Pain is highly prevalent in patients with multiple sclerosis (MS); it is chronic in 50% of cases and is classified as nociceptive, neuropathic, or mixed-type. Pain affects quality of life, sleep, and the activities of daily living. Electrotherapy is an interesting alternative or complementary treatment in the management of pain in MS, with new innovations constantly appearing.
Material and methodsThis study evaluates the effectiveness of treatment with monopolar dielectric transmission of pulsed electromagnetic fields (PEMF) for pain associated with MS. We performed a randomised, placebo-controlled clinical trial including 24 patients, who were assessed with the Brief Pain Inventory, the Multiple Sclerosis International Quality of Life questionnaire, the Beck Depression Inventory, and the Modified Fatigue Impact Scale.
ResultsStatistically significant improvements were observed in maximum and mean pain scores, as well as in the impact of pain on work, personal relationships, and sleep and rest. Not significant differences were found between the treatment and placebo groups.
ConclusionsTreatment with PEMF may be effective in reducing pain in patients with MS, although further research is necessary to confirm its effectiveness over placebo and to differentiate which type of pain may be more susceptible to this treatment.
El dolor presenta elevadas tasas de prevalencia en la población con Esclerosis Múltiple (EM), siendo el 50% de carácter crónico y clasificándose en tipo nociceptivo, neuropático o mixto. Afecta a la calidad de vida, al sueño y a las actividades de la vida diaria. La electroterapia se presenta como una interesante alternativa o complemento en el abordaje del dolor en EM, presentando constantes innovaciones.
Material y métodosEste estudio valora la eficacia de la terapia por señales electromagnéticas pulsadas y transmitidas de manera dieléctrica monopolar (SEDM) en procesos dolorosos asociados a EM mediante un ensayo clínico aleatorizado sobre 24 pacientes a los que se les medirá el dolor mediante BPI (Brief Pain Inventory), la calidad de vida con MusiQol, la depresión con BECK y la fatiga mediante MFIS.
ResultadosSe hallaron mejoras estadísticamente significativas en puntuaciones máximas y medias de dolor, así como en interferencia del dolor a nivel laboral, a nivel de relaciones personales, del sueño y el descanso. Las diferencias entre grupo de intervención y placebo no fueron significativas.
ConclusionesEl tratamiento con SEDM podría ser efectivo para reducir el dolor en pacientes con EM, siendo necesarios más estudios para mostrar su efectividad respecto al placebo y diferenciar en qué tipología de dolor puede ser más eficaz.
Multiple sclerosis (MS) is a neurodegenerative disease characterised by demyelination, axonal loss, and inflammation of the central nervous system. Although a wide variety of symptomatic and immunomodulatory therapies exist, the disease is still not curable as its aetiology is not fully understood.1–3 MS affects young and middle-aged adults, and is the leading non-traumatic cause of neurological disability in Spain.4
Prevalence studies estimate that 26% to 86% of patients with MS experience pain,5–7 which is chronic in at least 50%.6 Pain negatively impacts a variety of functional domains, including sleep, occupational and recreational activities, and activities of daily living in general, thereby worsening quality of life.8–10
Systems have been proposed that classify pain associated with MS according to the underlying pathophysiological mechanism6,7,11:
- -
Neuropathic pain: dysaesthesia caused by a lesion or disease of the nervous system.
- -
Nociceptive pain: musculoskeletal pain associated with actual or potential tissue injury that responds to conventional sensory mechanisms.
- -
Mixed pain: painful tonic spasms and muscle pain associated with spasticity.
