Neurodegeneration in Alzheimer disease (AD) begins decades before dementia and patients with mild cognitive impairment (MCI) already demonstrate significant lesion loads. Lack of information about the early pathophysiology in AD complicates the search for therapeutic strategies.
Subjective cognitive impairment is the description given to subjects who have memory-related complaints without pathological results on neuropsychological tests. There is no consensus regarding this heterogeneous syndrome, but at least some of these patients may represent the earliest stage in AD.
MethodWe reviewed available literature in order to summarise current knowledge on subjective cognitive impairment.
ResultsAlthough they may not present detectable signs of disease, SCI patients as a group score lower on neuropsychological tests than the general population does, and they also have a higher incidence of future cognitive decline. Depression and psychiatric co-morbidity play a role but cannot account for all cognitive complaints. Magnetic resonance imaging studies in these patients reveal a pattern of hippocampal atrophy similar to that of amnestic mild cognitive impairment and functional MRI shows increased activation during cognitive tasks which might indicate compensation for loss of function. Prevalence of an AD-like pattern of beta-amyloid (Aβ42) and tau proteins in cerebrospinal fluid is higher in SCI patients than in the general population.
ConclusionsMemory complaints are relevant symptoms and may predict AD. Interpatient variability and methodological differences between clinical studies make it difficult to assign a definition to this syndrome. In the future, having a standard definition and longitudinal studies with sufficient follow-up times and an emphasis on quantifiable variables may clarify aspects of early AD.
La neurodegeneración en enfermedad de Alzheimer (EA) empieza décadas antes que la demencia y algunos pacientes con deterioro cognitivo leve presentan una importante carga lesional. La ausencia de información sobre la fisiopatología temprana de la enfermedad dificulta la búsqueda de estrategias terapéuticas.
La queja cognitiva subjetiva (QCS) agrupa a sujetos con quejas mnésicas sin déficits significativos en test neuropsicológicos. Es un síndrome heterogéneo sobre el que no existe consenso, pero algunos de estos pacientes podrían representar el estadio más precoz de EA.
MétodoRealizamos una revisión bibliográfica para resumir el estado del conocimiento actual sobre quejas cognitivas subjetivas.
ResultadosAunque a nivel individual no presenten enfermedad objetivable, a nivel de grupo los pacientes con QCS rinden peor en test neuropsicológicos que la población general y tienen mayor incidencia de declive cognitivo futuro. La depresión y la comorbilidad psiquiátrica desempeñan un papel pero no son la única causa de quejas cognitivas. Estudios con resonancia magnética muestran un patrón de atrofia hipocampal similar al del deterioro cognitivo leve amnésico y en resonancia funcional hay aumento de activación en tareas cognitivas que podrían representar una compensación ante pérdida de función. Los pacientes con QCS presentan un patrón tipo EA de marcadores betaamiloide (Aβ42) y tau con mayor frecuencia que la población general.
ConclusionesLas quejas mnésicas son un síntoma relevante y podrían predecir EA. La heterogeneidad de los pacientes y de los ensayos clínicos ha dificultado la definición del síndrome. En el futuro, una definición estandarizada y estudios longitudinales con un seguimiento suficiente, y centrados en variables cuantificables, podrían clarificar aspectos tempranos de la EA.
Subjective cognitive impairment (SCI) affects a heterogeneous group of patients who present cognitive complaints with no evidence of neurodegenerative disease. The origins of the concept are found in level 2 of the Global Deterioration Scale described by Reisberg et al.1 SCI has been defined as a possible initial stage of neurodegenerative diseases which progress to dementia, especially Alzheimer disease (AD).2 Regardless of the epidemiological or conceptual value of this term, a large percentage of patients are diagnosed with SCI in normal clinical practice.3,4 However, methodological heterogeneity between studies and the lack of an unequivocal and standardised definition make this group difficult to categorise. The use of biomarkers and the deliberations of the National Institute of Aging (NIA) on the definition of a preclinical stage in AD represent a good start. However, the shift from an epidemiological or research concept to a real diagnosis of patients in clinical practice has not yet materialised.
