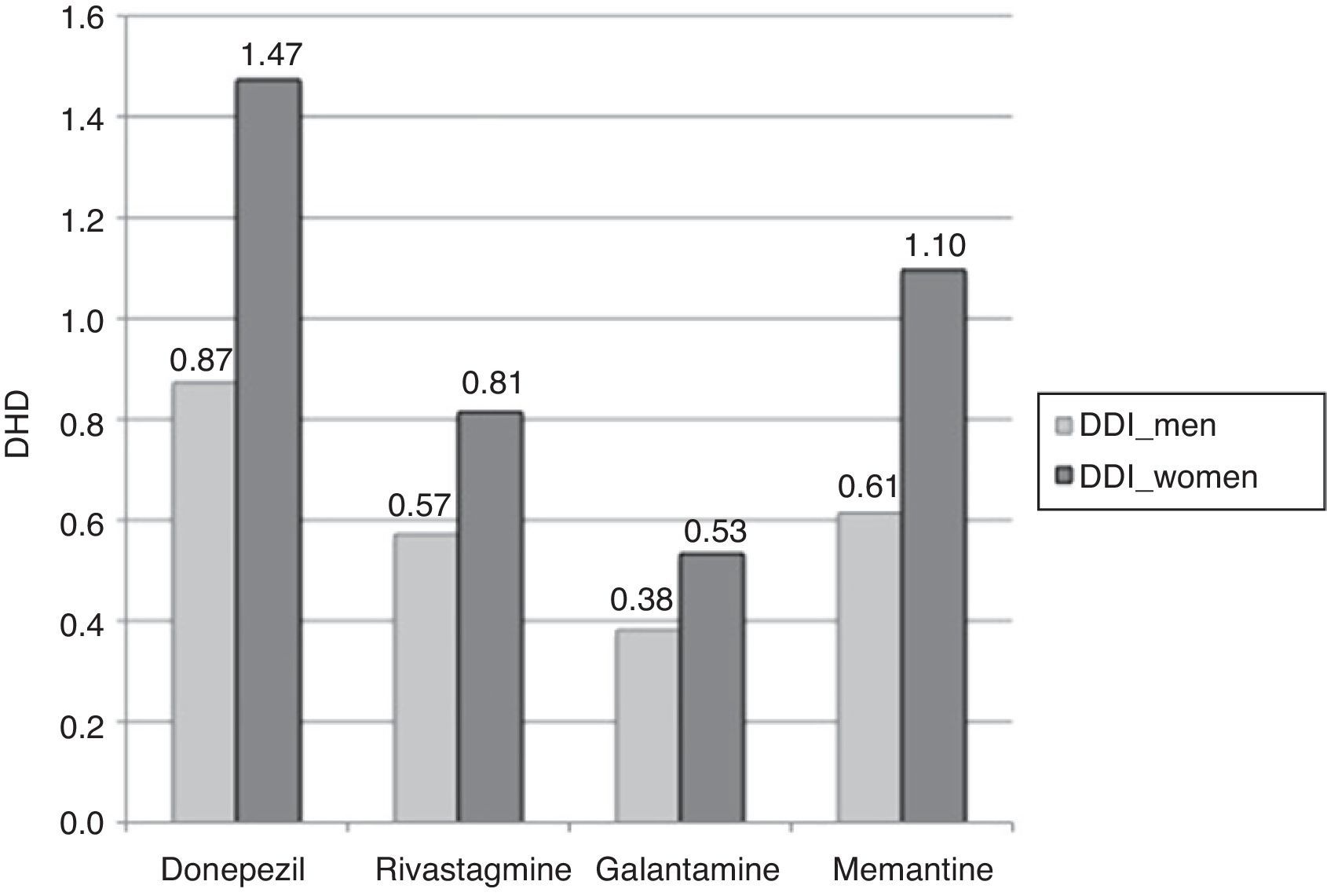
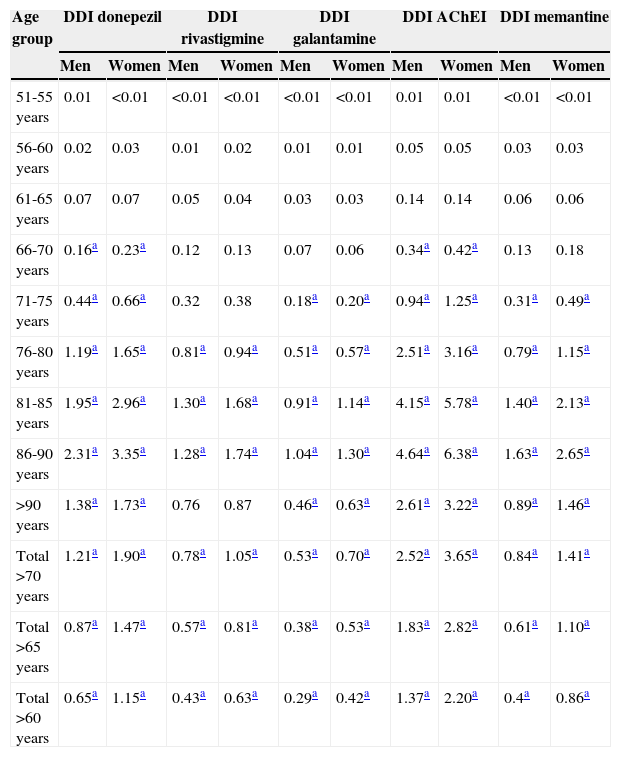
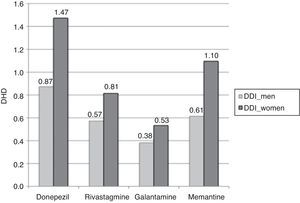
Consumption was higher in women than in men. In the group of patients older than 65 years, 2.82% of the women (95% CI, 2.78-2.87) consumed AChEI versus 1.83% of the men (95% CI, 1.79-1.87). Memantine was consumed by 1.10% of the women (95% CI, 1.07-1.12) versus 0.61% of the men (95% CI, 0.59-0.64). We found significant differences between sexes in patients older than 65 years regarding consumption of AChEI, and of memantine in patients older than 71 years. Increased consumption with age and decreased consumption in patients older than 90 years was observed both in men and women (Table 3, Figs. 3 and 4).
Age and sex distribution of consumption of specific anti-dementia drugs (cholinesterase inhibitors and memantine) in the region of Madrid in 2012.
| Age group | DDI donepezil | DDI rivastigmine | DDI galantamine | DDI AChEI | DDI memantine | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | Men | Women | Men | Women | |
| 51-55 years | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.01 | 0.01 | <0.01 | <0.01 |