Conventional treatments for pain in MS include analgesics, antiepileptics, antidepressants, opiates, and cannabinoids, which frequently do not achieve satisfactory results and increase the number of medical consultations, as well as decreasing patients’ satisfaction with their pain care.11 However, managing pain in MS requires assessment of the type, intensity, and cause of pain. Given its complexity and multifactorial origin, different strategies are required for effective treatment12; therefore, it may be beneficial to complement pharmacological treatment with physiotherapy.13
Physiotherapy frequently combines different approaches, including physical conditioning, improving postural compensations, therapeutic education, and strictly analgesic techniques, such as electrotherapy. Electrotherapy consists of the use of electricity and its derivative effects (thermotherapy, magnetic therapy, electrical stimulation, etc) for treatment purposes; several studies have shown the effectiveness of such techniques as transcutaneous electrical nerve stimulation (TENS) for alleviating pain in patients with MS.14–17 New, cutting-edge electroanalgesic techniques represent a significant step forward in terms of the transmission mechanism and the amount of energy emitted safely. One such technique is monopolar dielectric transmission of pulsed electromagnetic fields (PEMF), which combines various physiological effects by applying electromagnetic waves that are digitally modulated and adapted in terms of intensity, frequency, form, and duration in order to increase the nociceptive threshold,18,19 reduce inflammation,18,19 release endorphins,20,21 and locally clear proinflammatory and algogenic substances.22,23 These waves are administered transcutaneously by dielectric transfer and monopolar application,24,25 creating focused energy deposits25 in the areas affected by pain.
To date, no study has addressed the effectiveness of PEMF therapy for treating pain in MS patients, and only clinical experience on its use for neuropathic pain is available. Therefore, pilot studies are needed to assess its usefulness. If PEMF therapy is shown to be effective in patients with MS-associated pain, then it will represent a new, non-invasive tool with no apparent adverse reactions, which would complement the management of pain in MS patients.
