Lithium was proposed in 2008 as an effective candidate in the treatment of ALS after a report claimed that it was able to delay functional deterioration by 40% and that none of the 16 patients treated with a combination of lithium plus riluzole had died during a 15-month follow-up period. The excellent results of this pilot study engendered considerable optimism among patients, their families, patients’ associations, and the scientific community. This report sparked numerous phase ii clinical trials. Many patients who were not included in these studies used all resources at their disposal to access the drug as treatment under a compassionate use programme.
ObjectivesTo evaluate the effectiveness of lithium in ALS using a meta-analysis of the information reported in 12 studies which were examined for methodological quality.
Material and methodsSearches were performed using MEDLINE, EMBASE, the Cochrane Neuromuscular Disease Group Trials Register, ClinicalTrials.gov, and EudraCT (January 1996-August 2012).
ResultsTo date, we have information on more than 1100 patients treated with lithium. Unfortunately, the results do not confirm the positive effect described in the pilot study, which suggests that this drug is not effective at slowing disease progression. Two trials had to be suspended before the scheduled completion date due to the ineffectiveness of the drug as well as numerous adverse effects. A recently published study also ruled out any possible modest effect.
ConclusionsThere is evidence to suggest that lithium has no short-term benefits in ALS. A comparison of the group of patients treated with lithium+riluzole and the control group treated with riluzole alone showed no statistically significant differences in rates of functional decline, deterioration of respiratory function, or survival time. Furthermore, there was no evidence that it was more effective than the placebo.
El litio fue propuesto en 2008 como un candidato eficaz en el tratamiento de ELA tras reportarse que era capaz de retrasar el deterioro funcional en un 40% y que ninguno de los 16 pacientes tratados con una combinación de litio más riluzole falleció durante un periodo de seguimiento de 15 meses. Los excelentes resultados de este estudio piloto despertaron una gran expectativa en pacientes, familiares, asociaciones de enfermos y comunidad científica. Consecuencia directa de esta noticia fue la puesta en marcha de numerosos ensayos clínicos en fase ii. Muchos de los pacientes, que no pudieron ser incluidos en estos estudios, utilizaron todos sus recursos para acceder a este fármaco mediante uso compasivo.
ObjetivosEvaluar la eficacia del litio en ELA mediante un metaanálisis de la información reportada en 12 de estos estudios. Se analiza su calidad metodológica.
Material y métodosSe realizaron búsquedas en MEDLINE, EMBASE y Registros Cochrane del Grupo de Enfermedades Neuromusculares, ClinicalTrials.gov y EudraCT (enero de 1996-agosto de 2012).
ResultadosHasta la fecha disponemos de información de más de 1.100 pacientes tratados con litio. Lamentablemente los resultados obtenidos no confirman el efecto positivo descrito en el estudio piloto y sugieren que este fármaco es ineficaz para detener la progresión de la enfermedad. Dos ensayos tuvieron que ser suspendidos antes del plazo previsto por ineficacia del fármaco y por numerosos efectos adversos. En un estudio publicado recientemente se descarta también cualquier posibilidad de un mínimo efecto.
ConclusionesHay evidencia de que el litio no ofrece beneficios a corto plazo en ELA. Al comparar el grupo de pacientes tratados con litio+riluzole con el grupo control tratado con riluzole no se observan diferencias estadísticamente significativas en las tasas de deterioro funcional o de deterioro de la función respiratoria ni tampoco en la supervivencia. No hay tampoco evidencia de que sea superior al placebo.
Amyotrophic lateral sclerosis (ALS), a neurodegenerative disorder characterised by rapidly progressing muscle weakness, has a median survival time of 3 years and a considerable socioeconomic impact.1,2 No current treatments are able to stop disease progression and the effectiveness of riluzole, the only drug approved for treating ALS, is questionable since it only prolongs life expectancy a mean of 2 to 4 months. This fact, added to the high cost of riluzole, has led researchers to look for more effective pharmacological treatments.
A study published in 20083 reported that lithium, a drug used for treating bipolar disorders, significantly increased life expectancy (by 35%) when administered to SOD1G93A transgenic mice at symptom onset; these findings gave some hope to the ALS community. This therapeutic effect has been associated with the lithium's ability to enhance autophagy, increase the number of healthy mitochondria in motor neurons, reduce mutated SOD1 levels, increase the number of Renshaw cells (Lamina VII), and suppress reactive gliosis.3–16 The authors of the article mentioned above simultaneously reported the results of a pilot study conducted in 44 patients with ALS. The patients treated with lithium at doses producing plasma levels of 0.4-0.8mEq/L displayed a 43% delay in symptom progression compared to the control group.3,17 The ALS community found these results unusually promising and numerous clinical trials were conducted to corroborate the findings. Many patients with ALS, eager to benefit from effective treatment, requested lithium for compassionate use; other patients tried self-medicating without appropriate medical supervision since lithium could be easily acquired in pharmacies.
In 2009, 2 studies had to be interrupted due to ineffectiveness: there was no evidence that lithium slowed disease progression by the expected 40%.18–21 A third study, which was the first one to be completed, failed to report differences between 107 patients treated with lithium and 249 historical controls.22,23 These findings agree with the conclusions expressed by patients themselves on the Internet.24 A research group from the Netherlands recently proved that lithium has no beneficial effects on disease progression after a long period of treatment.25
The objectives of the present study are as follows: (1) to evaluate effectiveness of lithium for treating ALS by conducting a meta-analysis of the information obtained from different clinical trials published prior to August 2012; (2) to assess the methodological quality of each of these trials; and (3) to analyse the reasons for this new failure of translational medicine.