AD is the cause of 60% to 80% of all dementias. Worldwide prevalence was 24.3 million in 20056 and it will quadruple by 2050.7 The search for a cure has met with obstacles including late diagnosis, technical difficulties with treatments, and the pathophysiology of AD.8–11 One of the common objectives of the Dubois criteria,12,13 NIA recommendations,5 and the definition of mild cognitive impairment (MCI) is to provide a diagnosis before patients enter the dementia phase. If neurodegeneration begins decades before dementia is present14,15 and the MCI stage can last between 7 and 10 years with a conversion rate of 16% per year,16 we can expect to find a group of patients with AD and no apparent cognitive impairment.13 It seems reasonable to search for the earliest cases among subjects with cognitive complaints but no clinical signs of AD.
Identifying patients in the preclinical stage would be useful in 2 ways. First, for developing new therapeutic strategies. Delaying disease onset by only a year would mean a decrease in worldwide prevalence by 9 million cases in the next 40 years.7 Neuroprotective treatments are not effective in Alzheimer-type dementia and this is also probably true of MCI.16–20 Treatment would be more effective in patients with a lower amyloid load, and it could combat underlying pathophysiological mechanisms regardless of the stage of the disease.12
At the population level, such factors as depression, low educational level, hypertension, and diabetes increase the risk of AD. The percentage of the population at risk is between 10% and 25%, meaning that 1.1 to 3 million cases may be avoidable.21,22 Second, identifying subjects at risk would allow us to focus preventive efforts.
In this article, we present a review of the PubMed database using ‘subjective cognitive impairment’, ‘subjective cognitive complaints’, and ‘subjective memory impairment’ as search criteria. The wide scope of this subject and the heterogeneity of studies addressing it are incompatible with performing a systematic review.
Our aim is to summarise the current knowledge on this syndrome and discover new lines of research for the future.
DevelopmentDifficulties in defining a new syndromeAlzheimer disease still presents diagnostic challenges. It covers a heterogeneous spectrum: patients with anatomical pathology findings compatible with AD but with no symptoms, patients with atypical presentations, and even asymptomatic patients with AD biomarkers in cerebrospinal fluid (CSF). This spectrum is more complex in MCI, with its heterogeneous cohorts used in clinical trials, as can be seen in the different rates of conversion to AD.16,17,19,20,23 It is therefore reasonable for SCI to represent an even greater challenge, since there are no relevant clinical findings in this entity by definition.
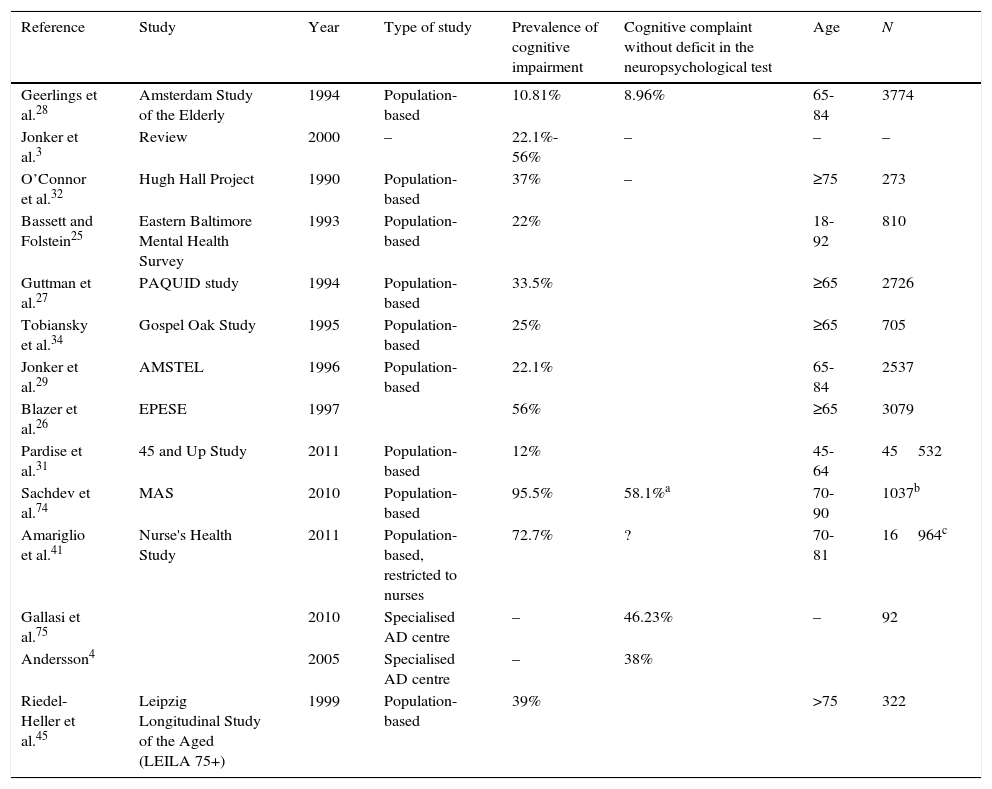