The main aim of this study is to analyse the effects of PEMF therapy for the treatment of pain in patients with MS-associated painful processes. As a secondary objective, we analyse the potential effects of reducing pain on different aspects of patients’ lives, such as depression and fatigue.
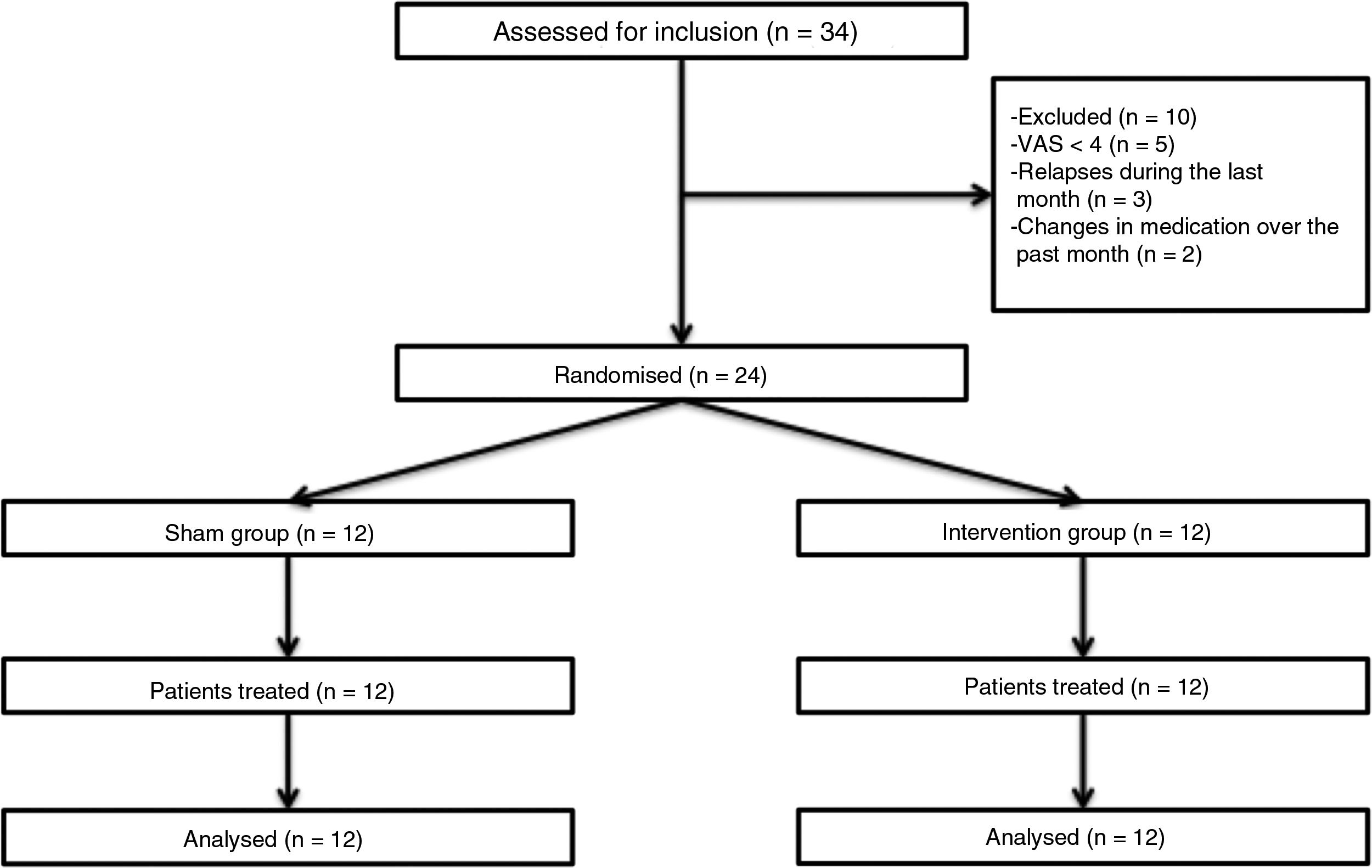
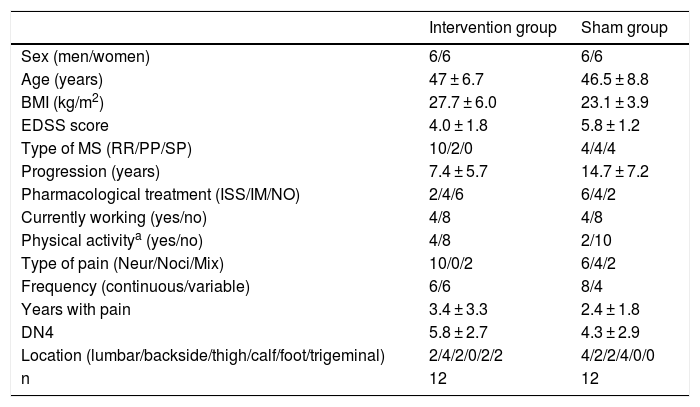
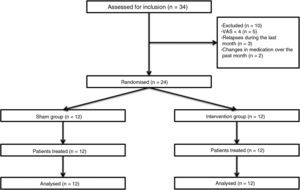
Patients and methodsWe performed a double-blind pilot study to analyse the effect of PEMF therapy in the management of pain in MS patients. We selected a sample of 34 patients attended at a neurological physiotherapy department (the hospital is not named to protect anonymity); after application of our inclusion and exclusion criteria, the final sample included 24 of these patients (Table 1).
Descriptive statistics for our sample.
| Intervention group | Sham group | |
|---|---|---|
| Sex (men/women) | 6/6 | 6/6 |
| Age (years) | 47 ± 6.7 | 46.5 ± 8.8 |
| BMI (kg/m2) | 27.7 ± 6.0 | 23.1 ± 3.9 |
| EDSS score | 4.0 ± 1.8 | 5.8 ± 1.2 |
| Type of MS (RR/PP/SP) | 10/2/0 | 4/4/4 |
| Progression (years) | 7.4 ± 5.7 | 14.7 ± 7.2 |
| Pharmacological treatment (ISS/IM/NO) | 2/4/6 | 6/4/2 |
| Currently working (yes/no) | 4/8 | 4/8 |
| Physical activitya (yes/no) | 4/8 | 2/10 |
| Type of pain (Neur/Noci/Mix) | 10/0/2 | 6/4/2 |
| Frequency (continuous/variable) | 6/6 | 8/4 |
| Years with pain | 3.4 ± 3.3 | 2.4 ± 1.8 |
| DN4 | 5.8 ± 2.7 | 4.3 ± 2.9 |
| Location (lumbar/backside/thigh/calf/foot/trigeminal) | 2/4/2/0/2/2 | 4/2/2/4/0/0 |
| n | 12 | 12 |
DN4: neuropathic pain questionnaire; EDSS: Expanded Disability Status Scale; IM: immunomodulator; ISS: immunosuppressant; Mix: mixed pain; MS: multiple sclerosis; n: total number of patients; Neur: neuropathic; NO: no pharmacological treatment; Noci: nociceptive; PP: primary progressive; RR: relapsing-remitting; SP: secondary progressive.
