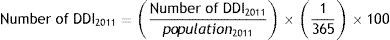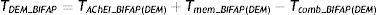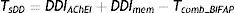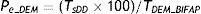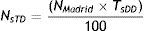The progressive rise in dementia prevalence increases the need for rapid methods that complement population-based prevalence studies.
ObjectiveTo estimate the prevalence of dementia in the population aged 65 and older based on use of cholinesterase inhibitors and memantine.
MethodsDescriptive study of use and prescription of cholinesterase inhibitors and/or memantine in 2011 according to 2 databases: Farm@drid (pharmacy billing records for the Region of Madrid) and BIFAP (database for pharmacoepidemiology research in primary care, with diagnosis and prescription records). We tested the comparability of drug use results from each database using the chi-square test and prevalence ratios. The prevalence of dementia in Madrid was estimated based on the dose per 100 inhabitants/day, adjusting the result for data obtained from BIFAP on combination treatment in the general population (0.37%) and the percentage of dementia patients undergoing treatment (41.13%).
ResultsCholinesterase inhibitors and memantine were taken by 2.08% and 0.72% of Madrid residents aged 65 and older. Both databases displayed similar results for use of these drugs. The estimated prevalence of dementia in individuals aged 65 and older is 5.91% (95% CI%, 5.85-5.95) (52287 people), and it is higher in women (7.16%) than in men (4.00%).
ConclusionsThe estimated prevalence of dementia is similar to that found in population-based studies. Analysing consumption of specific dementia drugs can be a reliable and inexpensive means of updating prevalence data periodically and helping rationalise healthcare resources.
El aumento progresivo de la demencia hace conveniente disponer de métodos rápidos que complementen los estudios poblacionales de prevalencia.
ObjetivoEstimar la prevalencia de demencia en la población mayor de 65 años a partir del consumo de anticolinesterásicos y memantina.
MétodosEstudio descriptivo del uso de anticolinesterásicos y/o memantina en 2011 en 2 bases de datos: Farm@drid, con registro de facturación en farmacias de la Comunidad Autónoma de Madrid (CAM) y Base de Investigación Farmacoepidemiológica en Atención Primaria (BIFAP), con registros sobre diagnóstico y prescripción. Se analizó la comparabilidad de resultados sobre utilización de fármacos de ambas bases mediante χ2 de Pearson y razón de prevalencias. La prevalencia de demencia en la CAM se estimó a partir de la dosis por 100 habitantes/día, ajustando el resultado con los datos obtenidos en la BIFAP sobre tratamiento combinado en población general (0,37%) y proporción de pacientes con demencia en tratamiento (41,13%).
ResultadosEl consumo de anticolinesterásicos y memantina entre la población > 65 años de la CAM fue del 2,08 y el 0,72% respectivamente. Ambas bases muestran resultados con similar uso de estos medicamentos. La prevalencia estimada de demencia en > 65 años en la CAM es del 5,91% (IC del 95%, 5,85-5,95) (52.287 personas), mayor en mujeres (7,16%) que en hombres (4,00%).
ConclusionesLa prevalencia estimada de demencia es similar a la encontrada en estudios poblacionales. El análisis del consumo de fármacos específicos para la demencia puede ser una herramienta fiable que actualice periódicamente esta prevalencia sin gran coste, ayudando en la planificación de recursos sociosanitarios.
Dementia, a syndrome mainly caused by Alzheimer disease (AD) and cerebrovascular disease, is responsible for the highest rates of disability, dependence, and use of healthcare resources among the elderly.1,2 Epidemiological studies of dementia prevalence estimate that the number of patients with dementia will double every 20 years, reaching 81.1 million patients in 2040. Western Europe has a dementia prevalence rate of 5.4% in patients older than 60 years (0.9% for patients aged 60-64 and 24.8% for patients aged≥85 years). In 2001, there were 4.9 million people with dementia, a number that will increase by 102% to reach 9.9 million by 2040.2 In Spain, the number of individuals aged 65 and older has doubled in 30 years; rapid population ageing may lead to an exponential increase in the incidence of neurodegenerative diseases, and especially dementia.3 Having quick methods for updating data on dementia prevalence may be helpful for planning and managing the resources required by this vulnerable population.