Materials and methodsWe conducted an electronic search of all clinical trials on lithium for patients with a clinical diagnosis of ALS using the following databases: (1) the Cochrane Neuromuscular Disease Group Specialised Register, (2) the international register of publicly and privately supported clinical studies (ClinicalTrials.gov), (3) the European Clinical Trials Database (EudraCT), (4) MEDLINE, and (5) EMBASE (from 1 January 1996 to 1 August 2012).26–30 The same search criteria were used to select relevant abstracts from among those presented at international congresses on ALS and the annual meetings of the American Academy of Neurology held between 1998 and 2012. The end date for the search was 1 August 2012. We also consulted the references listed by relevant reviews and trials to confirm additional unpublished data. Table 1 lists the terms used as keywords (single terms or combined). We checked all titles and abstracts; those studies considered potentially relevant were obtained in full-text format. The compiled data were verified and computerised. The methodological quality of the studies was graded according to the criteria recommended by Cochrane: A=adequate; B=unclear; C=inadequate; or D=not specified. The following points were especially relevant in the assessment of methodological quality: (1) inclusion criteria; (2) risk of bias in the included patients, especially regarding baseline characteristics; (3) patient classification and treatment blinding; (4) primary and secondary endpoints; (5) follow-up; and (6) criteria for objective evaluation of results.
Keywords used for the electronic search.
| Amyotrophic lateral sclerosis | Neurodegenerative diseases |
| ALS | Neuroprotection |
| Lou Gehrig's disease | Autophagy |
| Motor neuron disease | Apoptosis |
| MND | Oxidative stress |
| Lithium | Mitochondria |
| Riluzole | Calcium homeostasis |
| Placebo | Cytoskeleton |
| Clinical trial | Kinases |
| Randomised controlled trial | DNA damage |
| Comparative study | Calpain |
| Prospective studies | Signal transduction |
| Multicenter study | Tau kinases |
| Unicenter study | Cell cycle control |
| Follow up studies | GSK-3β |
| Results | Neuronal aggregates |
| Adverse event | Spinal cord neurogenesis |
| Human | Inositol monophosphatase |
| No human | Inhibition |
| Double blind procedure | Neuronal death |
| Single blind procedure | Endoplasmic reticulum stress |
| Crossover procedure | Caspases |
| Historical controls | |
| Compassionate use | |
| Unapproved drug use | |
| Neurodegeneration |
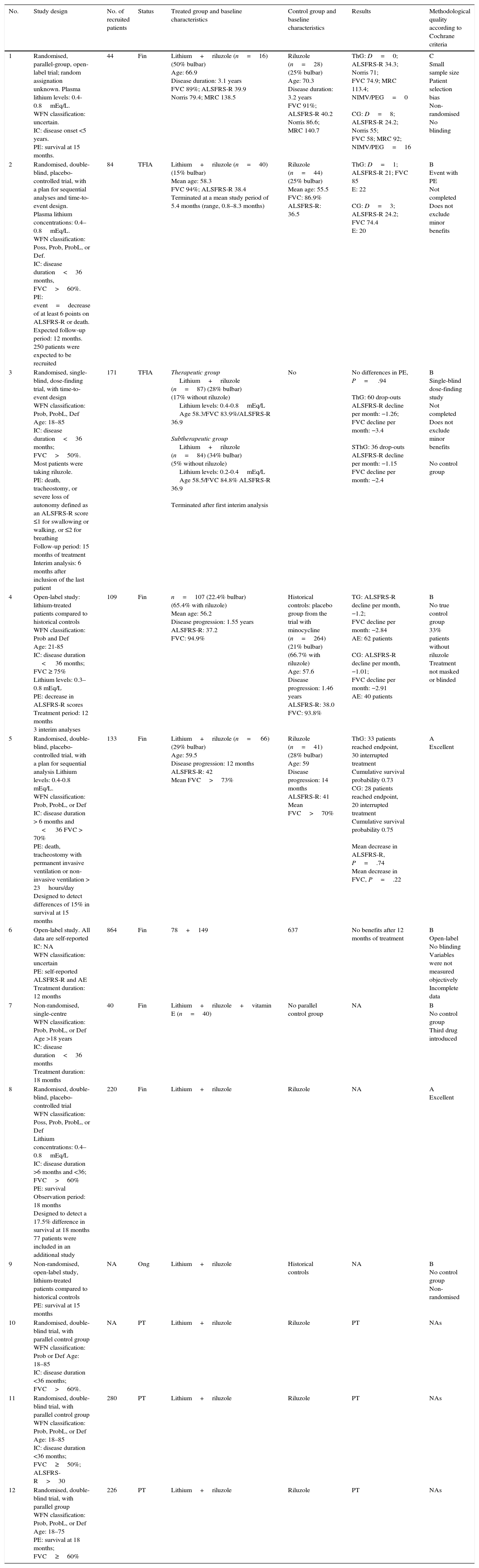
We identified 12 clinical trials; the results of 6 have already been published,3,19,21,23–25 2 are yet to report their findings (LICALS and EudraCT 2009-010060-41), one is still underway (EudraCT 2008-003707-32), and the remaining 3 (EudraCT 2008-005106-38, EudraCT 2008-006224-61, and EudraCT 2008-006722-34) have been ended prematurely (Table 2).
