Acetone cyanohydrin (ACH) is a toxic substance present in cassava roots (Manihot esculenta Crantz) which results from enzymatic hydrolysis of linamarin. Long-term consumption is associated with 2 neurological disorders: konzo and tropical ataxic neuropathy. Previous studies have evaluated behavioural alterations linked to ACH consumption, but the toxic effects of this substance on physiological processes remain unknown.
Method32 male Wistar rats were assigned to 4 experimental groups (n=8 per group): a vehicle group (0.3mL saline solution, IP) and 3 ACH groups (PubChem CID: 6406) dosed at 10, 15, and 20mM/24hours for 28 days. We evaluated spontaneous motor activity with the open field test and motor coordination with the rotarod and forced swimming tests at 0, 7, 14, 21, and 28 days of treatment. At the end of the assessment period (day 28), blood samples were collected by transcardiac puncture to evaluate kidney and liver function.
ResultsACH caused alterations in locomotor activity and promoted both lateral swimming and spinning in the forced swimming test at 21 and 28 days of treatment. Furthermore, it led to an increase in the levels of the parameters of kidney and liver function in a concentration-dependent manner, except for glucose and total bilirubin.
ConclusionOur results suggest that long-term consumption of this toxic compound present in cassava roots may be potentially dangerous for vulnerable subjects.
La acetona cianohidrina (ACH) es una sustancia tóxica resultante de la hidrólisis enzimática de linamarina, contenido en las raíces de yuca (Manihot esculenta Crantz); su consumo a largo plazo se asocia con 2 trastornos neurológicos: konzo y la neuropatía atáxica tropical. Estudios anteriores han evaluado las alteraciones conductuales después del consumo de esta sustancia, pero los efectos tóxicos sobre los procesos fisiológicos se desconocen.
MétodoSe asignaron 32 ratas Wistar macho a 4 grupos experimentales (n=8): un grupo vehículo (solución salina 0,3ml/rata, ip) y 3 grupos con ACH (PubChem CID: 6406) a concentraciones de 10, 15 y 20mM, durante 28 días, cada 24h. Se evaluó la actividad motora espontánea en campo abierto y la coordinación motora en pruebas de rotarod y nado a 0, 7, 14, 21 y 28 días de tratamiento. Al final de las pruebas conductuales (día 28) se tomaron muestras de sangre por punción transcardiaca para evaluar la función renal y hepática.
ResultadosLa ACH promovió alteraciones en la actividad locomotora y promovió tanto el nado lateral como la conducta de giro en la prueba de nado los días 21 y 28 del tratamiento. La ACH incrementó los parámetros de la función renal y hepática de una manera dependiente de la concentración, excepto la glucosa y la bilirrubina total.
ConclusiónEstos datos indican que el contenido de este compuesto tóxico contenido en las raíces de yuca podría ser potencialmente peligroso bajo el consumo a largo plazo en sujetos vulnerables.
Cassava (Manihot esculenta Crantz, from the family Euphorbiaceae) is a plant native to Latin America and the Caribbean. It was introduced to Africa and Asia due to its wide use in the food industry, its versatility, and ease of cultivation. Cassava is considered to be of great nutritional value due to its high carbohydrate content.1,2 The plant is widely grown in South America and Mexico, especially in the Mexican states of Guerrero, Tabasco, Michoacán, Morelos, and Veracruz.3 Cassava contains 2 cyanogenic compounds, linamarin and lotaustralin, whose metabolism produces cyanide. The enzyme linamarase, contained in the cell wall, hydrolyses linamarin, forming highly toxic metabolites including acetone cyanohydrin (ACH).4 In such products as cassava flour, ACH is unstable and may spontaneously decompose into acetone and hydrogen cyanide.5 Our research group has previously reported a close correlation between locomotor impairment and the chemical compounds contained in cassava.6–9 These experimental findings support epidemiological evidence of the association between excessive consumption of cassava or cassava derivatives and such central nervous system diseases as tropical ataxic neuropathy and konzo.10
Histological studies of tissue samples from male Wistar rats have shown that both cyanide and cyanogenic glycosides cause toxicity after long-term consumption of cassava juice; these compounds cause vacuolation of hepatocytes, alterations in the glomeruli, and renal tubule degeneration or necrosis.11 However, the effect of long-term ACH administration on kidney and liver function and its association with locomotor impairment are yet to be explored. The present study focuses on the effects of different concentrations of ACH on kidney and liver function, as well as the association between the compound's hepatotoxic and nephrotoxic effects and spontaneous motor activity in Wistar rats.