Population-based prevalence studies are costly in terms of time and resources. Screening tests followed by clinical examinations provide enough data in most of these studies,4 but results may vary greatly depending on the screening and diagnostic methods used. A systematic review of population-based dementia prevalence studies conducted in Spain reports rates ranging from 3.5% to 17.2% among patients aged over 70.3
An alternative method for estimating prevalence of a disease is based on consumption data for drugs specific to that disease; these data can be gathered from the database of official medical prescriptions invoiced to the Spanish Health System.5 In Spain, this method has already been used for other diseases, including tuberculosis,6,7 epilepsy,8 Parkinson's disease,9 hypothyroidism,10 and diabetes mellitus.11
In the case of dementia, there are several studies addressing drug use, but few of them have explored prevalence.12,13 Acetylcholinesterase inhibitors (AChEI: donepezil, rivastigmine, galantamine) and memantine are specific drugs for AD which are usually administered either in monotherapy or in combination treatment (one AChEI+memantine). These drugs are also administered for diseases with Lewy bodies (dementia with Lewy bodies and Parkinson's disease) and AD associated with vascular dementia, but they are not indicated for patients with frontotemporal dementia.14–16
In Spain, these drugs are listed as ‘hospital-prescribed drugs’,17 which means that they must initially be prescribed by a specialist in neurology, gerontology, or psychiatric medicine, and subsequently authorised by the medical regulatory authority. This ensures that they are prescribed to patients according to their indication; use of these drugs in a specific population therefore provides fairly reliable data on dementia prevalence. We must be mindful that some patients may be taking both drugs at the same time (combination treatment) in order not to overestimate the number of patients receiving specific treatment for dementia. Another factor to consider when estimating prevalence from drug use data is that not all patients diagnosed with dementia receive specific treatment.18
The Database for Epidemiological and Pharmacological Research in Primary Care (BIFAP)19 is a population-based database created as part of a project by the Spanish Agency for Medicines and Medical Devices (AEMPS) in cooperation with all Spanish regions; the region of Madrid alone contributes 57% of all patients. This database contains anonymous data from electronic medical histories of patients attended by 2239 participating primary care doctors. It includes data from more than 4 million medical histories (19976344 person-years of follow-up), with an age and sex distribution similar to that of the Spanish population. The purpose of this database is pharmacovigilance, including use in studies on drug consumption.18 Unlike pharmacy databases containing prescription invoicing data, BIFAP provides data on demographic characteristics, the diagnosis, and clinical events associated with the prescription. This may enable extrapolation of the proportion of untreated dementia patients to other databases.
The purpose of the present study is to estimate dementia prevalence in the region of Madrid (subsequently, Madrid) in 2011 based on consumption data for AChEIs and memantine, using the BIFAP database version 2011 as a method for adjusting data.
Material and methodsThis study uses 2011 data from 2 databases which have previously been used to analyse AChEI and memantine consumption18,20: BIFAP and Farm@drid, the system providing information on prescriptions and pharmaceutical services in the region of Madrid in the form of a population-based database of official medical prescriptions dispensed by pharmacies in that region and invoiced to the Spanish Health System. In BIFAP19 (http://www.bifap.org), healthcare visits are coded according to International Classification of Primary Care (ICPC) criteria, and prescriptions are coded following the Anatomical Therapeutic Chemical (ATC) classification system. The population included in the BIFAP is dynamic and may change from one year to another, depending on the number of doctors participating.
Using the Farm@drid database, we gathered the sex distribution and use of AChEIs (subgroup ATC N06DA) and memantine (subgroup N06DX) in 2011 in patients aged 66 and older.20
From the BIFAP database, we gathered information on official prescriptions of AChEIs and/or memantine issued by primary care doctors in 2011 to patients aged 65 and older, and invoiced to the Spanish Health System. We identified how many of these patients were treated with both AChEIs and memantine (combination treatment), as well as the number of patients diagnosed with dementia (ICPC code: P70) in 2011 or previously according to their medical histories. Prescription data broken down by sex was obtained for both groups (general population and patients with dementia).18
In both databases, AChEI consumption (N06DA) included donepezil, rivastigmine, and galantamine; ginkgo biloba (N06DX02) was not included in the N06DX subgroup since it is not a specific treatment for dementia.
