Given that epileptic seizures and non-epileptic paroxysmal events have similar clinical manifestations, using specific diagnostic methods is crucial, especially in patients with drug-resistant epilepsy. Prolonged video electroencephalography monitoring during epileptic seizures reveals epileptiform discharges and has become an essential procedure for epilepsy diagnosis. The main purpose of this study is to characterise paroxysmal events and compare patterns in patients with refractory epilepsy.
MethodsWe conducted a retrospective analysis of medical records from 91 patients diagnosed with refractory epilepsy who underwent prolonged video electroencephalography monitoring during hospitalisation.
ResultsDuring prolonged video electroencephalography monitoring, 76.9% of the patients (n=70) had paroxysmal events. The mean number of events was 3.4±2.7; the duration of these events was highly variable. Most patients (80%) experienced seizures during wakefulness. The most common events were focal seizures with altered levels of consciousness, progressive bilateral generalised seizures and psychogenic non-epileptic seizures. Regarding all paroxysmal events, no differences were observed in the number or type of events by sex, in duration by sex or age at onset, or in the number of events by type of event. Psychogenic nonepileptic seizures were predominantly registered during wakefulness, lasted longer, started at older ages, and were more frequent in women.
ConclusionsParoxysmal events recorded during prolonged video electroencephalography monitoring in patients with refractory epilepsy show similar patterns and characteristics to those reported in other latitudes.
La inexistencia de signos clínicos que diferencien entre crisis epilépticas y episodios paroxísticos no epilépticos hace necesario utilizar métodos diagnósticos específicos, principalmente en pacientes refractarios al tratamiento farmacológico. La monitorización prolongada con videoelectroencefalografía durante las crisis epilépticas evidencia descargas epileptiformes en el EEG ictal y constituye una prueba fundamental para su diagnóstico. La presente investigación pretende caracterizar los eventos paroxísticos y comparar los patrones encontrados en pacientes con diagnóstico de epilepsia refractaria.
MétodosSe realizó un estudio y análisis retrospectivo a partir de los registros médicos de la monitorización prolongada con video EEG de 91 pacientes diagnosticados con epilepsia refractaria durante su internamiento.
ResultadosDurante el videoelectroencefalograma prolongado el 76,9% (n=70) de los pacientes presentaron eventos paroxísticos. El número promedio de eventos fue 3,4 (±2,7) y su duración fue muy variable. La mayoría de los pacientes (80,0%) presentó las crisis durante vigilia y los principales tipos de eventos registrados fueron: focales con alteración de la conciencia, evolutivos a crisis convulsivas bilaterales y crisis psicógenas no epilépticas. Considerando la totalidad de los eventos paroxísticos, no se objetivan diferencias en cuanto al número o tipo de eventos descritos según el sexo, la edad de inicio de la enfermedad o el sexo y la duración de los eventos, o al número de eventos según el tipo. Las crisis psicógenas no epilépticas se registran predominantemente en vigilia, presentan mayor duración, se inician más tardíamente y ocurren principalmente en mujeres.
ConclusionesLos eventos paroxísticos observados durante la monitorización prolongada con videoelectroencefalograma de pacientes internados con epilepsia refractaria muestran patrones y características similares a los descritos en otras latitudes.