Inclusion criteria were age between 18 and 60, diagnosis of MS according to the 2005 revised McDonald criteria, Expanded Disability Status Scale score ≤ 7, and pain perception of ≥ 4 on the Visual Analogue Scale (VAS); all patients gave informed consent to participate in the study. We excluded patients who were receiving another physiotherapy intervention for pain, who had received oncological treatment in the past year, who had pacemakers, who presented a relapse or changed medication during the study period or in the 30 days prior, and those patients with comorbidities associated with pain (Fig. 1).
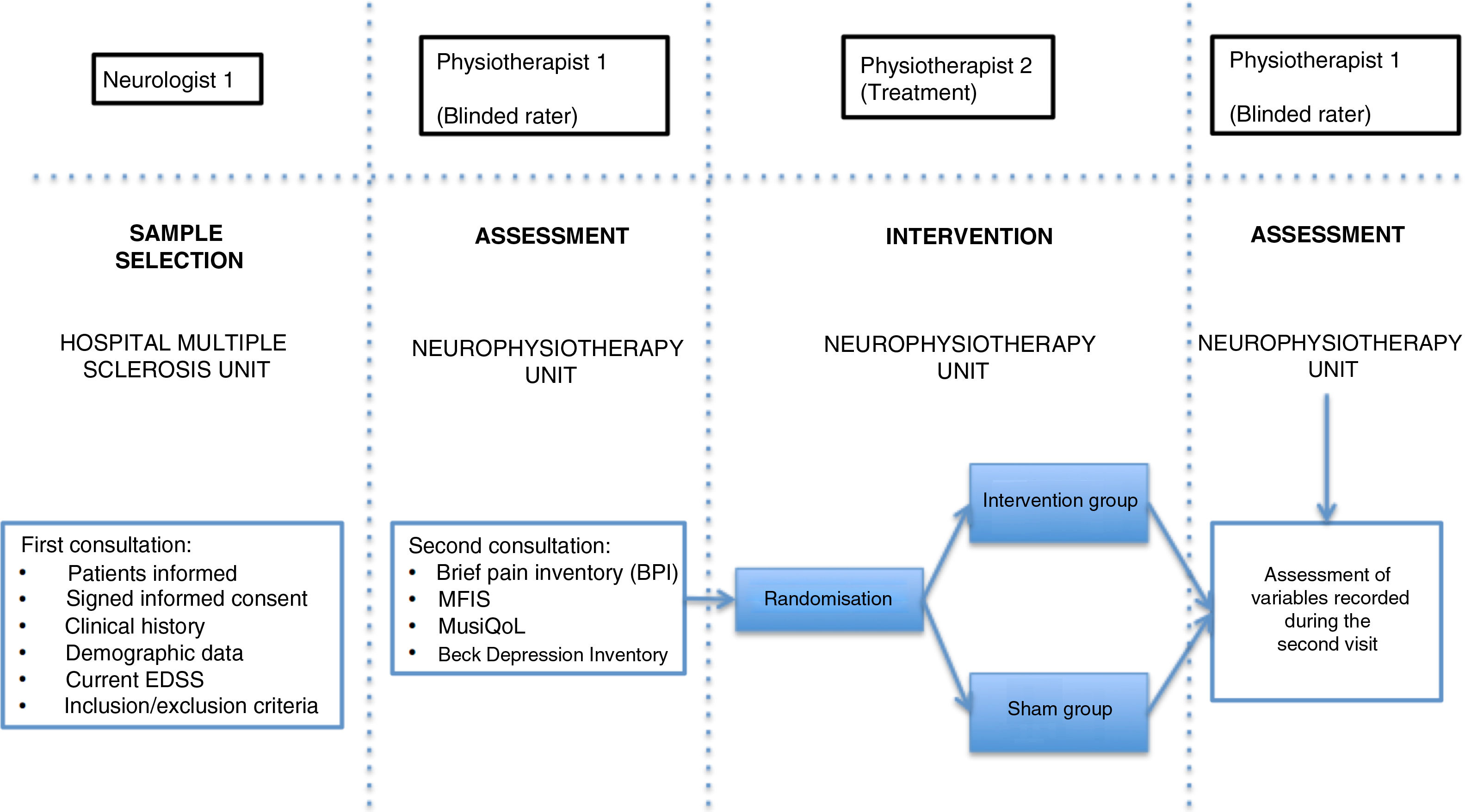
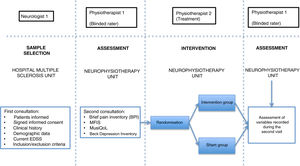
Patients were assessed by physiotherapist 1, who was blinded before starting the intervention. They were then randomly allocated to the intervention group (active treatment) or sham group (sham treatment) with version 1.1 of the Glaxo Wellcome C4-SDP software for Windows.