Using Farm@drid data, AChEI and memantine consumption was calculated based on the daily dose per inhabitant (DDI) for each active ingredient20 for all patients older than 65 and for both sexes, according to the following formula:
DDD is the defined daily dose of each drug for its main indication as set forth by the World Health Organization.21 Daily dose per inhabitant refers to the mean number of inhabitants receiving daily treatment with a given drug.17 We used the DDI adjusted for 100 inhabitants to compare our results with the data included in the BIFAP database. Populations used in calculating this rate were obtained from Farm@drid, according to health card holder records corresponding to 2011 (896722 people).In the BIFAP database, we analysed use of these drugs by the general population and by patients with dementia separately.18 Treatment with AChEIs (TAChEI_BIFAP) and memantine (Tmem_BIFAP) in the general population was calculated by analysing the proportion of patients aged ≥65 receiving at least one prescription in 2011 vs the population aged ≥65 included in the database in 2011, using the formulas:
Combination treatment in the general population (Tcomb_BIFAP) was calculated with the formula:
The 2011 population of the BIFAP database (BIFAP2011) used to calculate these rates included all patients aged ≥65 and at least one day of follow-up in 2011 (270470 patients).
In patients with dementia, we analysed the proportion of patients undergoing specific treatment with one of the drugs mentioned above (TDEM_BIFAP) using the formula:
TAChEI_BIFAP(DEM), Tmem_BIFAP(DEM), and Tcomb_BIFAP(DEM) are prescription rates for AChEIs, memantine, and combination treatment, respectively, in dementia patients aged ≥65.The population used to calculate these rates included all patients aged ≥65 years with at least one day of follow-up in 2011 and an episode of dementia in their medical histories (11271 patients).
The method for gathering drug consumption data used in both studies is explained in more detail in the original articles.18,20
We used Pearson's chi-square test and prevalence ratio (including 95% CIs) to assess homogeneity and comparability of prevalence results for AChEI and memantine consumption in both databases, in both the total population and in single sex subgroups.
After confirming homogeneity and comparability of both databases, we extrapolated BIFAP results for prescribed combination treatment and untreated dementia patients to the Farm@drid database.
The percentage of patients aged 66 and older in Madrid who were treated with specific drugs for dementia in 2011 (TsDD) was estimated applying the following formula to the Farm@drid database figures:
DDIAChEI is the percentage of patients ≥66 years treated with AChEIs in 2011 (Farm@drid); DDImem is the percentage of patients ≥66 years treated with memantine in 2011 (Farm@drid); Tcomb_BIFAP is the percentage of patients ≥65 years treated with combination treatment: AChEI+memantine (extrapolated from BIFAP 2011).To estimate prevalence of dementia in Madrid residents aged 66 or older (Pe-DEM) in 2011, we considered the percentage of dementia patients aged ≥65 who according to the BIFAP database were prescribed AChEIs and/or memantine in the same period (TDEM_BIFAP), using the formula:
We estimated the number of patients aged ≥66 under specific treatment for dementia (NsTD) and the number of patients aged ≥66 with dementia (Ne_DEM), using 2011 data for Spanish national health card holders and the following formulas:
NMadrid is the population aged ≥66 years in 2011 in Madrid.Data were analysed with SPSS statistical software, version 19.0; Excel® version 12.3.0 (110427); and Epidat 3.0.
Data extracted from both digital databases did not include any variables that could identify patients; therefore, our study preserves data confidentiality.