Study designAnimals, ACH concentrations, and experimental groupsThe study included 32 male Wistar rats weighing 250-300g at the beginning of the study. Rats were housed in acrylic cages at 25±2°C with a 12:12 light-dark cycle (lights on at 7:00 AM). They had ad libitum access to water and food. All experiments were conducted in accordance with international12 and Mexican guidelines for the care and use of laboratory animals (NOM-062-ZOO-1999).13 We made every effort to minimise the number of experimental animals used.
ACH (PubChem CID: 6406) concentrations were established based on previous studies reporting neuronal damage in the brain and spinal cord with the administration of 10 and 20mM ACH.14 We used these concentrations plus an intermediate concentration (15mM) to explore the effects of ACH on kidney and liver function and on spontaneous motor activity.
The study included a vehicle group (rats receiving 0.9% saline solution) plus the 3 experimental groups (10, 15, and 20mM ACH). Substances were administered every 24hours for 28 consecutive days, at a volume of 0.3mL/rat, as in the study by Machholz et al.15 We conducted daily check-ups and recorded rats’ body weights every 3 days. Rats performed behavioural tests every 7 days, from experimental day 0 (baseline) to experimental day 28. Blood samples were obtained by cardiac puncture on day 28 and used to evaluate biochemical parameters and liver function.
Behavioural testsLocomotor activity testEach rat was placed for 5minutes inside an opaque acrylic cage (44×33×20cm) whose base was divided into squares measuring 11×11cm. We assessed the following: 1) number of squares crossed (the rat was considered to have crossed a square when at least three-quarters of its body passed from one square to another); and 2) number of vertical behaviours (number of times when the rat stood on its hind legs). Crossed squares were an indicator of spontaneous motor activity; vertical behaviour was used to detect any alterations in motor coordination.16
Rotarod testBefore receiving ACH or the vehicle, rats were trained on 5 consecutive days on a rotarod (LE8300; Letica LSI, Panlab Scientific Instruments, Barcelona, Spain) at a speed of 18 rotations per minute. We recorded latency to fall (time elapsed from the moment the rat was placed on the rod to the moment it fell) on experimental days 0, 7, 14, 21, and 28. This variable is used to identify any alterations in motor coordination and balance.17
Forced swim testRats were placed in a glass tank (26×29×50cm) filled with water at a temperature of 25±1°C for 5minutes. The water level was such that rats could touch the bottom of the tank with their hind feet and tails. At the beginning of the test, the rat was placed in one corner of the tank. All rats swam vigorously as soon as they were placed in the water. No rat drowned. We evaluated the number of times rats displayed lateral swimming and spinning behaviour, 2 indicators of poor motor coordination.6 Lateral swimming is defined as a behaviour in which a rat swims slowly on one side rather than maintaining horizontal balance. Rats swim either on their right or their left side with the head remaining horizontal. The hind legs are extended, rigid, and parallel to the surface of the water for short periods of time, making uncoordinated movements to move through the water. One or both front legs remain flexed. After lateral swimming, rats eventually display normal swimming behaviour for short periods of time. Spinning behaviour is defined as a behaviour in which a rat does not move across water but rather spins on its own axis.6,16
Video footage was recorded during all sessions of the locomotor activity and forced swim tests. Two independent observers quantified locomotor activity test variables until reaching a concordance of at least 95%. In the forced swim test, the variable “number of spins” was quantified by analysing video feeds with a software tool (ANY-maze 4.73; Stoelting, Wood Dale, IL, USA).
Blood collectionBlood samples were obtained on day 28, after all behavioural tests were completed, using a 5-mL syringe with a 22-mm needle. Rats were deeply anaesthetised with pentobarbital sodium and placed in the supine position. We located the point of the strongest pulse on the chest and inserted the syringe into the heart at an angle of 20° to 30° through the lateral thoracic wall and the intercostal space. The needle was moved slowly, generating a slight negative pressure in the syringe and aspirating gently until the flow of blood stopped. Blood samples were stored without anticoagulant in Vacutainer tubes (BD Vacutainer; Mexico City, Mexico) until the biochemical analysis was performed.18
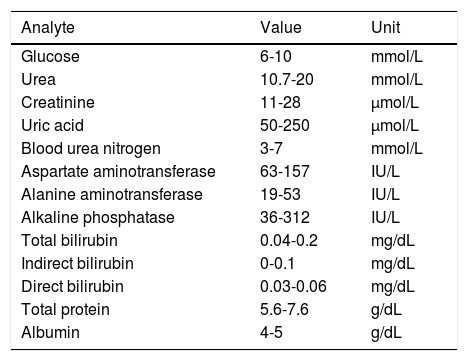
Biochemical studyThe biochemical study evaluated kidney (glucose, urea, creatinine, uric acid, blood urea nitrogen) and liver function (glutamate-oxaloacetate transaminase, glutamic-pyruvic transaminase, alkaline phosphatase, bilirubin, total protein, albumin). Blood samples were stored in Vacutainer tubes without anticoagulant, allowing blood to clot. They were then centrifuged at 3500rpm for 5minutes to obtain serum, which was immediately transferred with a pipette to the containers allocated for dry chemistry analysis (Vitros 250; Johnson & Johnson, Ramsey, MN, USA). We compared our results with normal reference values for each test to detect any alterations in kidney and renal function (Table 1).