Characteristics and design of clinical trials with lithium.
| No. | Study design | No. of recruited patients | Status | Treated group and baseline characteristics | Control group and baseline characteristics | Results | Methodological quality according to Cochrane criteria |
|---|---|---|---|---|---|---|---|
| 1 | Randomised, parallel-group, open-label trial; random assignation unknown. Plasma lithium levels: 0.4-0.8mEq/L. WFN classification: uncertain. IC: disease onset <5 years. PE: survival at 15 months. | 44 | Fin | Lithium+riluzole (n=16) (50% bulbar) Age: 66.9 Disease duration: 3.1 years FVC 89%; ALSFRS-R 39.9 Norris 79.4; MRC 138.5 | Riluzole (n=28) (25% bulbar) Age: 70.3 Disease duration: 3.2 years FVC 91%; ALSFRS-R 40.2 Norris 86.6; MRC 140.7 | ThG: D=0; ALSFRS-R 34.3; Norris 71; FVC 74.9; MRC 113.4; NIMV/PEG=0 CG: D=8; ALSFRS-R 24.2; Norris 55; FVC 58; MRC 92; NIMV/PEG=16 | C Small sample size Patient selection bias Non-randomised No blinding |
| 2 | Randomised, double-blind, placebo-controlled trial, with a plan for sequential analyses and time-to-event design. Plasma lithium concentrations: 0.4–0.8mEq/L. WFN classification: Poss, Prob, ProbL, or Def. IC: disease duration<36 months, FVC>60%. PE: event=decrease of at least 6 points on ALSFRS-R or death. Expected follow-up period: 12 months. 250 patients were expected to be recruited | 84 | TFIA | Lithium+riluzole (n=40) (15% bulbar) Mean age: 58.3 FVC 94%; ALSFRS-R 38.4 Terminated at a mean study period of 5.4 months (range, 0.8–8.3 months) | Riluzole (n=44) (25% bulbar) Mean age: 55.5 FVC: 86.9% ALSFRS-R: 36.5 | ThG: D=1; ALSFRS-R 21; FVC 85 E: 22 CG: D=3; ALSFRS-R 24.2; FVC 74.4 E: 20 | B Event with PE Not completed Does not exclude minor benefits |
| 3 | Randomised, single-blind, dose-finding trial, with time-to-event design WFN classification: Prob, ProbL, Def Age: 18–85 IC: disease duration<36 months; FVC>50%. Most patients were taking riluzole. PE: death, tracheostomy, or severe loss of autonomy defined as an ALSFRS-R score ≤1 for swallowing or walking, or ≤2 for breathing Follow-up period: 15 months of treatment Interim analysis: 6 months after inclusion of the last patient | 171 | TFIA | Therapeutic group Lithium+riluzole (n=87) (28% bulbar) (17% without riluzole) Lithium levels: 0.4-0.8mEq/L Age 58.3/FVC 83.9%/ALSFRS-R 36.9 Subtherapeutic group Lithium+riluzole (n=84) (34% bulbar) (5% without riluzole) Lithium levels: 0.2-0.4mEq/L Age 58.5/FVC 84.8% ALSFRS-R 36.9 Terminated after first interim analysis | No | No differences in PE, P= .94 ThG: 60 drop-outs ALSFRS-R decline per month: −1.26; FVC decline per month: −3.4 SThG: 36 drop-outs ALSFRS-R decline per month: −1.15 FVC decline per month: −2.4 | B Single-blind dose-finding study Not completed Does not exclude minor benefits No control group |
| 4 | Open-label study: lithium-treated patients compared to historical controls WFN classification: Prob and Def Age: 21-85 IC: disease duration < 36 months; FVC ≥ 75% Lithium levels: 0.3–0.8 mEq/L PE: decrease in ALSFRS-R scores Treatment period: 12 months 3 interim analyses | 109 | Fin | n=107 (22.4% bulbar) (65.4% with riluzole) Mean age: 56.2 Disease progression: 1.55 years ALSFRS-R: 37.2 FVC: 94.9% | Historical controls: placebo group from the trial with minocycline (n=264) (21% bulbar) (66.7% with riluzole) Age: 57.6 Disease progression: 1.46 years ALSFRS-R: 38.0 FVC: 93.8% | TG: ALSFRS-R decline per month, −1.2; FVC decline per month: −2.84 AE: 62 patients CG: ALSFRS-R decline per month, −1.01; FVC decline per month: −2.91 AE: 40 patients | B No true control group 33% patients without riluzole Treatment not masked or blinded |