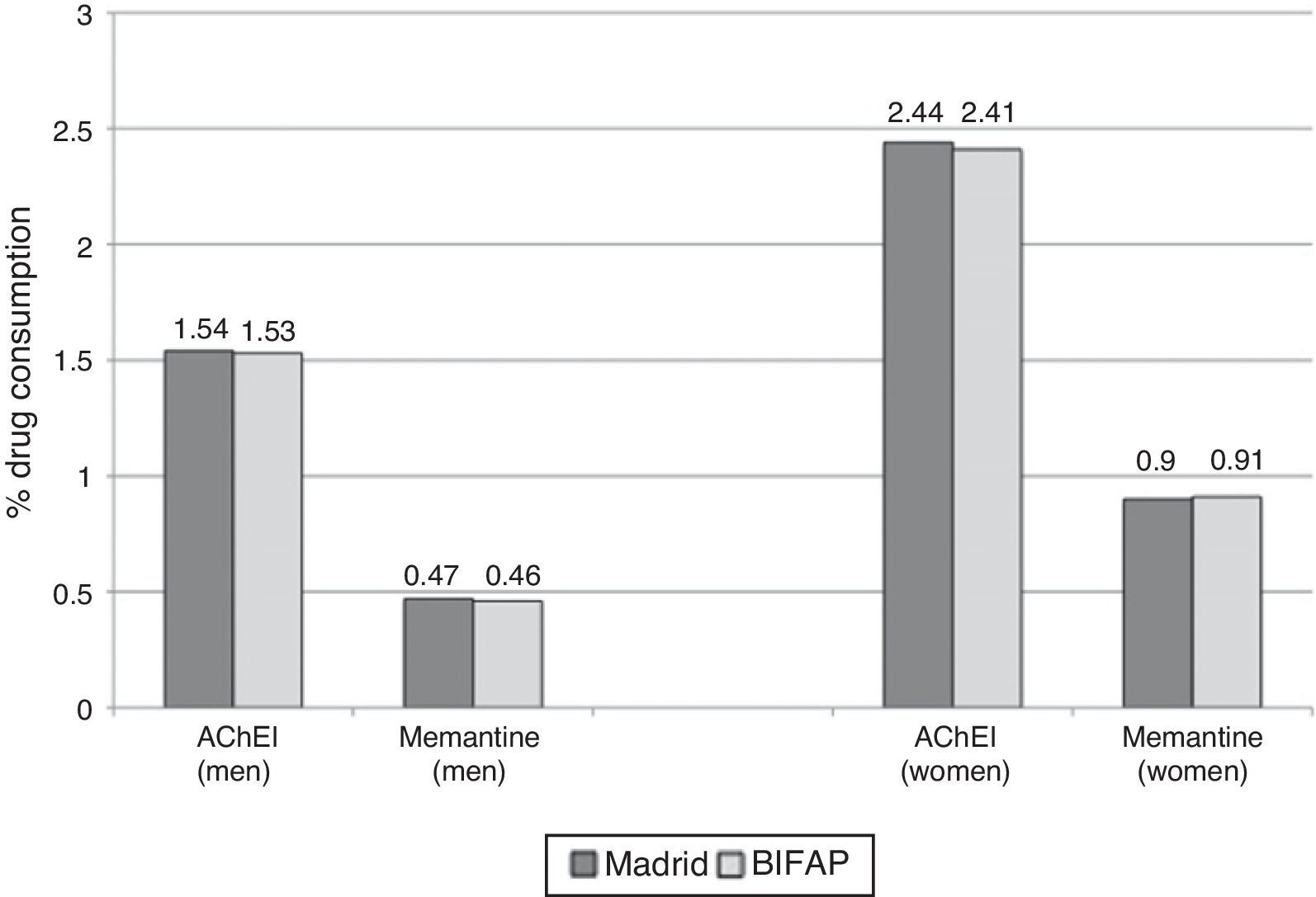
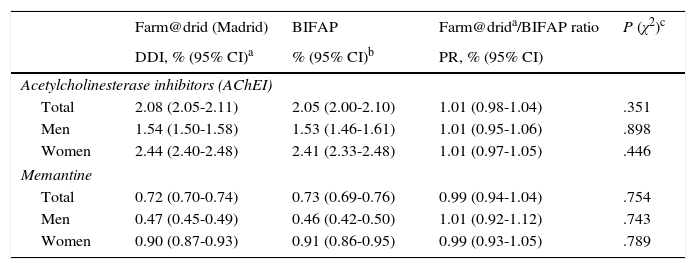
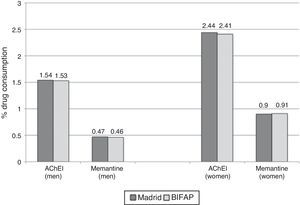
ResultsComparison of AChEI and memantine consumption data between the 2 studied databasesAccording to Farm@drid data for 2011, 2.08% (95% CI, 2.05-2.11) of patients older than 65 were treated with AChEIs and 0.72% (95% CI, 0.70-0.74) received memantine. According to the BIFAP database, the prescription rate for AChEI in patients ≥65 years was 2.05% (95% CI, 2.00-2.10) and for memantine, 0.73% (95% CI, 0.69-0.76). Use of AChEIs and memantine was greater among women (Fig. 1). We found no differences in the total population's consumption of AChEI (prevalence ratio 1.01; 95% CI, 0.98-1.04) and memantine (prevalence ratio 0.99; 95% CI, 0.94-1.04) between the 2 databases. Likewise, no significant differences were observed between sexes (Table 1).