Parameters evaluated and normal reference ranges for kidney and liver function tests in Wistar rats.
| Analyte | Value | Unit |
|---|---|---|
| Glucose | 6-10 | mmol/L |
| Urea | 10.7-20 | mmol/L |
| Creatinine | 11-28 | μmol/L |
| Uric acid | 50-250 | μmol/L |
| Blood urea nitrogen | 3-7 | mmol/L |
| Aspartate aminotransferase | 63-157 | IU/L |
| Alanine aminotransferase | 19-53 | IU/L |
| Alkaline phosphatase | 36-312 | IU/L |
| Total bilirubin | 0.04-0.2 | mg/dL |
| Indirect bilirubin | 0-0.1 | mg/dL |
| Direct bilirubin | 0.03-0.06 | mg/dL |
| Total protein | 5.6-7.6 | g/dL |
| Albumin | 4-5 | g/dL |
Data were analysed with the 2-way repeated measures ANOVA for behavioural tests, and one-way ANOVA for biochemical analysis. Statistical significance was set at P≤.05. We compared means with the Student-Newman-Keuls post hoc test. Data are expressed as means±SD. Statistical analysis was performed using the Sigma Stat software, version 3.5.
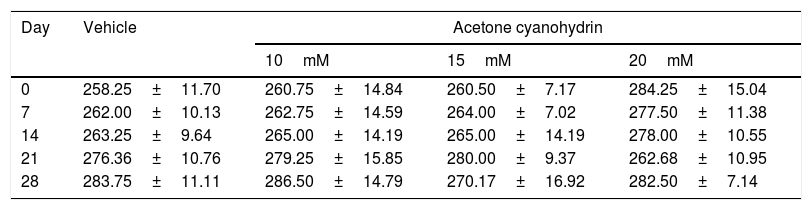
ResultsBody weightTable 2 shows the rats’ body weights at different time points over the study period. Our analysis found no significant differences in body weight for treatment type (F3,132=0.791, P=.501), days of treatment (F4,132=0.661, P=.620), or the interaction of factors (F21,132=0.674, P=.774).
Body weight of the rats included in our study, broken down by treatment received.
| Day | Vehicle | Acetone cyanohydrin | ||
|---|---|---|---|---|
| 10mM | 15mM | 20mM | ||
| 0 | 258.25±11.70 | 260.75±14.84 | 260.50±7.17 | 284.25±15.04 |
| 7 | 262.00±10.13 | 262.75±14.59 | 264.00±7.02 | 277.50±11.38 |
| 14 | 263.25±9.64 | 265.00±14.19 | 265.00±14.19 | 278.00±10.55 |
| 21 | 276.36±10.76 | 279.25±15.85 | 280.00±9.37 | 262.68±10.95 |
| 28 | 283.75±11.11 | 286.50±14.79 | 270.17±16.92 | 282.50±7.14 |
Results are expressed as means±SD. No statistically significant differences were found between factors or in the interaction of factors. Two-way ANOVA and Student-Newman-Keuls post hoc test.
Analysis of the number of squares crossed revealed no significant differences for treatment type (F3,132=0.679; P=.566), but did detect differences for day of treatment (F4,148=7.400; P<.001). The post hoc analysis showed that the number of squares crossed decreased significantly (P<.05) on experimental days 14 (42.06±3.66), 21 (47.23±6.5), and 28 (43.46±5.78) compared to days 0 (77.72±6.67) and 7 (62.41±5.28), whereas on day 7 it was significantly lower only with regard to day 0. An analysis of the interaction of factors did not reveal any significant differences (F12,148=1.443; P=.154).
Vertical behaviourAnalysis of the number of vertical behaviours revealed no significant differences for treatment type (F3,148=0.203; P=.894), but did detect differences for day of treatment (F4,148=2.486; P<.046). The post hoc test showed less vertical time on day 7 (208.68±9.54s) than on day 0 (247.73±7.93); no changes were observed on days 14 (213.37±14.17), 21 (235.95±10.11), or 28 (235.74±10.56) with respect to day 0. An analysis of the interaction of factors did not reveal any significant differences (F12,148=1.581; P=.105).
Rotarod testNo significant differences were observed in latency to fall for treatment group, day of treatment, or the interaction of factors (F128=0.678, P=.567; F148=0.750, P=.701); these data are not shown.