Diagnosis of epilepsy is usually based on the patient's medical history and physical examination, electroencephalography (EEG), and imaging findings.1–3 The International League Against Epilepsy defines an epileptic seizure as a sudden, transient occurrence of signs or symptoms due to abnormal, excessive, or hypersynchronous neuronal discharges in the brain.4
The intermittent nature of clinical manifestations and the variability of EEG findings make it difficult to establish a precise diagnosis of epilepsy. Presence of epileptiform discharges on the ictal EEG recording constitutes the most conclusive finding; prolonged video-EEG monitoring is therefore the most useful diagnostic tool.5,6 Video-EEG monitoring consists in the continuous, simultaneous, synchronous recording of the patient's symptoms, behaviour, and brain electrical activity. Patients may be monitored for several days; the technique aims to analyse interictal activity and to establish correlations between electrical activity and clinical alterations during epileptic seizures.2,7
According to the International League Against Epilepsy, the main indications for prolonged video-EEG monitoring are: (1) diagnostic assessment, (2) differential diagnosis between epileptic and non-epileptic episodes, (3) presurgical identification of the epileptogenic focus, (4) identification, quantification, and classification of the type of seizure and/or epileptic syndrome, and (5) quantitative assessment of seizure control.3,8,9 Video-EEG also helps determine the need for complementary tests, including specific neuroimaging, genetic, or metabolic studies. It is common for patients’ diagnosis and treatment to be changed following video-EEG monitoring.10–12 Despite the advantages of video-EEG, the technique is only available at specialised centres due to the high costs involved in terms of equipment and staff.13
The lack of pathognomonic signs distinguishing epileptic from non-epileptic seizures makes traditional diagnostic tools insufficient for determining the nature of the paroxysmal event.14–16 Psychogenic seizures, anoxic seizures, paroxysmal sleep disorders, and motor disorders constitute the main types of non-epileptic paroxysmal events.17 Psychogenic non-epileptic seizures (PNES), also known as pseudoseizures, are sudden events resembling epileptic seizures which are triggered by psychological factors. Although these events are not associated with abnormal neuronal discharges, the psychopathogenic mechanisms are poorly understood and there is no evidence that the symptoms constitute a single syndrome.18
The prevalence of PNES ranges from 2 to 33 cases per 100000 population,19 and up to 70% of cases are associated with psychiatric comorbidities.20–22 Identifying PNES constitutes a clinical challenge and is of great importance in the differential diagnosis of epilepsy: up to 32% of patients diagnosed with refractory epilepsy and assessed in monitoring units actually have PNES.23–25
A lack of ictal changes in conventional EEG during episodes has traditionally been considered a typical feature of PNES; however, this is insufficient for diagnosis as a considerable percentage of cases of frontal lobe epilepsy, frontomesial seizures, and focal seizures show no changes in surface EEG.16,26 While video-EEG is the standard technique for diagnosing PNES, diagnosis is also based on the patient's medical history, symptoms, and findings from complementary examinations, including neuropsychological tests. Video-EEG validates the diagnosis of PNES when seizures are not accompanied by epileptiform EEG activity.27
Diagnosis of PNES takes a mean of 7 years from the onset of paroxysmal events; the condition has severe consequences and a considerable impact on patients’ quality of life28,29 and is associated with a high social cost.30–32 Diagnosis of PNES does not rule out the presence of epileptic seizures; the 2 entities coexist in 4-6 cases per 1000 patients.20,23
The purpose of this study is to describe paroxysmal events and analyse the characteristics of those associated with abnormal neuronal discharges or psychogenic seizures; to this end, a series of patients with a diagnosis of refractory epilepsy were monitored with video-EEG at a specialised epilepsy unit.
Patients and methodsWe analysed all medical records of patients diagnosed with refractory epilepsy. Patient video-EEG monitoring was carried out between 1 August 2012 and 31 October 2014 at the epilepsy monitoring unit of the neurology department at Hospital San Juan de Dios, in San José, Costa Rica. The study includes no patients younger than 13 years, since our centre does no assess these patients.
Patients stayed at the unit for 6 days for video-EEG monitoring. Seizures were induced either by reducing the doses of antiepileptic drugs or by depriving patients of sleep before or during their stay. As per our unit's protocol, pharmacological treatment was resumed if a patient presented seizures entailing potential risks. Patients were monitored at all times by trained nursing staff.
They were monitored with a 44-channel unit using surface electrodes, which were placed at the locations described by the international 10-20 system. Additional positions were used as necessary. Pharmacological treatment was reintroduced after patients left the unit; patients remained hospitalised for at least 24 hours after treatment was resumed or until they reached their baseline condition.