| 56-60 years | 0.02 | 0.03 | 0.01 | 0.02 | 0.01 | 0.01 | 0.05 | 0.05 | 0.03 | 0.03 |
| 61-65 years | 0.07 | 0.07 | 0.05 | 0.04 | 0.03 | 0.03 | 0.14 | 0.14 | 0.06 | 0.06 |
| 66-70 years | 0.16a | 0.23a | 0.12 | 0.13 | 0.07 | 0.06 | 0.34a | 0.42a | 0.13 | 0.18 |
| 71-75 years | 0.44a | 0.66a | 0.32 | 0.38 | 0.18a | 0.20a | 0.94a | 1.25a | 0.31a | 0.49a |
| 76-80 years | 1.19a | 1.65a | 0.81a | 0.94a | 0.51a | 0.57a | 2.51a | 3.16a | 0.79a | 1.15a |
| 81-85 years | 1.95a | 2.96a | 1.30a | 1.68a | 0.91a | 1.14a | 4.15a | 5.78a | 1.40a | 2.13a |
| 86-90 years | 2.31a | 3.35a | 1.28a | 1.74a | 1.04a | 1.30a | 4.64a | 6.38a | 1.63a | 2.65a |
| >90 years | 1.38a | 1.73a | 0.76 | 0.87 | 0.46a | 0.63a | 2.61a | 3.22a | 0.89a | 1.46a |
| Total >70 years | 1.21a | 1.90a | 0.78a | 1.05a | 0.53a | 0.70a | 2.52a | 3.65a | 0.84a | 1.41a |
| Total >65 years | 0.87a | 1.47a | 0.57a | 0.81a | 0.38a | 0.53a | 1.83a | 2.82a | 0.61a | 1.10a |
| Total >60 years | 0.65a | 1.15a | 0.43a | 0.63a | 0.29a | 0.42a | 1.37a | 2.20a | 0.4a | 0.86a |
DDI, defined dose per 100 inhabitants per day.