Consumption of specific drugs for dementia in the region of Madrid and in the BIFAP database in 2011.
| Farm@drid (Madrid) | BIFAP | Farm@drida/BIFAP ratio | P (χ2)c | |
|---|---|---|---|---|
| DDI, % (95% CI)a | % (95% CI)b | PR, % (95% CI) | ||
| Acetylcholinesterase inhibitors (AChEI) | ||||
| Total | 2.08 (2.05-2.11) | 2.05 (2.00-2.10) | 1.01 (0.98-1.04) | .351 |
| Men | 1.54 (1.50-1.58) | 1.53 (1.46-1.61) | 1.01 (0.95-1.06) | .898 |
| Women | 2.44 (2.40-2.48) | 2.41 (2.33-2.48) | 1.01 (0.97-1.05) | .446 |
| Memantine | ||||
| Total | 0.72 (0.70-0.74) | 0.73 (0.69-0.76) | 0.99 (0.94-1.04) | .754 |
| Men | 0.47 (0.45-0.49) | 0.46 (0.42-0.50) | 1.01 (0.92-1.12) | .743 |
| Women | 0.90 (0.87-0.93) | 0.91 (0.86-0.95) | 0.99 (0.93-1.05) | .789 |
BIFAP: Database for Epidemiological and Pharmacological Research in Primary Care; DDI: dose per 100 inhabitants/day; Farm@drid (Madrid): pharmacy billing records for the region of Madrid; PR: prevalence ratio.
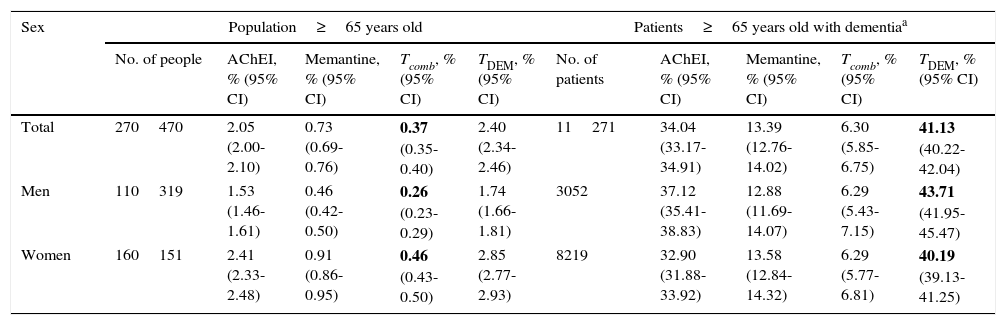
The percentage of the population aged ≥65 receiving combination treatment (AChEI+memantine) in the BIFAP database in 2011 was 0.37% (95% CI, 0.35-0.40). Of all patients aged ≥65 and diagnosed with dementia (ICPC code: P70), 41.13% were treated with those drugs in 2011 (95% CI, 40.22-42.04). Table 2 shows the results of BIFAP in 2011 in more detail.
Consumption of specific drugs for dementia in the general population and in patients diagnosed with dementia in BIFAP in 2011.