| 5 | Randomised, double-blind, placebo-controlled trial, with a plan for sequential analysis Lithium levels: 0.4-0.8 mEq/L. WFN classification: Prob, ProbL, or Def IC: disease duration > 6 months and < 36 FVC > 70% PE: death, tracheostomy with permanent invasive ventilation or non-invasive ventilation > 23hours/day Designed to detect differences of 15% in survival at 15 months | 133 | Fin | Lithium+riluzole (n= 66) (29% bulbar) Age: 59.5 Disease progression: 12 months ALSFRS-R: 42 Mean FVC>73% | Riluzole (n=41) (28% bulbar) Age: 59 Disease progression: 14 months ALSFRS-R: 41 Mean FVC>70% | ThG: 33 patients reached endpoint, 30 interrupted treatment Cumulative survival probability 0.73 CG: 28 patients reached endpoint, 20 interrupted treatment Cumulative survival probability 0.75 Mean decrease in ALSFRS-R, P=.74 Mean decrease in FVC, P=.22 | A Excellent |
| 6 | Open-label study. All data are self-reported IC: NA WFN classification: uncertain PE: self-reported ALSFRS-R and AE Treatment duration: 12 months | 864 | Fin | 78+149 | 637 | No benefits after 12 months of treatment | B Open-label No blinding Variables were not measured objectively Incomplete data |
| 7 | Non-randomised, single-centre WFN classification: Prob, ProbL, or Def Age >18 years IC: disease duration<36 months Treatment duration: 18 months | 40 | Fin | Lithium+riluzole+vitamin E (n=40) | No parallel control group | NA | B No control group Third drug introduced |
| 8 | Randomised, double-blind, placebo-controlled trial WFN classification: Poss, Prob, ProbL, or Def Lithium concentrations: 0.4–0.8mEq/L IC: disease duration >6 months and <36; FVC>60% PE: survival Observation period: 18 months Designed to detect a 17.5% difference in survival at 18 months 77 patients were included in an additional study | 220 | Fin | Lithium+riluzole | Riluzole | NA | A Excellent |
| 9 | Non-randomised, open-label study, lithium-treated patients compared to historical controls PE: survival at 15 months | NA | Ong | Lithium+riluzole | Historical controls | NA | B No control group Non-randomised |
| 10 | Randomised, double-blind trial, with parallel control group WFN classification: Prob or Def Age: 18–85 IC: disease duration <36 months; FVC>60%. | NA | PT | Lithium+riluzole | Riluzole | PT | NAs |
| 11 | Randomised, double-blind trial, with parallel control group WFN classification: Prob, ProbL, or Def Age: 18–85 IC: disease duration <36 months; FVC≥50%; ALSFRS-R>30 | 280 | PT | Lithium+riluzole | Riluzole | PT | NAs |
| 12 | Randomised, double-blind trial, with parallel group WFN classification: Prob, ProbL, or Def Age: 18–75 PE: survival at 18 months; FVC≥60% | 226 | PT | Lithium+riluzole | Riluzole | PT | NAs |
A, excellent; B, uncertain; C, inadequate; IC, inclusion criteria; Fin, finalised; Def, definitive; E, event (decrease ≥6 points on ALSFRS-R or death); PE, primary endpoint; D, deaths; TFIA, prematurely terminated at first interim analysis; CG, control group; TG, treated group; NA, data not available; NAs, not assessable; Ong, ongoing; PEG, percutaneous endoscopic gastrostomy; Poss, possible; Prob, probable; ProbL, clinically probable laboratory supported; PT, prematurely terminated; SThG, subtherapeutic group; ThG, therapeutic group; NIMV, non-invasive mechanical ventilation.
In general, the study design for the included trials varies greatly, especially regarding inclusion criteria, sample size, randomisation, type of control population, primary and secondary endpoints, blinding, discontinuation criteria, analysis of results, and such other methodological details as type of blinding, open or closed clinical trial, drugs used for the control group, dose-finding design, sequential or non-sequential design, parallel or cross-over groups, and how disease progression was measured. According to data provided by the studies listed above, approximately 1100 patients have been treated with lithium, but results are only available for 627 of them. Lithium is therefore the drug that has been tested on the greatest number of patients and in the greatest number of clinical trials in the search for an effective treatment for ALS. Unfortunately, none of these trials has been able to replicate the unparalleled results reported by Fornai et al., and the hypothesis that lithium has a positive effect on disease progression has therefore been ruled out. In this study, we will describe the main characteristics and results of each clinical trial.