The most widely used active ingredient in 2012 in both men and women of all ages was donezepil; the least used was galantamine. Rivastigmine was the drug with the least pronounced sex difference; differences in use by sex were only identified in patients aged 76 to 90.
DiscussionOverall consumption of specific drugs used to treat dementia in the region of Madrid increased by a mean of 50% annually between 2002 and 2012. Mean annual increase during the first 6 years (2002-2007) was 48% versus 15% for the last 6 years (2007-2012). Compared to similar periods, the consumption increase in the region of Madrid was higher than that reflected by other studies performed in Spain using the same analysis methods. For example, DDD consumption around Madrid increased by 25% annually from 2004 to 2008, while in Castille-La Mancha this increase reached 11.5% (in a study which included ginkgo biloba23). Likewise, the annual increase between 2006 and 2011 was 20% in the region of Madrid, versus 12% in the Basque country during the same period, as reported by a study in patients older than 60.24
Donepezil was the most widely used active ingredient in the region of Madrid throughout the study period, a tendency that was also observed in other Spanish studies22,23,28,33,34 as well as in international studies.26,35,36 This trend has been linked to the drug's ease of use and low rate of adverse effects.14 However, the literature shows considerable variability in the proportions and consumption trends for these drugs.23,24,34,37
The standstill in galantamine consumption in the region of Madrid coincides with the increase in the use of rivastagmine. Increased use of rivastagmine during the past few years was also observed by other consumption studies24,26 and may be explained by the marketing of rivastigmine transdermal patches in 2008.
The indication of memantine for moderate to advanced dementia suggests that the prescription profile might show a use curve tending more towards advanced ages than is the case for AChEI. However, as in other studies of overall consumption,28 the memantine consumption profile by age group was similar to that of AChEI in 2012. This might indicate that memantine could be used in the same progression stages of dementia, whether in combination treatment or as sole treatment in cases of moderate dementia with a contraindication of AChEI. Testing this hypothesis would require a different study design.
In the last year of our study period (2012), AChEI consumption per inhabitant and day among patients older than 65 years was 2.82%, versus 1.10% in the case of memantine. Considering that combined treatment is used some patients, we have to assume that less than 3.92% of patients older than 65 consume specific anti-dementia drugs (consumption prevalence). Despite progressive increases in consumption, this percentage is lower than might be expected if we compare it to prevalences of AD (4.6%) or of dementia (5.8-7.6%)38,39 in patients older than 65, as reported by other population studies conducted in nearby settings. This difference is more pronounced if we consider only those patients older than 70, since consumption of AChEI (3.13%) and memantine (1.16%) indicate that 4.29% of all patients older than 70 consume these drugs. Meanwhile, the prevalence of dementia in that age group is much higher.2
Women older than 65 consume more AChEI and memantine that men, which may be explained by the higher prevalence of dementia among women. Likewise, consumption increases with age, since prevalence of dementia displays the same tendency. However, consumption tapers off at more advanced ages, a trend that has also been observed in other studies in the general population28,40 and in institutionalised patients.41,42 The reason may be that dementia prevalence no longer increases exponentially in patients older than 90 years; the tendency may also result from decisions to limit treatment (due to comorbidities, potential toxicity, very advanced dementia), and therefore withdrawal and/or reduced use of these drugs in this age group.3,17
Consumption of different active ingredients varies according to sex and age, which seems to indicate a sex-related prescription profile, as also observed in other studies.28,40 Confirming sex differences in how these drugs are prescribed will require further studies of consumption among patients with dementia to determine whether differences persist after adjusting by dementia subtype and stage of progression. Either one of these circumstances may determine which of the active ingredients is the most appropriate.