Any sudden EEG or clinical alteration was considered a paroxysmal event. When patients displayed more than one event of the same type during video-EEG monitoring, we calculated the mean duration. When the video-EEG detected different types of event, we recorded the most frequent type. Non-conclusive events were those not adequately documented in the patient's medical record.
We analysed the following variables: sex, age at disease onset, number of events during monitoring, duration of the event, type of event, and state during the event (asleep/awake). We estimated the absolute and relative frequencies of categorical variables, and measures of central tendency (means and medians) and dispersion (standard deviations and ranges) for quantitative variables. Unless otherwise indicated, data are expressed as absolute quantities and percentages (qualitative variables) or mean±standard deviation (quantitative variables). After checking for normal distribution and homogeneity of variance, we compared means between groups using either the t test or ANOVA. Where tests did not show a normal distribution or homoscedasticity, analyses were performed using non-parametric tests followed by post hoc analysis to study significant correlations. P-values<.05 were considered statistically significant. Statistical analysis was conducted using the SPSS software, v. 22.0 (SPSS; Armonk, NY, USA). Graphs were created using Sigmaplot v. 11.0 (Systat Software; San Jose, CA, USA). The study protocol was approved by our hospital's Bioethics Committee and complies with the ethical standards established by the Declaration of Helsinki.
ResultsDuring the study period, we identified 91 medical records of patients diagnosed with refractory epilepsy; these patients were monitored with video-EEG. The sample included 43 men (47.3%) and 48 women (52.7%). Patients were young, with a mean age of 33.6 years. Eleven patients (12.1%) had initially been diagnosed with PNES.
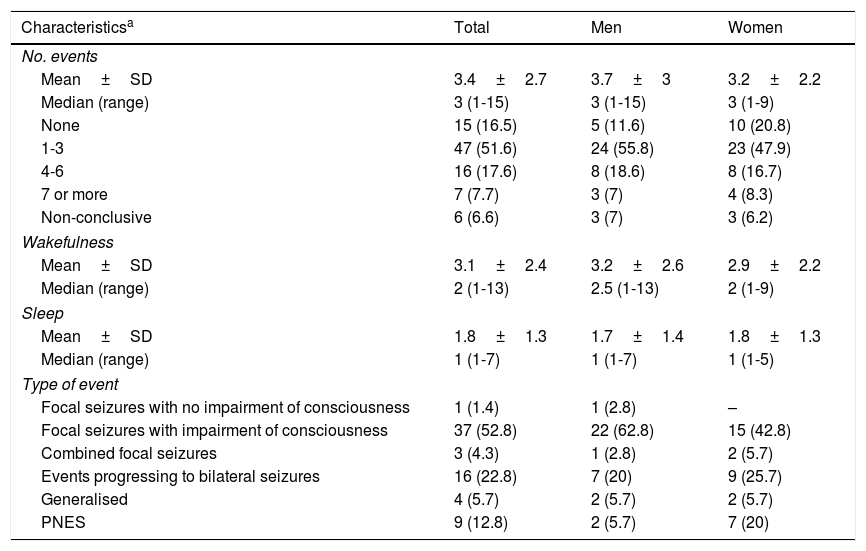
During video-EEG monitoring, 70 patients (76.9%) presented paroxysmal events (3.4±2.7 events; range, 1-15). Fifteen patients (16.5%) presented no events during monitoring; this was more frequent in women (20.8%) than in men (11.6%). We recorded 6 (6.6%) non-conclusive events (Table 1).