Pilot study by Fornai et al.In February 2008, this Italian research group reported the results of a pilot clinical study (randomised single-blind parallel-group study) of 44 patients presenting a disease duration <5 years and meeting the El Escorial revised diagnostic criteria.3 Sixteen patients were randomly selected to receive riluzole+lithium carbonate, and the remaining patients (n=28) were administered riluzole only. At time of inclusion, the distribution of patients with bulbar ALS was similar in both groups, and patients and controls were matched for forced vital capacity (FVC). Patients were assessed on 6 occasions (at baseline and every 3 months during the 15-month study period). The primary endpoint was survival rate. The secondary endpoints were changes on the ALSFRS-R and the Norris scales. Muscle strength (MRC scale), FVC, and quality of life (SF-36) were also evaluated. Lithium concentrations had to range from 0.4 to 0.8mEq/L. Surprisingly, all patients receiving lithium were alive at the end of the study, while 29% of the patients receiving riluzole died. The researchers concluded that lithium delays progression of ALS. When measuring disease progression in the lithium-treated group, the ANOVA test showed no statistically significant differences in the scores achieved on either the Norris scale (baseline score 79.4±2.8; final score 71.0±3.9) or the ALSFRS-R (baseline score 39.9±1.2; final score 34.3±2.3). Likewise, FVC was not found to decrease significantly (baseline value 89.0±2.6; final value 74.9±3.6). The only significant decrease was found in progression of MRC scores (baseline score 138.5±1.6; final score 113.4±4.9). The patients treated with riluzole only scored significantly lower on both the Norris scale (baseline score 86.6±2.1; final score 55.3±3.2) and the ALSFRS-R (baseline score 40.2±0.8; final score 24.2±1.8). A significant deterioration in FVC was also found in this group (baseline value 91.0±1.9; final value 58.0±3.0). Results for MRC scores were similar (baseline value 140.7±1.2; final value 92.0±5.3). According to Fornai et al., lithium significantly delayed disease progression during the 15-month follow-up. Changes were detectable on the functional scales beginning in the third month of treatment, and beginning in the sixth month on the MRC scale and FVC. After 15 months of follow-up, none of the 16 lithium-treated patients required BiPAP, while 8 of the 28 controls receiving riluzole required BiPAP and/or PEG before death. No significant adverse effects were reported in the lithium-treated group.17
ClinicalTrials.gov NCT00818389This research group conducted a randomised double-blind placebo-controlled trial. Patients were recruited from 21 ALS clinics in North America (11 in the US and 10 in Canada).19 Lithium levels required for this study were the same as those in the study by Fornai et al. The primary endpoint was the time elapsed to an event defined as either death or a decrease ≥6 points from baseline on the ALSFRS-R. Patients in the placebo group were switched to lithium when they displayed a drop of 6 points. The secondary endpoints were ALSFRS-R, ALSSQOL, QIDS-SR16, and tracheostomy-free survival. The NEALS database of past clinical trial placebo groups was used as a reference; these placebo groups showed a mean decrease of 1 point per month on the ALSFRS-R. Thus, a 6-month period of active treatment may be considered the minimum period to determine therapeutic efficacy. In-person consultations were scheduled for weeks 4, 8, 12, 20, 28, 36, 44, and 52 of follow-up. Telephone visits were scheduled for weeks 16, 24, 32, 40, and 48. The study was designed to detect a 40% decrease in the rate of decline (ALSFRS-R). The study design also included 3 interim analyses calculated according to a mathematical function that is proportional to the number of events predicted to have occurred. A total of 167 events were expected for a population of 250 patients. The first interim analysis was planned for after the inclusion of the first 84 patients. At that point, the trial could either be halted or continue to follow the 84 patients and analyse their data 6 months later. The stopping boundary was defined based on the following contrasting hypothesis: one-sided P-value <.5 would favour lithium while P-values >.5 would favour the placebo. At the first interim analysis, the futility stopping boundary was established at P-values ≥.68, whereas the P-value for the efficacy stopping boundary was .001. From January 2009 to June 2009, 84 patients were randomised to lithium+riluzole (n=40) or to placebo+riluzole (n=44). All patients were monitored during a mean of 5.4 months until the study was prematurely terminated due to futility in September 2009. The first interim analysis, which was conducted at that time, favoured the placebo (P=.78); these values exceeded those indicating futility (P≥.68). This analysis also found that 22 of the 40 patients in the lithium-treated group experienced an event compared to 20 of the 44 patients in the placebo group. Of the 5 patients who died during the study, 3 were in the placebo group. Regarding ALSFRS-R scores, 38 patients showed a drop ≥6 points from the baseline value (21 in the lithium-treated group and 17 in the placebo group). The most frequent adverse effects were generalised muscle weakness, fatigue, nausea, falls, dyspnoea, limb oedema, fasciculations, dysphagia, headache, and back pain. The authors concluded that their trial failed to reproduce the exceptional results reported by the Italian research group, as they observed no significant delays in disease progression in lithium-treated patients. Nevertheless, they suggested that future studies investigate the possibility of less marked beneficial effects of lithium for patients with ALS.
EudraCT 2008-001094-15In August 2010, an Italian research group reported the results from a multicentre single-blind randomised dose-finding study conducted in 21 centres and including 171 patients.21 The inclusion criteria were ages between 18 and 75 years, disease onset ≤36 months prior, mild to moderate disability (ALSFRS-R), FVC≥50%, and evidence of disease progression in the previous 3 months. Patients were randomly assigned to 2 different groups. One group received lithium at doses sufficient to maintain serum levels between 0.4 and 0.8mEq/L (therapeutic group, TG). Patients included in the second group were dosed with lithium to achieve lithium levels between 0.2 and 0.4mEq/L (subtherapeutic group, STG). The primary endpoint was survival (outcomes other than death or tracheostomy), or severe loss of autonomy, defined as ALSFRS-R scores ≤1 for swallowing, ≤1 for walking, or ≤2 for breathing. The secondary endpoints were mean monthly changes in the following: (1) total ALSFRS-R score; (2) FVC; (3) number of treatment failures (termination for futility, adverse effects, or both); and (4) quality of life (McGill QLQ). The follow-up period covered 15 months, and consultations were scheduled at 0, 1, 3, 6, 9, 12, and 15 months. The study included an interim analysis of the efficacy and safety of lithium; it was conducted 6 months after the last patient had enrolled. Early termination of the trial was recommended on 2 November 2009 due to the results from the interim analysis, which showed that 117 patients (68.4%) had left the study (60 from the TG and 57 from the STG). The reasons for patient drop-outs were death (21), tracheostomy (14), severe disability (15), adverse effects (32), lack of efficacy (31), and poor compliance (4). Adverse effects were observed in both groups (35 patients from the TG and 36 from the STG). Deep venous thrombosis was commonly reported among patients in the TG, while gastrointestinal disorders were more frequent among patients in the STG. The most frequent adverse effects attributable to lithium were tremor, thyroid disorders, and polyuria. The authors conclude that the frequency of adverse effects, even at subtherapeutic doses, raises serious doubts about the safety of lithium for the treatment of ALS. Lithium efficacy could not be adequately evaluated due to early termination of the trial. In any case, monthly changes in ALSFRS-R and FVC were similar in the TG and STG, which suggests that the therapeutic dose of lithium was no more efficient than the subtherapeutic dose. Likewise, no intergroup differences were observed in the primary endpoint. The authors recognised that lack of a placebo group constituted an important limitation.