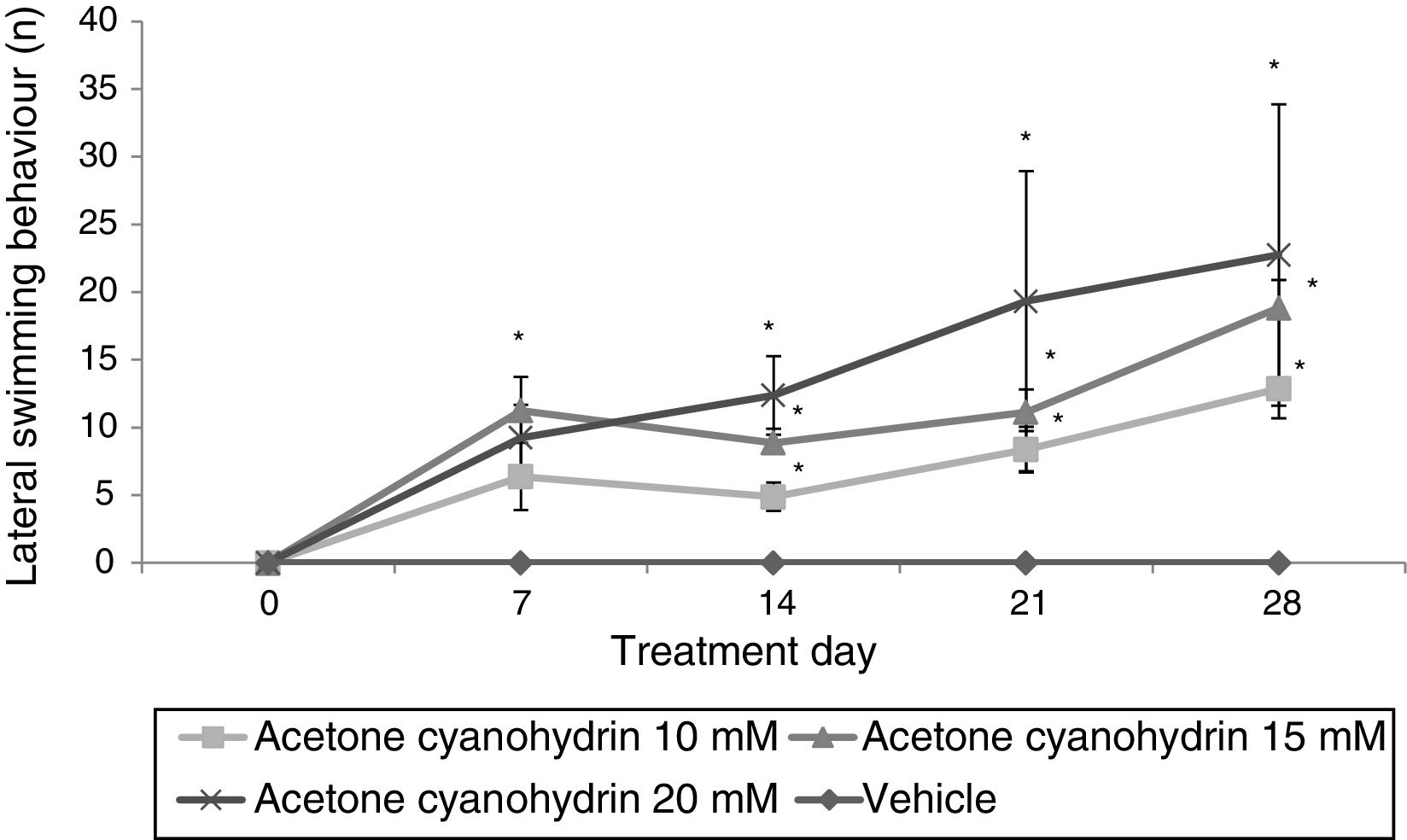
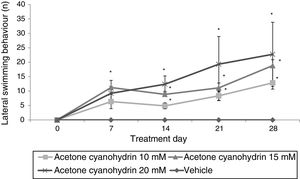
Forced swim testLateral swimmingSignificant differences were found between treatment groups (F3,148=9.592; P<.001). The 3 groups receiving ACH showed lateral swimming behaviour, whereas the vehicle group did not. According to the post hoc test, 15mM and 20mM ACH significantly increased lateral swimming compared to the group receiving 10mM ACH. We also identified significant differences for day of treatment (F4,148=5.654; P<.001). The post hoc test shows that the number of times rats displayed lateral swimming behaviour was significantly larger on days 7 (6.72±1.63), 14 (6.53±1.12), 21 (9.07±2.47), and 28 (11.80±2.94) than on day 0 (P<.05), where no lateral swimming was observed. An analysis of the interaction of factors did not reveal any significant differences (F12,148=1.472; P=.142). According to the post hoc test, however, this variable increased significantly from day 7 in all ACH groups compared to the vehicle group; this effect was more pronounced in the group receiving 20mM ACH (Fig. 1).