To differentiate the type of pain, we used the DN4 questionnaire, which includes a series of characteristic signs of neuropathic pain.26 Data on pain were gathered with the Brief Pain Inventory (BPI) questionnaire, which includes various categories on pain variability throughout the day (worst, average, and least pain), as well as the impact of pain on different aspects of daily living (relationships with other people, normal work, enjoyment of life, etc).27 We also used the validated Spanish-language version of the Multiple Sclerosis International Quality of Life (MusiQoL) questionnaire, which assesses patients’ quality of life through 31 multidimensional questions, with a maximum score of 100 points.28 Depression was quantified with the validated Spanish-language version of the Beck Depression Inventory version II (BDI-II), which uses 21 questions, each with 4 possible responses, to determine how the patient has been feeling in the last 2 weeks; depression is classified as minimal, mild, moderate, or severe.29 Fatigue was assessed with the Spanish-language version of the Modified Fatigue Impact Scale (MFIS), which includes 3 subscales (physical, cognitive, and psychosocial fatigue) with 4 possible answers per question.30
In the intervention phase, physiotherapist 1 conducted patient interviews and thorough examinations to establish their medical history and the type of pain (neuropathic, nociceptive, or mixed), classifying pain according to the DN4 and BPI questionnaire criteria. As well as pain, fatigue was assessed with the self-administered MFIS questionnaire, quality of life with the MusiQoL questionnaire, and depression with the BDI-II questionnaire (Fig. 2). Record sheets were completed for each patient at each session to record any adverse effects presenting during the intervention.
In the intervention, we used a PEMF unit (Physicalm®) provided by Biotronic Advance Develops® (Granada, Spain), with pulsed emission in frequency ranges of 800−900 kHz and peak intensity of 30 V. A second professional (physiotherapist 2) performed the intervention in an air-conditioned room separated from the neurological physiotherapy room of the MS unit. Treatment consisted of 15 sessions of PEMF (5 days per week for 3 weeks) lasting 20 minutes each. PEMF was administered transcutaneously using a flat, rounded applicator measuring 4 cm in diameter, applied to the painful area with longitudinal and transverse movements; 5 mL of 100% pure sweet almond oil (a dielectric material)31 was applied to the skin. Sham treatment used the same procedure, but the applicator did not emit any electromagnetic signal, despite the green light being lit.
Given the lack of previous studies on this therapy, we could not calculate the appropriate sample size. Data from our study will help establish sample sizes in future studies, based on the differences found between variables.
Data analysisWe tested for normal distribution using the Shapiro-Wilk test. As variables were quantitative and groups were normally distributed, we used the t test for paired samples to compare results between pre- and post-treatment values for the variables in both treatment groups. Subsequently, we used one-way ANOVA to compare the improvement observed between the intervention and the sham group. We also calculated the effect size for each intervention, which enabled us to determine the clinical significance of the treatment effects. To do this, we used the Cohen d statistic, considering a value of 0.2 to 0.49 as a small effect size, 0.5 to 0.79 as a medium effect size, and 0.80 as a large effect size.