Methodological heterogeneity between studies and its impact on the results for prevalence of subjective cognitive impairmentCognitive impairment is a frequent motive for consultation and a significant percentage of patients seen in memory units worldwide report SCI. Jonker et al.3 found an incidence rate of 25% to 56%. In the Karolinska Memory Clinic (Huddinge, Sweden), SCI accounted for 38% of all cases in 2005.4 At the cognition and behaviour unit at Hospital Clínico San Carlos, SCI cases made up 7.52% of the total between 2008 and 2011 (Manzano, personal observations, not published). The reason for this percentage being so low is because the memory unit does not focus on early diagnosis. The concept of SCI needs a precise definition considering how many patients receive this diagnosis.
However, none is available. The term is often used to refer to a symptom or set of symptoms, but it can also refer to an independent entity other than AD or MCI. Many studies do not include an optimal battery of neuropsychological tests and many others have included patients with subjective cognitive problems in their healthy control groups.24
There is no homogeneous criterion for patient selection. Some studies select individuals from the general population to interview them about cognitive problems.25–34 Other studies select patients seeking treatment in memory units but whose neuropsychological evaluations do not show significant impairment.35–39 Considering that these patients themselves choose to visit the memory unit due to memory concerns, these 2 control groups are probably not equivalent.
Studies including a screening of the general population did not use standardised or validated questions. Geerlings et al.28 used the question ‘do you have complaints about your memory?’.b Answers were coded in four categories: no; sometimes, but is no problem; yes, is a problem; yes, is a serious problem. The Rotterdam study used the same question but with dichotomous answers.40 In the Eastern Baltimore Mental Health Survey25 and the PAQUID study,27 the question was ‘do you find that you have trouble with your memory’; possible answers were ‘yes/no’. The AMSTEL,29 Hugh Hall Project,32 and Gospel Oak studies34 used similar questions to those in the CAMDEX study. The only question in the EPESE project was ‘is your memory worsening?’. The prevalence rate of SCI was logically higher, at 56%.26 Paradise et al.31 used a Likert-type scale for memory function, and Jorm et al.30 used 4 questions, each of which was answered on a scale of 0 to 4. The substudy within the Nurse's Health Study by Amariglio et al.41 used 7 questions, some of which were clearly related to normal ageing and not to the disease. For this reason, the incidence of cognitive complaints was very high. The only study conducted in our setting was the one by Montejo et al.33 who performed their screening in the context of the Health Survey of the City of Madrid (ESCM 05) and used 3 questions from the SCAN42 and CAMDEX43 studies: ‘do you have memory problems?’, ‘do you forget where you have left things?’, and ‘do you have difficulty recalling the names of family members or acquaintances?’.44
Methodological heterogeneity also explains variations in incidence rates between studies, which are summarised in Table 1.