Events recorded during video-EEG monitoring in patients with refractory epilepsy at our epilepsy monitoring unit between 2012 and 2014.
| Characteristicsa | Total | Men | Women |
|---|---|---|---|
| No. events | |||
| Mean±SD | 3.4±2.7 | 3.7±3 | 3.2±2.2 |
| Median (range) | 3 (1-15) | 3 (1-15) | 3 (1-9) |
| None | 15 (16.5) | 5 (11.6) | 10 (20.8) |
| 1-3 | 47 (51.6) | 24 (55.8) | 23 (47.9) |
| 4-6 | 16 (17.6) | 8 (18.6) | 8 (16.7) |
| 7 or more | 7 (7.7) | 3 (7) | 4 (8.3) |
| Non-conclusive | 6 (6.6) | 3 (7) | 3 (6.2) |
| Wakefulness | |||
| Mean±SD | 3.1±2.4 | 3.2±2.6 | 2.9±2.2 |
| Median (range) | 2 (1-13) | 2.5 (1-13) | 2 (1-9) |
| Sleep | |||
| Mean±SD | 1.8±1.3 | 1.7±1.4 | 1.8±1.3 |
| Median (range) | 1 (1-7) | 1 (1-7) | 1 (1-5) |
| Type of event | |||
| Focal seizures with no impairment of consciousness | 1 (1.4) | 1 (2.8) | – |
| Focal seizures with impairment of consciousness | 37 (52.8) | 22 (62.8) | 15 (42.8) |
| Combined focal seizures | 3 (4.3) | 1 (2.8) | 2 (5.7) |
| Events progressing to bilateral seizures | 16 (22.8) | 7 (20) | 9 (25.7) |
| Generalised | 4 (5.7) | 2 (5.7) | 2 (5.7) |
| PNES | 9 (12.8) | 2 (5.7) | 7 (20) |
PNES: psychogenic non-epileptic seizures; SD: standard deviation.
Values are expressed as numbers and percentages unless otherwise indicated.
Combined focal seizures: focal seizures with and without impairment of consciousness.
Paroxysmal events were observed during wakefulness in 56 patients (80%; 3.1±2.4 events; range, 1-13). Events occurred during sleep in 39 patients (55.7%; 1.8±1.3 events; range, 1-7) (Table 1). Twenty-five patients (35.7%) presented paroxysmal events during both wakefulness and sleep, although more frequently during wakefulness.
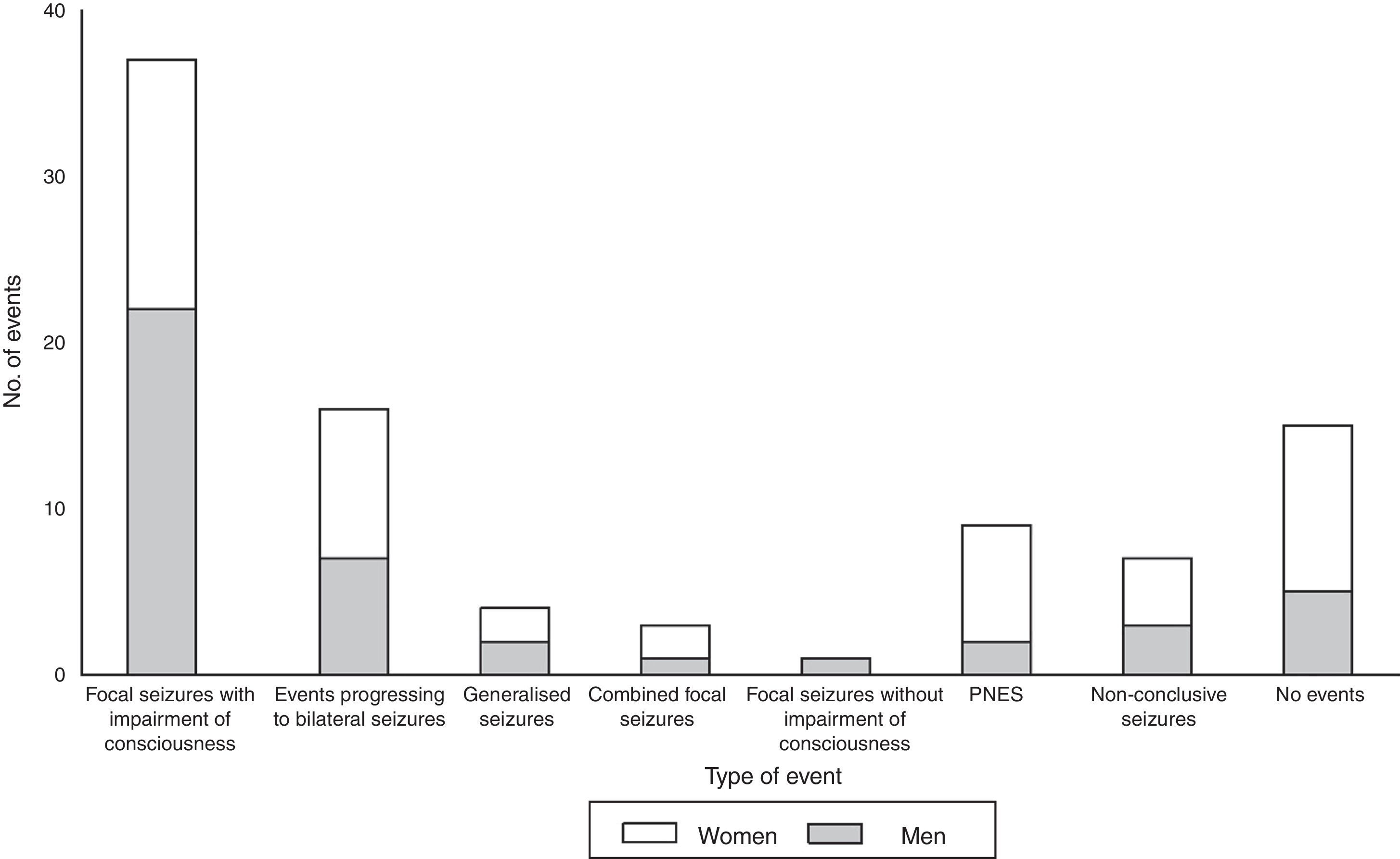
The main types of events described were focal seizures associated with alterations in the level of consciousness (n=37, 52.8%), events progressing to bilateral convulsive seizures (n=16, 22.8%), and PNES (n=9, 12.8%). Fig. 1 summarises the types of events recorded by sex; sex differences are more marked among patients with PNES and those not presenting paroxysmal events during video-EEG monitoring.