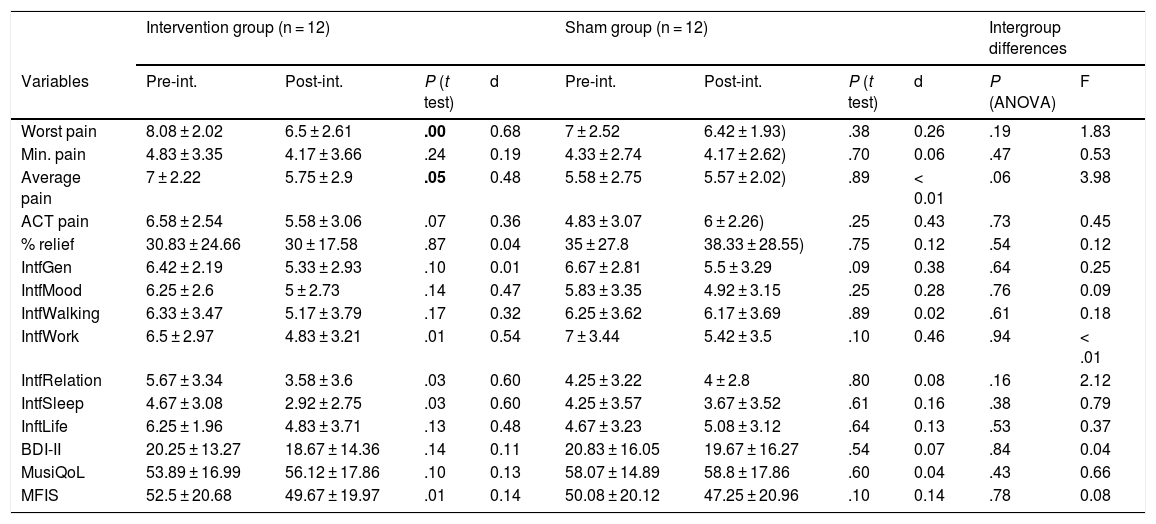
ResultsIn the intervention group, we detected statistically significant differences after treatment for the variables worst pain, average pain, interference with normal work, interference with relationships with others, interference with sleep quality, and fatigue as measured with MFIS (Table 2). Treatment with PEMF showed a medium effect size for the variables worst pain, average pain, interference with normal work, interference with relationships with others, and interference with sleep (Table 2).
Effect size and differences between variables before and after treatment by group.
| Intervention group (n = 12) | Sham group (n = 12) | Intergroup differences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Pre-int. | Post-int. | P (t test) | d | Pre-int. | Post-int. | P (t test) | d | P (ANOVA) | F |
| Worst pain | 8.08 ± 2.02 | 6.5 ± 2.61 | .00 | 0.68 | 7 ± 2.52 | 6.42 ± 1.93) | .38 | 0.26 | .19 | 1.83 |
| Min. pain | 4.83 ± 3.35 | 4.17 ± 3.66 | .24 | 0.19 | 4.33 ± 2.74 | 4.17 ± 2.62) | .70 | 0.06 | .47 | 0.53 |
| Average pain | 7 ± 2.22 | 5.75 ± 2.9 | .05 | 0.48 | 5.58 ± 2.75 | 5.57 ± 2.02) | .89 | < 0.01 | .06 | 3.98 |
| ACT pain | 6.58 ± 2.54 | 5.58 ± 3.06 | .07 | 0.36 | 4.83 ± 3.07 | 6 ± 2.26) | .25 | 0.43 | .73 | 0.45 |
| % relief | 30.83 ± 24.66 | 30 ± 17.58 | .87 | 0.04 | 35 ± 27.8 | 38.33 ± 28.55) | .75 | 0.12 | .54 | 0.12 |
| IntfGen | 6.42 ± 2.19 | 5.33 ± 2.93 | .10 | 0.01 | 6.67 ± 2.81 | 5.5 ± 3.29 | .09 | 0.38 | .64 | 0.25 |
| IntfMood | 6.25 ± 2.6 | 5 ± 2.73 | .14 | 0.47 | 5.83 ± 3.35 | 4.92 ± 3.15 | .25 | 0.28 | .76 | 0.09 |
| IntfWalking | 6.33 ± 3.47 | 5.17 ± 3.79 | .17 | 0.32 | 6.25 ± 3.62 | 6.17 ± 3.69 | .89 | 0.02 | .61 | 0.18 |
| IntfWork | 6.5 ± 2.97 | 4.83 ± 3.21 | .01 | 0.54 | 7 ± 3.44 | 5.42 ± 3.5 | .10 | 0.46 | .94 | < .01 |
| IntfRelation | 5.67 ± 3.34 | 3.58 ± 3.6 | .03 | 0.60 | 4.25 ± 3.22 | 4 ± 2.8 | .80 | 0.08 | .16 | 2.12 |
| IntfSleep | 4.67 ± 3.08 | 2.92 ± 2.75 | .03 | 0.60 | 4.25 ± 3.57 | 3.67 ± 3.52 | .61 | 0.16 | .38 | 0.79 |
| InftLife | 6.25 ± 1.96 | 4.83 ± 3.71 | .13 | 0.48 | 4.67 ± 3.23 | 5.08 ± 3.12 | .64 | 0.13 | .53 | 0.37 |
| BDI-II | 20.25 ± 13.27 | 18.67 ± 14.36 | .14 | 0.11 | 20.83 ± 16.05 | 19.67 ± 16.27 | .54 | 0.07 | .84 | 0.04 |
| MusiQoL | 53.89 ± 16.99 | 56.12 ± 17.86 | .10 | 0.13 | 58.07 ± 14.89 | 58.8 ± 17.86 | .60 | 0.04 | .43 | 0.66 |
| MFIS | 52.5 ± 20.68 | 49.67 ± 19.97 | .01 | 0.14 | 50.08 ± 20.12 | 47.25 ± 20.96 | .10 | 0.14 | .78 | 0.08 |