Lateral swimming. The rats receiving acetone cyanohydrin displayed lateral swimming significantly more times after treatment than at baseline and significantly more times than rats in the vehicle group on the same treatment day.
*P<.05 vs day 0 and the same day in the vehicle group. Two-way ANOVA, post hoc Student-Newman-Keuls test.
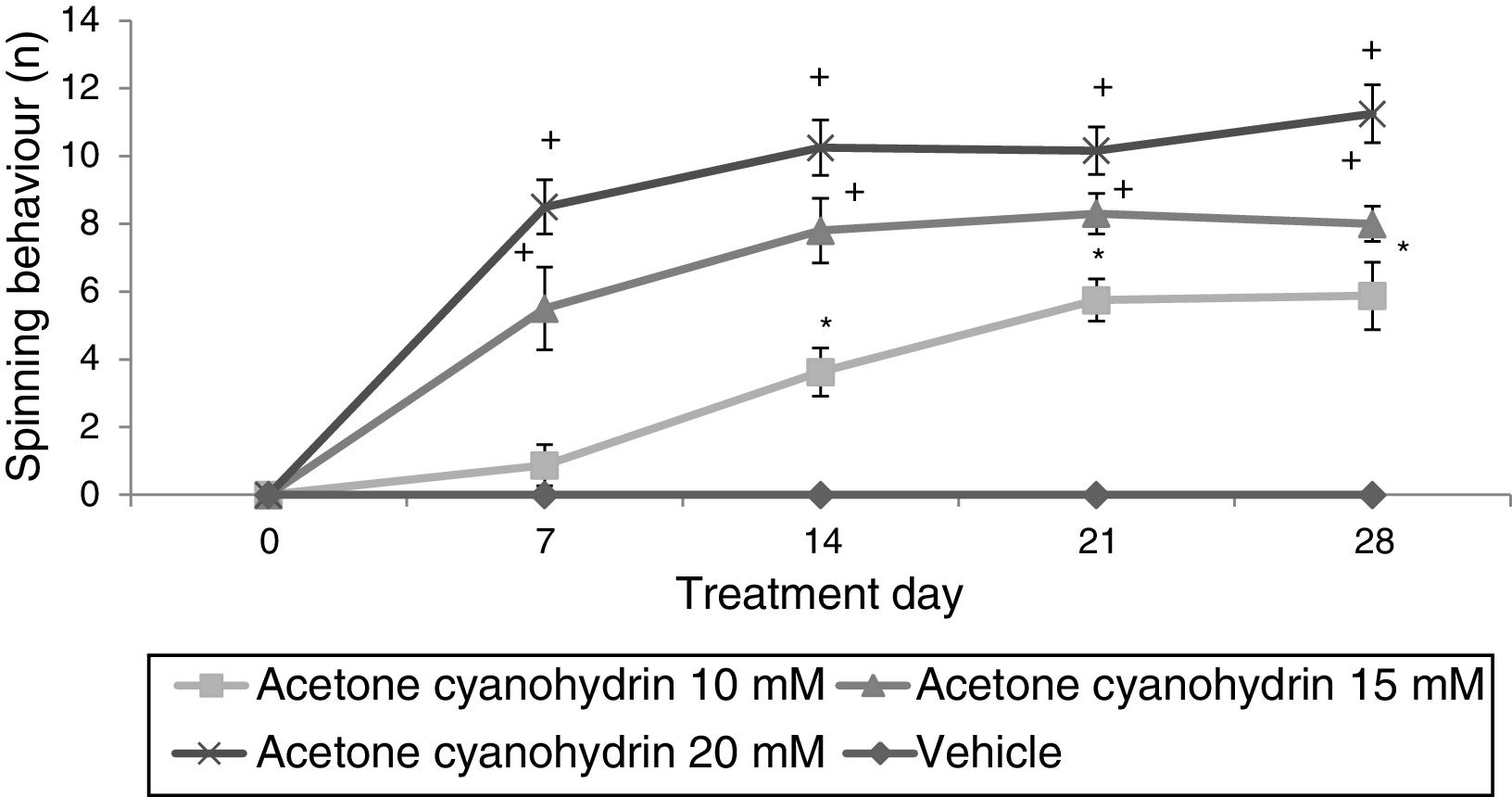
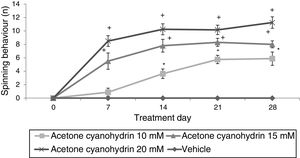
Significant differences in the number of spins were found for treatment type (F3,148=36.943; P<.001), day of treatment (F4,148=4.569; P<.002), and the interaction of factors (F12,148=12.263; P<.001). In the post hoc test, this variable was found to increase significantly in all ACH groups, from day 14 in the 10-mM-ACH group and from day 7 in the 15-mM- and 20-mM-ACH groups compared to day 0 and to the vehicle group on the same treatment day (Fig. 2).