Event duration was highly variable, with a median of 90.5 seconds and a range of 14 to 811seconds. Combined focal events (with and without alterations in the level of consciousness) were the shortest, whereas PNES were the longest (Table 2). No differences in duration were observed between sexes by type of event.
Duration of the events recorded during video-EEG monitoring in patients with refractory epilepsy at our epilepsy monitoring unit between 2012 and 2014, broken down by type of event.
| Mean±SD | 132.8±137.4 |
| Median (range) | 90.5 (14-811) |
| Focal seizures with no impairment of consciousness | 45 (–) |
| Focal seizures with impairment of consciousness | 91.5 (43.2) |
| Combined focal seizures | 40.0 (36.8) |
| Events progressing to bilateral seizures | 211.9 (211.6) |
| Generalised | 69.2 (38.6) |
| PNES | 299.2 (159.3) |
PNES: psychogenic non-epileptic seizures; SD: standard deviation.
Duration of the events, in seconds, is expressed as mean±SD. No SD is provided for single events.
Combined focal seizures: focal seizures with and without impairment of consciousness.
After monitoring, the number of antiepileptic drugs used decreased by 56.8%.
The medical records analysed showed no statistically significant differences in terms of number or type of event by sex, duration of event by sex or age at disease onset, or number of events by type of event.
During prolonged video-EEG monitoring, 9 of the patients with an initial diagnosis of PNES (81.8%) presented paroxysmal events not associated with epileptiform discharges on EEG; the mean number of events was 3.1±2.9 (range, 1-9). All events occurred during wakefulness (2.9±3.0 events per patient; range, 1-9). Two patients (18.2%) also presented an isolated seizure during sleep. In our sample, PNES lasted between 131 and 600 seconds; 77.8% were recorded in women (Fig. 1).