ClinicalTrials.gov NCT00790582This 13-month phase II open multicentre study was conducted at 10 locations from the Western ALS Study Group (WALS) and included a group of historical controls.23 Patient inclusion criteria were as follows: (1) a diagnosis of probable or definite ALS with clinical onset <3 years prior; (2) age within the range of 21 to 85 years; and (3) FVC≥75%. The initial dose of lithium carbonate was 150mg twice daily. This dose was adjusted to reach serum lithium levels ranging between 0.3 and 0.8mEq/L. The primary endpoint was a change in ALSFRS-R scores. The secondary endpoints included changes in FVC, weight loss, quality of life (ALSQLS), and survival time (defined as time until death, invasive ventilation, or use of non-invasive ventilation 23hours/day for 14 days). The study was designed to detect reductions ≥30% in the mean slope of ALSFRS-R scores. Three interim analyses of safety were to be conducted after 30 patients completed 6 months of treatment, after 60 patients completed 6 months of treatment, and after 60 patients had completed 9 months of treatment. In addition to lithium, riluzole was administered to 65.4% of the patients. Of the 107 patients included in the study, only 78 completed the full treatment period. Some of the reasons for interruption were death (14 patients, although these deaths were not lithium-related) and adverse effects (12 patients). The mean change in ALSFRS-R scores was −1.20 per month in the lithium-treated group and −1.01 per month in the control group. This decline was more marked in the 37 patients taking lithium without riluzole (−1.53 per month) than in the 70 patients taking lithium plus riluzole (−1.03 per month). In contrast, no differences were observed in FVC, weight loss, quality of life, or time to death/tracheostomy/use of non-invasive ventilation for 23hours/day. Adverse effects were frequent among patients taking lithium (62 patients vs. the 40 expected), although they were attributable to lithium toxicity in only one case. Based on their findings, the researchers concluded that lithium has no beneficial effects for patients with ALS and discouraged any further trials of this drug.
EudraCT 2008-002110-22 (LITRA)This phase IIb sequential trial was a randomised double-blind placebo-controlled parallel-group trial. Eligibility criteria were as follows: having probable ALS, clinically probable laboratory supported ALS, or definite ALS; ages between 18 and 85 years; symptom onset ≥6 months and ≤36 months before inclusion; and FVC>70%.25 Patients were recruited from 3 centres in the Netherlands. The primary endpoint was efficacy, defined as survival time until death, invasive ventilation, or use of non-invasive ventilation for more than 16hours/day. The secondary endpoint was deterioration according to ALSFRS-R and FVC results. An independent biostatistician sequentially monitored the data to determine at each interim analysis whether or not the trial should continue. During the inclusion period (November 2008 to June 2011), a total of 133 patients were randomised to receive lithium (66) or placebo (67). The mean follow-up time was 16 months for the lithium group and 185 months for the placebo group. Medication had to be discontinued in 30 lithium-treated patients (45%) and in 20 controls (30%), with a mean of 10 months of treatment. Some of the causes for discontinuation were fatigue, general unease, tremor, psychological disorders, polyuria, nocturia, nausea, restless leg syndrome, headache, palpitations, elevated transaminases, and skin changes. Of the 61 patients reaching the primary endpoint (51 died, 1 underwent a tracheostomy, and 9 used non-invasive mechanical ventilation >16hours/day), 33 were lithium-treated patients and 28 were placebo controls. The cumulative survival probability at 12 months was 0.73 in the lithium group and 0.75 in the placebo group, and at 16 months it was 0.62 and 0.67, respectively. These results rule out the possibility of a modest positive effect for lithium, defined as a 15% increase in the cumulative survival rate. The researchers concluded that lithium plus riluzole did not improve survival times in patients with ALS. No differences were observed in the decline of mean ALSFRS-R scores (P=.74) and FVC (P=.22).