Spinning behaviour. The vehicle group did not show spinning behaviour at any time during the study period, whereas the rats receiving acetone cyanohydrin did display this behaviour in a dose-dependent manner.
*P<.05 vs days 0 and 7 in the same group, and the same treatment day in the vehicle group.
**P<.05 vs day 0 in the same group, and the same treatment day in the vehicle and 10-mM-ACH groups. Two-way ANOVA and Student-Newman-Keuls post hoc test.
The effects of ACH on kidney and liver function are shown in Tables 3 and 4.
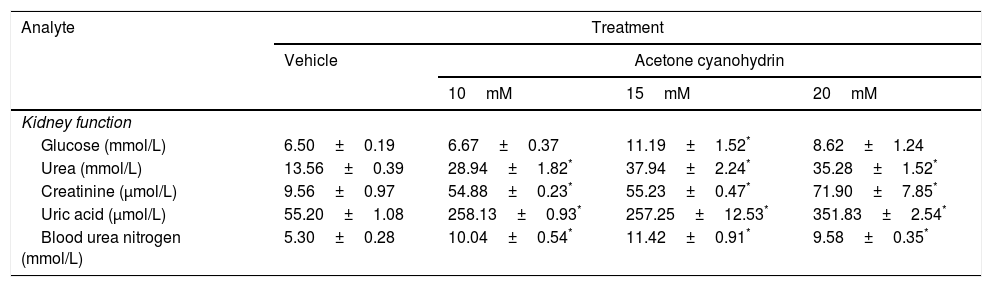
Effects of acetone cyanohydrin on kidney function in Wistar rats.
| Analyte | Treatment | |||
|---|---|---|---|---|
| Vehicle | Acetone cyanohydrin | |||
| 10mM | 15mM | 20mM | ||
| Kidney function | ||||
| Glucose (mmol/L) | 6.50±0.19 | 6.67±0.37 | 11.19±1.52* | 8.62±1.24 |
| Urea (mmol/L) | 13.56±0.39 | 28.94±1.82* | 37.94±2.24* | 35.28±1.52* |
| Creatinine (μmol/L) | 9.56±0.97 | 54.88±0.23* | 55.23±0.47* | 71.90±7.85* |
| Uric acid (μmol/L) | 55.20±1.08 | 258.13±0.93* | 257.25±12.53* | 351.83±2.54* |
| Blood urea nitrogen (mmol/L) | 5.30±0.28 | 10.04±0.54* | 11.42±0.91* | 9.58±0.35* |
Results are expressed as means±SD.
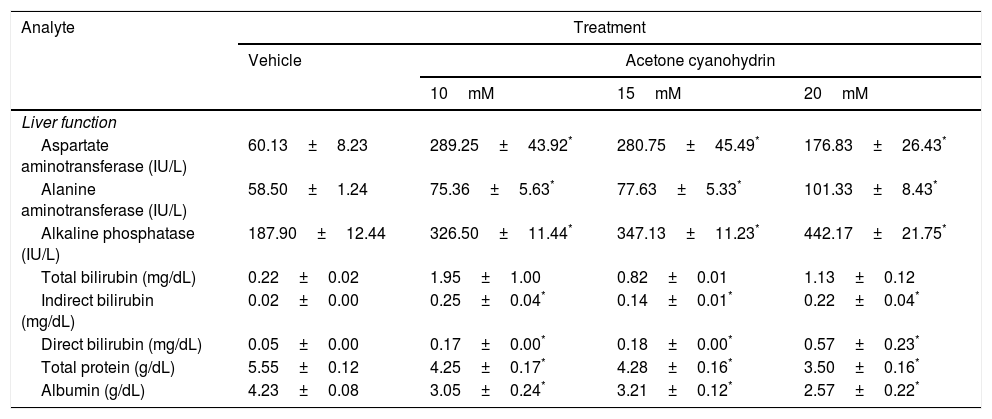
Effects of acetone cyanohydrin on liver function in Wistar rats.