| Sex | Population≥65 years old | Patients≥65 years old with dementiaa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of people | AChEI, % (95% CI) | Memantine, % (95% CI) | Tcomb, % (95% CI) | TDEM, % (95% CI) | No. of patients | AChEI, % (95% CI) | Memantine, % (95% CI) | Tcomb, % (95% CI) | TDEM, % (95% CI) | |
| Total | 270470 | 2.05 (2.00-2.10) | 0.73 (0.69-0.76) | 0.37 (0.35-0.40) | 2.40 (2.34-2.46) | 11271 | 34.04 (33.17-34.91) | 13.39 (12.76-14.02) | 6.30 (5.85-6.75) | 41.13 (40.22-42.04) |
| Men | 110319 | 1.53 (1.46-1.61) | 0.46 (0.42-0.50) | 0.26 (0.23-0.29) | 1.74 (1.66-1.81) | 3052 | 37.12 (35.41-38.83) | 12.88 (11.69-14.07) | 6.29 (5.43-7.15) | 43.71 (41.95-45.47) |
| Women | 160151 | 2.41 (2.33-2.48) | 0.91 (0.86-0.95) | 0.46 (0.43-0.50) | 2.85 (2.77-2.93) | 8219 | 32.90 (31.88-33.92) | 13.58 (12.84-14.32) | 6.29 (5.77-6.81) | 40.19 (39.13-41.25) |
Modified from Bonis Sanz et al.18
Data extrapolated to estimate prevalence of dementia in the region of Madrid are shown in bold.
BIFAP: Database for Epidemiological and Pharmacological Research in Primary Care; AChEI: acetylcholinesterase inhibitors; Tcomb: combination treatment (AChEI+memantine); TDEM: specific treatment for dementia (AChEI+memantine – combination treatment).
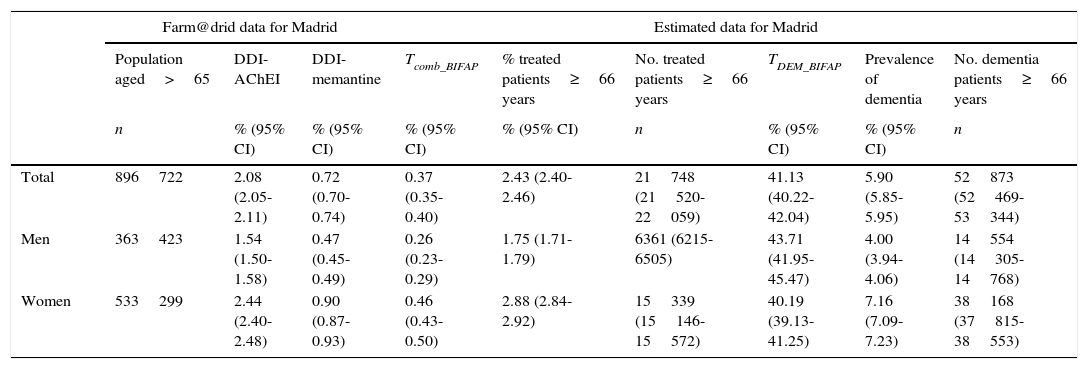
After adjusting results from Farm@drid using data extrapolated from BIFAP, 2.43% (95% CI, 2.40-2.46) of the Madrid population aged 66 and older were estimated to use specific drugs for dementia in 2011; prevalence of dementia for that same year in Madrid residents aged ≥66 was estimated at 5.90% (95% CI, 5.85-5.95), with a prevalence of 4% for men (95% CI, 3.94-4.06) and 7.16% for women (95% CI, 7.09-7.23) (Table 3).
Patients aged 66 and older with dementia in the region of Madrid in 2011. Estimation based on consumption of specific drugs for dementia.
| Farm@drid data for Madrid | Estimated data for Madrid | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Population aged>65 | DDI-AChEI | DDI-memantine | Tcomb_BIFAP | % treated patients≥66 years | No. treated patients≥66 years | TDEM_BIFAP | Prevalence of dementia | No. dementia patients≥66 years | |
| n | % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | n | % (95% CI) | % (95% CI) | n | |
| Total | 896722 | 2.08 (2.05-2.11) | 0.72 (0.70-0.74) | 0.37 (0.35-0.40) | 2.43 (2.40-2.46) | 21748 (21520-22059) | 41.13 (40.22-42.04) | 5.90 (5.85-5.95) | 52873 (52469-53344) |
| Men | 363423 | 1.54 (1.50-1.58) | 0.47 (0.45-0.49) | 0.26 (0.23-0.29) | 1.75 (1.71-1.79) | 6361 (6215-6505) | 43.71 (41.95-45.47) | 4.00 (3.94-4.06) | 14554 (14305-14768) |
| Women | 533299 | 2.44 (2.40-2.48) | 0.90 (0.87-0.93) | 0.46 (0.43-0.50) | 2.88 (2.84-2.92) | 15339 (15146-15572) | 40.19 (39.13-41.25) | 7.16 (7.09-7.23) | 38168 (37815-38553) |
Madrid: region of Madrid; Farm@drid: pharmacy billing records for Madrid; DDI: dose per 100 inhabitants/day; AChEI: acetylcholinesterase inhibitors; Tcomb_BIFAP: combination treatment in patients aged ≥65 years (BIFAP 2011); TDEM_BIFAP: percentage of dementia patients aged 65 years who were treated with specific drugs for dementia (BIFAP 2011).