Studies evaluating prevalence of subjective cognitive impairment.
| Reference | Study | Year | Type of study | Prevalence of cognitive impairment | Cognitive complaint without deficit in the neuropsychological test | Age | N |
|---|---|---|---|---|---|---|---|
| Geerlings et al.28 | Amsterdam Study of the Elderly | 1994 | Population-based | 10.81% | 8.96% | 65-84 | 3774 |
| Jonker et al.3 | Review | 2000 | – | 22.1%-56% | – | – | – |
| O’Connor et al.32 | Hugh Hall Project | 1990 | Population-based | 37% | – | ≥75 | 273 |
| Bassett and Folstein25 | Eastern Baltimore Mental Health Survey | 1993 | Population-based | 22% | 18-92 | 810 | |
| Guttman et al.27 | PAQUID study | 1994 | Population-based | 33.5% | ≥65 | 2726 | |
| Tobiansky et al.34 | Gospel Oak Study | 1995 | Population-based | 25% | ≥65 | 705 | |
| Jonker et al.29 | AMSTEL | 1996 | Population-based | 22.1% | 65-84 | 2537 | |
| Blazer et al.26 | EPESE | 1997 | 56% | ≥65 | 3079 | ||
| Pardise et al.31 | 45 and Up Study | 2011 | Population-based | 12% | 45-64 | 45532 | |
| Sachdev et al.74 | MAS | 2010 | Population-based | 95.5% | 58.1%a | 70-90 | 1037b |
| Amariglio et al.41 | Nurse's Health Study | 2011 | Population-based, restricted to nurses | 72.7% | ? | 70-81 | 16964c |
| Gallasi et al.75 | 2010 | Specialised AD centre | – | 46.23% | – | 92 | |
| Andersson4 | 2005 | Specialised AD centre | – | 38% | |||
| Riedel-Heller et al.45 | Leipzig Longitudinal Study of the Aged (LEILA 75+) | 1999 | Population-based | 39% | >75 | 322 |
It is still to be determined whether cognitive complaints in adults can predict cognitive impairment or if they are only a manifestation of depressive states and psychiatric comorbidity. However, despite this symptom being frequent among depressive patients, scientific literature has reported differences between individuals with SCI and the general population. These differences apply not only to psychiatric comorbidity but also to results of neuropsychological tests, future cognitive decline, neuroimaging and functional neuroimaging tests, and CSF markers. We will analyse these considerations in detail in the following sections.
Relationship between cognitive impairment and cognitive performanceStudies of the relationship between cognitive complaints and performance yield contradictory results.2,27,29,32 Depression is a confounder.3,29–32,41,45–47 The Mini-Mental State Examination (MMSE) may be too simple a tool to detect impairment in these patients, who do in fact display alterations on other tests.45 On most of these evaluations, cognitive complaints are related to poorer cognitive performance27,29,33,35,41,48 and more severe decline in the previous 4 years.46 However, results are contradictory and some other studies do not show this association.27,29–32,49
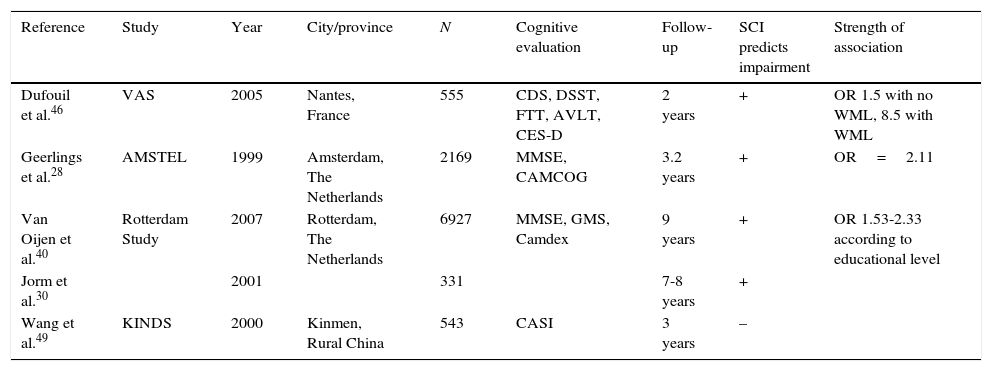
Longitudinal studies shed some light on this question, although contradictory results persist; this is probably due to methodological differences and insufficient follow-up time (Table 2). Jorm et al.30 did not find an association between cognitive complaints and baseline cognitive performance, but they were able to establish an association with follow-up. Tobianski et al.,34 Geerlins et al.,28 and Amariglio et al.41 found that individuals with cognitive complaints and no evidence of decline did not display a higher incidence rate of AD in the follow-up period after controlling for confounders such as depression. Schmand et al.47,50 found an association between cognitive complaints and incidence of dementia, although they did not distinguish between cognitively healthy subjects and those with impairment.