Costs of AChEI and memantine have been increasing since they were first marketed due to the progressive increase in consumption, considering that prices have remained relatively stable. These drugs’ share of the pharmaceutical expenditure on prescriptions dispensed in the region of Madrid43 also increased from 1.31% in 2003 to 3.04% in 2010 according to our results. This was in line with findings from the Basque country.24 In the region of Madrid, this percentage has been increasing since 2011, despite expenditure have been contained by the appearance of generic versions of these drugs. Therefore, considering a total prescription expenditure amounting to 1.057 billion43 in 2012, we estimate that 4.50% would correspond to these drugs (3.23% if we consider generic drugs). In return, appropriate use of AChEI and/or memantine could help reduce the indiscriminate use of other drugs used to treat cognitive impairment and dementia (such as ginkgo biloba, piracetam, or citicoline), which have shown no benefits for controlling symptoms in patients with dementia.
This study presents some inherent limitations. Firstly, data regarding AChEI and memantine consumption refer to the total population, without mentioning patients with a confirmed diagnosis of dementia.
Since we do not have data regarding combined treatment (AChEI with memantine), only an estimated prevalence of patients treated with specific anti-dementia drugs (AChEI and/or memantine) in the region of Madrid could be obtained, unlike in other studies.
The study does not include prescriptions given by private practices or within the framework of special healthcare programmes such as the general Spanish civil service mutual insurance company, general mutual insurance scheme for the judiciary, and the social insurance institute of the armed forces. These groups make up such a small percentage of the population living in the region of Madrid (0.87%) and covered by the Spanish Health System that they do not impact the validity of our data whether in terms of the number of prescriptions or in population figures.44
Using DDD in our study involves the limitations inherent to this kind of indicator, considering that it is a technical unit of measurement that does not necessarily reflect the true prescribed dose.30,31 However, since these drugs are only indicated for dementia and are used according to an established protocol, data reflect consumption quite reliably.
Yearly cost estimations were performed assuming similar consumption of all available commercial formats (capsules, solution, and patches). However, there is probably a tendency towards using one type more than others. The very low variation in the price of DDDs for each active ingredient during the 11-year period, before generic drugs became available, justifies our method.
Data regarding AChEI and memantine dispensed by pharmacies upon presentation of official prescriptions do not fully ensure treatment compliance among patients. However, since these drugs must be prescribed by a specialist and authorised by the medical inspection service, the statistics provide information about consumption that is quite reliable. This fact, in addition to having the consumption data for all drugs covered by the Spanish Health System and finding coherent results, lets us state that data accurately reflect consumption tendencies for specific drugs used to treat dementia in the region of Madrid.
The appearance of generic formats of these drugs beginning in 2011, approval of different regulations to rationalise drug use and thus control pharmaceutical expenditure (for example, prescribing active ingredients rather than brand names under Legislative Royal Decree 9/201145), and the changes applied to the reference price system in 2013 are all measures able to temporally reduce costs and slow the progressive increase in expenditure.
However, an increase in the number of dementia patients due to population ageing is to be expected, together with gradual growth in the percentage of dementia patients receiving treatment.28 Both situations lead us to believe that the trend found by our study, i.e. increasing consumption of cholinesterase inhibitors and memantine, will continue. This being the case, it is also foreseeable that expenditure on these drugs will also increase in the next few years. It is therefore important to continue performing cost-benefit studies analysing the role of AChEI and memantine in the control of symptoms in different dementia stages. These studies should assess how these drugs contribute to reducing use of neuroleptics and to delaying disease progression and institutionalisation.
FundingThis study has not received funding from any sources.
Conflicts of interestThe authors have no conflicts of interest to declare.
Directorate General for Pharmacy and Healthcare Products, Region of Madrid.
Juan José de la Cruz Troca, Universidad Autónoma de Madrid.
Please cite this article as: de Hoyos-Alonso MC, Tapias-Merino E, Meseguer Barros CM, Sánchez-Martínez M, Otero A. Evolución del consumo de fármacos específicos para la demencia en la comunidad autónoma de Madrid durante el periodo 2002-2012. Neurología. 2015;30:416–424.
This article has not been submitted to any other Spanish or international journals. Our results have not been published either totally or partially. Part of this study was presented at the 18th annual meeting of the Spanish Psychogeriatric Society, held in Madrid in October 2011 (partial data as of 2010). Part of this study was also presented at the 34th Congress of the Spanish Society of Primary Care Doctors (SEMERGEN) in September 2012 (partial data as of 2011).