| Analyte | Treatment | |||
|---|---|---|---|---|
| Vehicle | Acetone cyanohydrin | |||
| 10mM | 15mM | 20mM | ||
| Liver function | ||||
| Aspartate aminotransferase (IU/L) | 60.13±8.23 | 289.25±43.92* | 280.75±45.49* | 176.83±26.43* |
| Alanine aminotransferase (IU/L) | 58.50±1.24 | 75.36±5.63* | 77.63±5.33* | 101.33±8.43* |
| Alkaline phosphatase (IU/L) | 187.90±12.44 | 326.50±11.44* | 347.13±11.23* | 442.17±21.75* |
| Total bilirubin (mg/dL) | 0.22±0.02 | 1.95±1.00 | 0.82±0.01 | 1.13±0.12 |
| Indirect bilirubin (mg/dL) | 0.02±0.00 | 0.25±0.04* | 0.14±0.01* | 0.22±0.04* |
| Direct bilirubin (mg/dL) | 0.05±0.00 | 0.17±0.00* | 0.18±0.00* | 0.57±0.23* |
| Total protein (g/dL) | 5.55±0.12 | 4.25±0.17* | 4.28±0.16* | 3.50±0.16* |
| Albumin (g/dL) | 4.23±0.08 | 3.05±0.24* | 3.21±0.12* | 2.57±0.22* |
Results are expressed as means±SD.
Aspartate aminotransferase (AST) levels were significantly different between treatment groups (F3,26=9.59; P<.001). According to the post hoc test, AST levels were higher in all ACH groups after 28 days than in the vehicle group. We also found significant differences in alanine aminotransferase (ALT) levels (F3,26=9.78; P<.001). According to the post hoc test, all ACH concentrations increased ALT levels compared to the vehicle. Significant differences between treatment groups were also observed in alkaline phosphatase (ALP) levels (F3,26=54.75; P<.001). According to the post hoc test, ALP levels were higher in all ACH groups than in the vehicle group.
Significant differences were observed between treatment groups in glucose levels (F3,26=5.39; P<.005). Only 15mM ACH significantly increased glucose levels compared to the vehicle. The analysis also revealed significant differences between treatment groups in the levels of urea (F3,26=44.286; P<.001), creatinine (F3,26=72.18; P<.001), uric acid (F3,26=327.86; P<.001), and blood urea nitrogen (F3,26=20.52; P<.001). The post hoc analysis of these 4 parameters revealed that all 3 ACH concentrations led to three-, six-, seven-, and two-fold increases in urea, creatinine, uric acid, and blood urea nitrogen, respectively, compared to the vehicle (P<.05).
Total protein (F3,26=29.54; P<.001) and albumin levels (F3,26=15.73; P<.001) also showed significant differences between treatment groups. The post hoc test indicated higher total protein and albumin levels in all ACH groups than in the vehicle group. Indirect bilirubin (F3,26=11.759; P<.001) and direct bilirubin levels (F3,26=5.1; P<.007) also showed significant differences between treatment groups. According to the post hoc test, direct and indirect bilirubin levels were higher in all ACH groups than in the vehicle group.
Total bilirubin levels were not significantly different between treatment groups (F3,26=1.90; P=.155), although we did observe a trend towards statistical significance for 10mM and 20mM ACH.
DiscussionWhen studying the toxicity of a chemical substance, it is essential to determine not only the clinical and biological signs of intoxication, but also the substance’s action mechanism, detecting the initial lesion (enzymatic effect) responsible for the anatomical and physiological alterations that may cause toxicity-related behaviours.22 Toxicity studies in experimental animals aim to detect unusual behaviours prior to the occurrence of irreversible neurological damage,23 as observed with excessive cassava consumption, which may cause tropical ataxic neuropathy and konzo. In our study, the locomotor activity test identified hypoactivity unrelated to treatment, which is suggestive of habituation associated with test repetition (as reported in previous studies)24 but not with the effect of treatment on spontaneous motor activity.
Vertical behaviour is considered an exploratory behaviour, although some authors evaluate this parameter to identify locomotor alterations, as the vertical position requires balance.25,26 Our study found significant decreases in vertical activity in the vehicle and 10-mM-ACH groups, which may be associated with reduced exploration, and increased vertical activity in the 15-mM- and 20-mM-ACH groups, possibly associated with exploratory hyperactivity secondary to ACH neurotoxicity.27 The toxic effects of ACH on locomotor activity test performance may be associated with increased exploratory behaviour (ie, vertical behaviour) but not with displacement (ie, crossed squares); this hypothesis is supported by the absence of locomotor alterations in the rotarod test.