ACT: pain during general activity; aver.: average; Beck: Beck Depression Inventory; d: effect size, Cohen’s d; F: ratio between groups (ANOVA); IntfGen: general interference; IntfLife: interference with life; IntfMood: interference with mood; IntfRelation: interference with relationships; IntfSleep: interference with sleep; IntfWalking: interference with walking; IntfWork: interference with work; MFIS: fatigue measured with MFIS; min.: minimum; MusicQoL: quality of life; Post-int.: after intervention; Pre-int.: before intervention; % relief: percentage of relief.Figures in bold denote statistically significant differences (P < .05).
However, we found no significant differences between groups in the improvements observed (Table 2).
Regarding type of pain, we found no statistically significant differences between the different subgroups (nociceptive, neuropathic, or mixed) (P < .05 for all variables).
DiscussionConsidering the high cost of providing basic care and maintaining the independence and quality of life of patients with MS, both for the public health system and for patients and their family members, physiotherapy addressing these aspects is particularly relevant in these patients. If we also consider the frequent absences from work or inability to work, there is a clear need for research in various healthcare sectors in order to improve the management of MS patients. The implementation of new electrotherapy technologies improves the quality and variety of treatments that may be performed by physiotherapists during interventions for MS patients, with the validation of new techniques against pharmacological6 and non-pharmacological14 alternatives being necessary before they are included in treatment guidelines.
No previous studies address the effectiveness of PEMF therapy for treating pain in MS patients. Studies analysing non-pharmacological alternatives focus on physiotherapy and patient education for better management of fatigue.14 With regard to physiotherapy, low- and high-frequency TENS therapy shows particular effectiveness in treating pain,14,17 and is recommended by Sawant et al.17 to treat central pain in MS due to its infrequent side effects. The studies conducted by Al-Smadi et al.16 and Warke et al.15 are of good methodological quality; however, therapists were not blinded, which could partially compromise results. Therefore, we ensured patient, therapist, and assessor blinding in the present study. We also used more precise measurement tools than the VAS, including the BPI questionnaire, which provides data on pain variability throughout the day and its influence on the different aspects of the patients’ life; this has not been considered in other studies.15,16 In terms of secondary variables, we also assessed the possible effects on patients’ fatigue (using the MFIS questionnaire), depression (BDI-II), and quality of life (MusiQoL questionnaire); the effect was very small or non-existent.