Patients-like-meA great number of patients requested treatment with lithium for compassionate use due to there being easy access to the drug, the high expectations generated by the pilot study by Fornai et al., and the difficulty of being included in any of the trials mentioned above (estimates in the US show that 20% of patients with ALS are included in a clinical trial).24 A group of researchers therefore decided to analyse the data reported by patients with ALS on PatientsLikeMe.31 In a preliminary report, they analysed data from 75 patients who had been prescribed lithium.32 This study also included 75 patients not taking lithium who served as controls. No changes in progression were found at 12 months according to the ALSFRS-R. The final study (May 2011) included the information from 348 lithium-treated patients. Of the total, 78 patients had completed 12 months of treatment and 149 had discontinued the medication at some point. The study also included 637 patients not treated with lithium as the control group. After 12 months of treatment, lithium was observed to have no impact on disease progression. According to these authors, observational studies based on Internet data represent a fast, cost-effective tool for measuring the impact of new treatments.24
EudraCT 2009-010060-41This non-parallel-group non-randomised non-controlled single-centre interventional clinical trial was conducted in Italy starting in February 2009. The expected duration was 6 months for the recruitment phase and 18 months to complete the study. It included 40 patients. Although the trial was finalised its results are not available. This trial is particular in that patients had to be under treatment with riluzole and vitamin E before inclusion. The primary endpoint was survival time with complications (PEG or tracheostomy) or without them; the secondary endpoint was drug safety.
EudraCT 2008-006891-31 (LiCALS; UKCRN ID 5745)This trial was planned to be completed in October 2011. It was a multicentre double-blind randomised placebo-controlled trial including 220 patients who were randomly assigned to lithium or placebo. The follow-up period was 18 months. The primary outcome measure was survival at 18 months, and the secondary outcome measures were adverse effects, safety, functional capacity (ALSFRS-R), quality of life (EQ-5D), and psychological distress (HADS). The study was conducted in 10 centres in the UK and recruited 97% of the anticipated 220 patients.33 An extension study (UKCRN ID 9349) was planned for those patients participating in the LiCALS who were still alive at 18 months. Data collection was expected to close by 31 November 2011. According to our last query, 77 patients had been recruited for that phase.34
EudraCT 2008-003707-32 (LISLA)Starting in October 2008, this open interventional non-randomised controlled clinical trial includes historical patients as its parallel group and is currently underway in France. The primary endpoint is survival at 15 months; any changes in ALSFRS-R between inclusion and completing 15 months of treatment constitute the secondary endpoint.28
EudraCT 2008-006224-61This interventional randomised double-blind parallel-group clinical trial, funded by the Spanish Institute of Health Carlos III (research fund EC08/00077), was terminated prematurely.28
EudraCT 2008-005106-38 (LIELA)This was the second Spanish study to be funded by the Institute of Health Carlos III (health research fund EC08/00016) and it was also terminated prematurely. It was a closed randomised double-blind parallel-group trial including 280 patients.28,35
EudraCT 2008-006722-34This closed multicentre randomised double-blind parallel-group study included 226 patients from 22 centres in Italy and was designed to take place over 24 months.28
DiscussionThe pilot study by Fornai et al. reports the most marked therapeutic effect (a 43% reduction in disease progression) that has ever been achieved in patients with ALS. According to preclinical studies of SOD1G93A− mice, this beneficial effect was attributed to lithium's ability to promote autophagy. One of the direct consequences of the expectations generated by these results was the pressure from patients with ALS on doctors in order to receive this promising treatment. Another consequence was the unusual number of clinical trials that were conducted to confirm those findings. The debate on the efficacy and safety of lithium for patients with ALS started after the earliest experiences of patients treated with lithium for compassionate use were reported on the website PatientsLikeMe and the results of the first ad hoc clinical trial were published; this clinical trial was terminated prematurely after the first interim analysis showed that lithium failed to decrease the rate of decline in ALSFRS-R by 40%.18,19,24,32,36–43 The study by Aggarwal et al. was in fact designed to detect a beneficial effect similar to that reported by the pilot study (a 40% decrease in the rate of functional decline). However, the study was unable to rule out any modest or delayed beneficial effects of lithium since it was terminated at 6 months.19,25 A novel aspect of this trial was the introduction of the ‘time-to-event’ endpoint as a primary outcome measure, which was defined as a decrease of at least 6 points on the ALSFRS-R or death. However, the decrease in ALSFRS-R scores, which was one of the 2 primary endpoints, does not follow a linear curve. This is especially frequent among patients who have recently been diagnosed with ALS and those in advanced stages of the disease, whose scores may remain stable for months. Other relevant aspects of the design of this study were the interim analyses that were planned and the fact that patients in the placebo group were switched to lithium after the time to event endpoint was reached.44 No significant safety-related issues were observed. The study by Chiò et al., a dose-finding trial, lacked a placebo group. This study confirmed the lack of efficacy of lithium at any dose. The rate of death or disability was similar in both groups. This trial, which was also terminated for futility, provided class II evidence that the therapeutic and subtherapeutic doses did not yield different results for the primary outcome of efficacy, but the high drop-out rate (68.4%) reduced the impact of the trial.25,36,44
The trial by Miller et al.45 had a novel design in that the control group consisted of patients from a historical database: the patients from the placebo group of a minocycline trial. This was the study requiring the greatest FVC for inclusion. A positive feature of this study was that it defined criteria for treatment failure, especially the number of hours a patient needed non-invasive ventilation. Lithium was not superior to placebo in terms of survival, decline in FVC, and weight loss. However, the decline in ALSFRS-R scores was 20% faster in patients treated with lithium (P=.04, class IV evidence). These researchers concluded that their findings did not support any beneficial effects of lithium for ALS progression. Although they acknowledge that comparing survival between the lithium-treated group and a group of historical controls is a limitation, this type of design allows everyone treated with the study drug to be included in the patient group.23,44 Another limitation is that the lithium-treated group included numerous patients who were not treated with riluzole (37 of 107).