The forced swim test was more sensitive at detecting treatment-related motor alterations, as the motor functions involved in swimming require the integration of muscles, reflexes, and specific brain regions. Animals receiving 10, 15, and 20mM ACH showed lateral swimming and spinning behaviour; the former is attributed to poor motor coordination and imbalance secondary to spinal cord demyelination and cerebellar damage.28–30 Interestingly, this abnormal swimming pattern is observed in rats receiving cassava juice for long periods.6 Spinning behaviour may be associated with spinal cord demyelination, which disrupts communication between neurons, leading to poor limb coordination and abnormal swimming as rats attempt to maintain their balance. Spinning behaviour has also been observed in rats receiving methylazoxymethanol, a neurotoxic compound present in cycad seeds.16 In summary, our results show that ACH causes motor alterations, suggesting that the compound may also be involved in the development of neurological disorders associated with cassava consumption, such as konzo and tropical ataxic neuropathy.
Toxicity studies measure a wide range of biochemical parameters to evaluate and identify physiological and metabolic changes in organ or tissue function.31 Certain common biochemical parameters may provide data on damage to a particular organ (for instance, the activity of such enzymes as AST and ALT may indicate hepatotoxicity, while urea and creatinine levels provide information on glomerular function).32 Our results indicate that ACH has hepatotoxic and nephrotoxic effects in rats when administered at concentrations of 10, 15, and 20mM for 28 days. Previous studies have shown that different concentrations of potassium cyanide and cassava extract cause high thiocyanate levels, associated with kidney and liver damage, in murine models.11,33
Liver damage may be caused by direct exposure to toxic substances due to the organ’s involvement in the detoxification of xenobiotics and metabolic products.34 In our study, ACH exposure was associated with elevated levels of ALT, AST, ALP, and direct and indirect bilirubin, and low albumin and total protein levels, suggesting liver damage.35–38 Liver damage was found to depend on ACH concentration. According to previous studies in dogs and goats, higher concentrations of cassava juice and cyanide are associated with greater liver damage, characterised by vacuolation of periportal hepatocytes.39,40 High direct and indirect bilirubin levels indicate poorer functional capacity of liver tissue, mainly due to the attempt to metabolise xenobiotics. The biochemical analysis results for indirect bilirubin revealed poor bilirubin uptake and conjugation by the liver due to hepatocyte dysfunction.41,42 High AST and ALT levels may be attributed to hepatotoxicity, which leads to vacuolar degeneration and fatty liver. These effects were also observed in male Wistar rats receiving aqueous and methanolic extracts of cassava for 14 days.11 Low total protein and albumin levels may be associated with considerable hepatocyte damage or renal insufficiency. Long-term exposure to cyanide in rabbits has also been reported to produce these effects, observed through histological analysis of the liver, quantification of cell metabolism enzymes, and assessment of blood parameters. Decreased total protein levels have been associated with renal insufficiency, which prevents adequate blood filtration, leading to increased blood creatinine and urea levels.43
Kidneys are excretory organs that clear unnecessary or toxic substances through the urine (eg, urea in amino acid deamination, bilirubin from haemoglobin catabolism, creatinine from muscle fibre creatine, uric acid from nucleic acid catabolism, and such other substances as drugs and environmental toxins).44 We detected high levels of blood urea nitrogen, urea, creatinine, and uric acid, which suggests that ACH caused kidney damage. Significant increases in blood concentrations of these substances are associated with functional damage to the kidneys. This may be due to a decrease in the glomerular filtration rate and renal tubular dysfunction.44 A significant increase in creatinine concentration is strongly correlated with histopathological changes in the kidney, characterised by bleeding, congestion, and other degenerative changes.
Our results show that ACH, a metabolite of linamarin present in cassava, causes liver and kidney damage. These alterations may be associated with the formation of cyanide through metabolic processes. Cyanide, a powerful inhibitor of the electron transport chain, promotes oxidative stress. Our results provide valuable data on the toxic effects of compounds present in cassava and its derivatives. Future research should study the toxicity mechanisms of these compounds, including distribution, absorption, metabolism, and clearance, to explore ways to prevent neurotoxicity associated with consumption of cassava or cassava derivatives.
ConclusionLong-term administration of ACH causes kidney and liver damage, which may be associated with or have a long-term impact on locomotor impairment in Wistar rats. These findings point to the potential toxicity of cassava. Further toxicity studies should be conducted to identify which presentations are toxic and which are safe for consumption.
FundingThis study was partially supported by the study group for the biology, chemistry, and molecular functionality of vegetable metabolites (UV-GC-368) at Universidad Veracruzana.
Conflicts of interestThe authors have no conflicts of interest to declare.
During the study period, C.J. Rosas-Jarquín received a postgraduate grant from the Mexican National Council of Science and Technology (CONACYT; Reg. 714879).
Please cite this article as: Rivadeneyra-Domínguez E, Rosas-Jarquín CJ, Vázquez-Luna A, Díaz-Sobac R, Rodríguez-Landa JF. Efecto de la acetona cianohidrina, un derivado de la yuca, sobre la actividad motora y la función renal y hepática en ratas Wistar. Neurología. 2019;34:300–308.