Compared with TENS therapy, PEMF therapy better focuses its action, with greater depth of emission when using a mechanism of dielectric transmission and monopolar application rather than a bipolar conductive or resistive unit, such as a conventional electrotherapy system.25 This may explain the better pain management results obtained in patients with MS in this study compared with those reviewed by Sawant et al.17 for patients receiving TENS treatment; however, the scarce evidence on PEMF limits the comparison of results. Regarding quality of life, we found no significant improvements, as other authors have reported with TENS.15 We also observed no differences in fatigue and depression variables before and after treatment; this finding could not be compared against those reported in other articles, as we have found no other studies on electrotherapy that assess these variables.
Despite significant improvements in pain variables in the PEMF intervention group, we could not show that these differences were significantly greater than those observed in the sham group. However, intergroup differences in such variables as average pain were nearly significant (P = .058); therefore, we believe that a larger sample size may yield significant values in some variables.
In the light of the data contributed by this study, caution should be applied when determining the effectiveness of PEMF therapy for treating pain in MS. PEMF therapy seems to be effective for treating both perceived worst pain and average pain, and the improvement in pain seems to positively influence the negative effects of MS on social relationships (which seem to improve, potentially due to decreased irritability and functional improvement) and on rest and restorative sleep, with the treatment showing a medium-sized effect on both. This is especially relevant given that patients with better sleep quality present better pain outcomes32; other studies have not shown significant differences in sleep quality in patients receiving TENS.15,16 We also observed improvements in interference with normal work, although similar changes were observed in the sham group. No remarkable effect size was observed for the improvements in fatigue; therefore, a larger sample size is needed to determine the effect of the treatment on this variable; this improvement in fatigue was significant, however.
However, no significant differences were observed between improvements in the different types of pain: neuropathic, nociceptive, and mixed (P = .71). This may be because our sample was too small to reflect differences, or because PEMF therapy acts on mechanisms common to all 3 types of pain. It also remains to be determined whether changing the application pattern and increasing the duration of exposure to the therapy may have achieved significant differences between the different types of pain.
One of the main factors supporting the use of such electrotherapy systems as TENS in pain management is the absence of adverse reactions when compared to pharmacological treatment; the same seems to be true of PEMF therapy, since no such reaction was observed during our study.
Despite the positive results, due to the small sample size available in the unit where this study was performed, there is a need to increase the number of participants and to create larger treatment subgroups according to pain type and location. The statistically significant results recorded, despite the limited number of participants, suggest that PEMF may be an effective alternative to prevent higher polypharmacy rates in patients with MS; therefore, further studies should determine whether the trend towards significance observed for some variables is confirmed in the intergroup comparison. Our study represents a good start, considering the lack of previous studies on PEMF therapy; our results point to the need to further increase our knowledge on the subject.
Based on these results, we may state that PEMF therapy improves pain in patients with MS-associated painful processes, although it remains to be determined whether it is more effective than sham or other treatments. No clear effects were observed for the interference of pain in daily life, depression, or fatigue. Further studies are needed to clarify these facts and to differentiate effectiveness by pain type.
Ethical considerationsThis study complies with the principles of the Declaration of Helsinki, and was developed according to protocol and in compliance with good clinical practice guidelines, as described by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); it was approved by the Ethics Committee of the Hospital Virgen de la Macarena (Seville, Spain).
This study involves no additional risk for patients beyond the limits of normal clinical practice. Participants were informed of the study characteristics, measurements, aims, and procedures. The researcher responded to all patients’ queries and informed them that their participation was voluntary. Once the researcher confirmed that the patient had correctly understood the process, participants were given informed consent forms to be signed prior to their inclusion in the study, authorising the inclusion of their data.
Data protectionThe research team conducted the study in accordance with protocol, preserving the confidentiality of patient data and ensuring anonymisation; patients were informed according to Spanish legislation (Organic Law 15/1999 of 13 December and Royal Decree 1720/2007). Patient data obtained during the study are confidential and were used exclusively for research purposes.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Hochsprung A., Escudero-Uribe S., Ibáñez-Vera A.J., Izquierdo-Ayuso G.. Eficacia de la terapia con señales electromagnéticas pulsadas y transmitidas de manera dieléctrica monopolar en procesos dolorosos asociados a esclerosis múltiple. Estudio piloto. Neurología. 2021;36:433–439.