We observed statistically significant differences in event duration between types of events (P<.01). Unlike seizures associated with abnormal neuronal discharges, which have a mean duration of 120.9±118.8 seconds, PNES last a mean of 299.2±159.3 seconds. Significantly fewer events were observed during sleep than during wakefulness in patients with psychogenic seizures (P<.03). Age at seizure onset is 24.6±10.5 years in patients with PNES, compared to 12.3±10 years in patients showing abnormal neuronal discharges (P<.005).
DiscussionStudies using prolonged video-EEG for epilepsy diagnosis show a slight predominance of the condition among women and a similar age distribution to that observed in our sample.33–35 The percentage of patients with PNES in our sample (12.1%) is also consistent with those reported in the literature.19,23,32
Around 90% of patients present paroxysmal events during the first days of monitoring,36–38 although they often take more than a week to develop in a high percentage of patients.39,40 In some studies, 15% to 25% of patients present no events during monitoring.24,36,41,42 In our study, 16.5% of patients presented no events during the 6-day monitoring period; this may point to the need for longer monitoring times, greater reductions in antiepileptic drug use, or more effective seizure induction techniques.43
Our study is consistent with several others which found considerable differences in the duration of different types of epileptic seizures. There is no consensus on the mean duration of epileptic seizures or the main factors influencing seizure duration.43,44 However, in line with the literature, we found paroxysmal events progressing to bilateral convulsive seizures to be the epileptic events of longest duration.43
No correlations have been observed between sex and duration, number, or type of event; the differences described in event duration and age at disease onset are mainly due to differences in the study methodology and the population included. Seizures associated with abnormal neuronal discharges last longer in children43,45; this population was not included in our study.
PNES tend to last longer than seizures associated with abnormal neuronal discharges; this is reflected in our results.14,46 In line with the literature, PNES were found to be more common among women, with a female-to-male ratio of approximately 3.5:1, the ratio usually reported in the literature.14
We found significant differences in age at onset between PNES and epileptic seizures. Although epileptic seizures may appear at any age, they generally present at younger ages, frequently before the age of 5.46 PNES, in contrast, have been reported to appear during adolescence (10-19 years) and between the ages of 15 and 35 years.13 In our study, age at onset was older in patients with PNES, corresponding with the lower limit for the second and third decades of life, when this type of seizures is most prevalent.13,14,18 Psychogenic seizures in infants or preschool children are rare compared to epileptic seizures, which are much more frequent.47
Similarly to other studies of patients with refractory epilepsy, we found differences in the number of paroxysmal events in each phase of the sleep-wake cycle, according to seizure type.48,49 In our study, PNES were more numerous during wakefulness and significantly less numerous during sleep than epileptic seizures; these findings are consistent with those reported in the literature.50–52
The reduction in the percentage of antiepileptic drugs used after prolonged video-EEG monitoring is due to treatment changes in patients with epilepsy and to antiepileptic drug withdrawal in patients with PNES. Treatment changes after video-EEG are reported in 80% of patients.36,53
FundingThis study received no public or private funding; all expenses were covered by the authors themselves. This study has not been presented at the SEN's Annual Meeting or at any other meeting or congress.
Conflicts of interestDr Henríquez-Varela has received fees as a speaker from Abbott, Asopharma, Merck, Novartis Pharma, and Roche. Dr Sanabria-Castro has worked as a scientific consultant for Novartis Pharma.
We would like to thank Dr Ileana Alvarado-Echeverría, from our hospital's research department, for her support during the development of this study.
Please cite this article as: Sanabria-Castro A, Henríquez-Varela F, Monge-Bonilla C, Lara-Maier S, Sittenfeld-Appel M. Eventos paroxísticos durante la monitorización prolongada con videoelectroencefalografía en epilepsia refractaria. Neurología. 2019;34:234–240.