The data reported by patients themselves on the Internet was also consistent with the results from the 3 previous clinical trials. Researchers and patients agreed that lithium is not well tolerated and none was able to confirm the efficacy reported by the pilot study.24
The results from the fifth clinical trial were published in May 2012. This is one of the 2 trials designed to detect any modest effects of lithium (15%) on cumulative survival.25,33 Both studies are randomised double-blind placebo-controlled trials with death or severe respiratory failure as the primary endpoint. The sequential design of LITRA required fewer patients than traditional trials with the same power and enabled discontinuation of the study as soon as it provided enough evidence of the efficacy of lithium. This study was expected to provide a definitive answer on the efficacy of lithium for patients with ALS (class I evidence), but the drug showed no beneficial effects for either survival or functional decline.25 The sixth trial, LiCALS, was also designed to detect modest beneficial effects (17.5%) on survival; although it is now completed, its results have not been published.33,34
As the results from these clinical trials were published, criticism of the pilot study by Fornai et al. increased, especially regarding its methodology and patient selection criteria. Some suggest that its optimistic results were due to the small sample size. The study does not provide an explanation of its patient selection process and inclusion criteria. Likewise, it does not explain why there were fewer lithium-treated patients than patients who did not receive lithium. Lithium-treated patients were younger and presented shorter disease duration times than controls. Additionally, the study population was atypical: mean disease duration exceeded 3 years and baseline ALSFRS-R scores were high (39.9 in lithium-treated patients and 40.1 in controls). These values correspond to patients with very slow disease progression (0.2 ALSFRS-R points/month).23,44,46 All these factors may have contributed to the false-positive results reported by these authors.23
There is also controversy regarding the time needed to achieve lithium concentrations within the therapeutic range. Lithium is an accessible, affordable FDA-approved drug for the treatment of bipolar psychosis which has been in use for more than 50 years. Clinical response at treatment onset is typically delayed (possibly due to the drug's effect on the expression of neuronal genes), which makes it difficult to reach therapeutic lithium concentrations. This pharmacokinetic peculiarity requires strict monitoring of lithium concentrations and adjustment of doses, which negatively affects patients’ adherence to treatment.5,6,12,47–49 According to several studies, only a third of all psychiatric patients comply with the dose prescribed by their doctors.12,50–52 Patients with ALS seem to behave in a similar way. For example, in the trial by Aggarwal et al. only 36.8% of patients reached plasma lithium concentrations between 0.4 and 0.8mEq/L during the fourth week of treatment. This percentage rose to 47.4% in the eighth week. Six patients never reached lithium concentrations within the therapeutic range. Interestingly, 2 of the patients in the placebo group were found to have therapeutic lithium concentrations at their 12-week follow-up visit.19 The study by Chiò et al. does not specify how quickly therapeutic lithium concentrations were reached, but 4 patients in the TG never achieved the established plasma concentrations although the dose was increased to 1200mg/day. In the study by Miller et al., the mean lithium concentration at the end of the initial titration period was 0.36mEq/L (range, 0.2-0.8); however, the authors do not provide the percentage of patients with blood levels below the target for this study (0.3-0.8mEq/L). Mean blood lithium levels at 6 and 12 months were 0.35 and 0.37mEq/L, respectively. The maximum dose administered was 450mg/day: Fornai et al. reported confusion and vertigo in 2 patients taking 600mg (the maximum dose in the pilot study). Similar results were reported in the LITRA study: only 73% of the patients in the lithium-treated group presented lithium concentrations within the target range (0.4-0.8mEq/L). In their pilot study, Fornai et al. do not indicate the rate at which patients achieved therapeutic lithium concentrations or the percentage of patients able to do so.
The adverse effects most frequently associated with lithium were fatigue, general unease, tremor, dizziness, polyuria, nocturia, falls, and nausea. The lithium group in the pilot study by Fornai et al. did not exhibit more adverse effects than the untreated group. Differences in severe adverse effects between the lithium-treated group and the placebo group were also non-significant in the study by Aggarwal et al. (10 patients and 8 controls). In the study by Chiò et al., adverse effects or severe adverse effects were experienced by 35 patients in the TG and 36 in the STG. Miller et al. observed that severe adverse effects were more frequent in the lithium group (62 patients vs. the 40 expected), although only one case was directly associated with lithium. Likewise, the study conducted in the Netherlands reported more adverse effects in lithium-treated patients.
In conclusion, the hypothesis that lithium may be effective for treating ALS comes from a pilot study with numerous limitations. None of the clinical trials conducted subsequently has confirmed claims that lithium produces benefits or shown even a moderate delay in disease progression. One might argue that the first 3 clinical trials19,21,23,43 were designed to confirm the beneficial effects reported by Fornai et al. However, the LITRA study, designed to detect more modest effects, demonstrated that lithium provides no benefits for survival or functional decline in patients with ALS.25,36,44,53–55
FundingThe present study received financial support from the Spanish health research fund (FIS 10/01070).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gamez J, Salvado M, Martínez de la Ossa A, Badia M. Litio para el tratamiento de la esclerosis lateral amiotrófica: mucho ruido para nada. Neurología. 2016;31:550–561.