Using 2011 population data for Madrid, the number of residents with dementia and aged ≥66 in that year was estimated at 52,873 (95% CI, 52,469-53,344), with 14,554 men and 38,168 women; 21,748 of those patients (95% CI, 21,520-22,059) were treated with AChEIs and/or memantine (6361 men and 15,339 women). Table 3 shows estimated data for Madrid using BIFAP data.
DiscussionThe prevalence rates for dementia reported in this study (5.90%; 95% CI, 5.85-5.95) agree with those presented in population-based studies including patients ≥66 from a specific setting: 5.8% (95% CI, 5.2-6.5) in the NEDICES study4 and 7.6% (4.6% for AD) in the Toledo study.22 Estimation of prevalence data in our study was nevertheless faster and less costly. Our data are also similar to those reported for the population aged 65 and older in Western Europe: 6.4% for dementia (all causes) and 4.4% for AD.23
A study conducted in our setting using a similar estimation method (based on drug consumption data) reported a dementia prevalence of 2.98 per 100 inhabitants in 2008 for the region of Castille-La Mancha.13 The authors of that study consider this figure to be the minimum prevalence of AD (treated patients), although it may be overestimated since they include patients treated with gingko biloba and do not adjust for combination treatment, as they indicate in the discussion section. They estimate AD prevalence at 2.46% to 4.63% when comparing their data on treated patients with those from other studies conducted in different years. Our study estimated the prevalence of dementia in general, not only AD; using BIFAP results, we were able to extrapolate the proportion of patients with dementia and specific treatment (41.13%) for the same study year (2011), which yields a more precise estimation of prevalence. When comparing consumption percentages only, crude consumption of AChEI (2.08%) plus memantine (0.72%) in Madrid reaches 2.80%, without correcting for combination treatment; this percentage is similar to that reported in the study mentioned above, which follows the same method. Since consumption of these drugs is increasing,13,18,20,28 it may be surprising to learn that the 2008 percentages are somewhat higher than those found in Madrid in 2011. This finding might be explained by the inclusion of ginkgo biloba, which is the second most frequently prescribed drug in that study, after donepezil.13
Our study has several limitations. Firstly, our study assumes that all drugs recorded in both databases were administered to patients with dementia (and are therefore correctly prescribed). This assumption is common to all prevalence studies based on drug consumption data. However, these drugs must initially be prescribed by specialists and prescriptions must be authorised by the medical regulatory authority, so the likelihood of a prescription error is low. We therefore feel that both prescription invoicing data from pharmacies (Farm@drid) and prescription data submitted by primary care doctors (BIFAP) reflect real consumption of AChEIs and memantine more accurately than they would for other less-regulated drugs, for which prescription or invoicing data may not be indicative of real consumption.
Since BIFAP is a secondary database based on records kept in normal clinical practice, some degree of error may be expected in dementia data. According to published literature, dementia is difficult to diagnose and is therefore more likely to be underdiagnosed or under-reported than overdiagnosed.24 This being the case, prevalence estimates based on drugs specifically prescribed for dementia and directly associated with the recorded diagnosis may be lower than those reported by population-based studies conducting screening and clinical diagnostic tests to detect new cases of dementia. Studies following this prescription-based method let researchers analyse global data without data loss, unlike population-based studies.
Neither of the 2 databases analyse consumption and/or prescriptions given by private practices or within the framework of special healthcare programmes such as the Spanish civil service mutual insurance, general mutual insurance scheme for the judiciary, or the social insurance institute of the armed forces. These groups make up such a small percentage of the population living in Madrid (0.87%) and covered by the Spanish Health System that they do not impact the validity of our data whether in terms of the number of prescriptions or in population figures.25 In the BIFAP database, treatments initiated in specialist clinics (first prescription) but discontinued due to poor tolerance are not recorded as prescriptions. We have no data on drug withdrawals after the initial prescription but we feel that it does not affect the final consumption data: the literature reports a mean treatment duration of 74.9 weeks in the same setting (primary care).26
Finally, our study compares prevalence rates estimated using different methods: total consumption in the population using DDI (Farm@drid), and first prescription issued by the patient's primary care doctor, corresponding to the second prescription overall since these drugs must initially be prescribed by specialists (BIFAP). However, since the final data for AChEI and memantine consumption are similar between the 2 databases, we believe this comparison is justified.
Our study also has several strong points. Unlike other drugs with more variable dosing schemes, the DDD of AChEIs and/or memantine coincides with the maintenance dose in dementia, so there are no substantial changes in the daily doses prescribed for each patient. The DDI is therefore equivalent to the number of patients under treatment. As these drugs are specific for dementia, treated patients represent patients with dementia, an advantage which is absent from similar studies of other drugs, such as antiepileptic and antiparkinsonian drugs, that may be used for treating a number of diseases.27
To calculate DDI in patients aged 66 and older, we used only the DDD consumed by patients ≥66 years and the population aged 66 and older. Other consumption studies calculate the DDD consumed by the whole population based on the reference population for those drugs (>60 years 28 or >65 years,13 depending on the study) or understand the retired population to be equivalent to the group aged 65 and older.27
Although we have used 2 different databases featuring slightly different age groups (Farm@drid does not include patients 65 years old), homogeneity and comparability of results of drug consumption are high and enable extrapolation of BIFAP results for combination treatment and the proportion of untreated dementia patients to the population included in Farm@drid.
We feel that using data from the same period (2011) constitutes another strength, since the percentage of patients under treatment and prescription criteria for combination treatment will change with time.18,29 Likewise, both databases include similar populations (social security health card holders, equivalent to the general population in 2011), which is also important since the percentage of patients receiving treatment varies greatly depending on the data source (hospital records or specialised AD centres,29 primary care centres,18 or nursing homes30,31).
Regarding the geographical setting, although some differences have been described for prescriptions of AChEIs (donepezil, rivastigmine, or galantamine) or in the percentage of patients treated with memantine,13,28,32 the overall distribution of AChEIs and memantine is similar in Farm@drid and in BIFAP, which suggests that data on combination treatment may be extrapolated. This finding may be explained in part by the fact that roughly half of all BIFAP records correspond to patients from Madrid.
Studies estimating dementia prevalence based on drug consumption should not replace population-based prevalence studies since the former cannot evaluate patients directly and are therefore likely to harbour such errors as underdiagnosis or including patients without dementia affected by a prescription error. They provide, however, useful complementary data. These studies may be helpful for quicker identification of any changes in the number of treated patients and in drug consumption patterns by age group, sex, and active ingredient. Another important feature of these studies is that they allow comparisons of drug consumption by geographical area and/or estimates of prevalence in regions with few population-based prevalence studies for dementia but having available drug consumption data, as is the case in southern Spain.3
In conclusion, consumption figures for specific drugs for dementia may be a reliable source for calculating dementia prevalence in a specific setting when data from different drug databases are combined. This is a fast and inexpensive means of complementing population-based prevalence studies and updating records, which in turn can help with the rational management of healthcare resources.
FundingThis study has not received funding from any sources.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Juan José de la Cruz Troca (Universidad Autónoma de Madrid), all primary care doctors who submitted data to the Database for Epidemiological and Pharmacological Research in Primary Care (BIFAP), and the Directorate General for Pharmacy and Healthcare Products of the Region of Madrid.
Please cite this article as: de Hoyos-Alonso MC, Bonis J, Tapias-Merino E, Castell MV, Otero A. Estimación de la prevalencia de demencia a partir del análisis de bases de datos sobre uso de fármacos. La situación en la Comunidad de Madrid (España). Neurología. 2016;31:1–8.
Results from this study have not been published either totally or partially. Part of this study was presented at the 23rd Congress of the Madrid Society of Family and Community Medicine (SoMaMFyC) in April 2014.