Longitudinal studies. Relationship between cognitive complaints and impairment during follow-up.
| Reference | Study | Year | City/province | N | Cognitive evaluation | Follow-up | SCI predicts impairment | Strength of association |
|---|---|---|---|---|---|---|---|---|
| Dufouil et al.46 | VAS | 2005 | Nantes, France | 555 | CDS, DSST, FTT, AVLT, CES-D | 2 years | + | OR 1.5 with no WML, 8.5 with WML |
| Geerlings et al.28 | AMSTEL | 1999 | Amsterdam, The Netherlands | 2169 | MMSE, CAMCOG | 3.2 years | + | OR=2.11 |
| Van Oijen et al.40 | Rotterdam Study | 2007 | Rotterdam, The Netherlands | 6927 | MMSE, GMS, Camdex | 9 years | + | OR 1.53-2.33 according to educational level |
| Jorm et al.30 | 2001 | 331 | 7-8 years | + | ||||
| Wang et al.49 | KINDS | 2000 | Kinmen, Rural China | 543 | CASI | 3 years | – |
AVLT: auditory verbal learning test; Camcog: Cambridge cognitive exam; Camdex: Cambridge examination for mental disorders of the elderly; CASI: cognitive abilities screening instrument; CES-D: Center for Epidemiologic Studies Depression Scale; CSD: cognitive difficulties scale; DSST: digit symbol substitution test; FTT: finger tapping test; GDS-S: geriatric depression scale short form; GMS: Geriatric Mental State schedule; KINDS: Kinmen Neurological Disorders Survey; WML: white matter lesions; MMSE: Mini-Mental State Examination.
The study by Geerlings et al.28 included an elderly population randomly recruited from primary care centres throughout Amsterdam. They examined 3774 subjects, analysing demographic data and cognitive complaints, and conducting 4 cognitive state tests per subject, including MMSE. Authors excluded individuals with dementia and the subsample included patients with normal cognitive levels (MMSE 26-30) and borderline levels (MMSE 21-25). At the first evaluation, 10.8% of the patients presented cognitive complaints. Mean follow-up time until the second evaluation was 3.2 years, and 77 patients had progressed to AD by that point. Presence of cognitive complaints, advanced age, female sex, depression, low level of education, and baseline cognitive impairment were significant factors in the univariate analysis. The most relevant factors were older age (odds ratio [OR] 6.17), presence of cognitive decline at baseline with a MMES<26 versus MMES of 26 to 30 (OR 3.77), and female sex (OR 2.22). Presence of cognitive complaints was associated with progression to AD, with an OR of 2.11. In the multivariate analysis, cognitive complaints were associated with AD in patients with MMSE>26 during the initial evaluation, but not in those with dementia or borderline cognitive level. However, this result could have been affected by the high number of patients lost to follow-up among the dementia and borderline cognitive level groups. Neither depression nor level of education had any effect on results. Schofield et al.51 found an association between AD and cognitive complaints in patients who already displayed impairment at the first evaluation, but not in patients with SCI. This study covered a follow-up period of only one year, while the study by Geerlings et al.28 lasted 3.2 years.
Dufouil et al.46 also found a relationship between cognitive complaints and deficit on several tests, with a higher OR in those patients also presenting white matter lesions.
Van Oijen et al.40 conducted a longitudinal study of 6927 subjects, within the Rotterdam Study. The hypothesis suggested that, due to the ceiling effect of neuropsychological tests, cognitive complaints have a higher predictive value for impairment in patients with higher educational levels. The follow-up period was long at 10.8 months. The study revealed that higher educational levels protect against developing AD, especially in men. Cognitive complaints were a risk factor for dementia, even after adjusting for educational level, baseline MMSE score, ApoE allele, and depression. As expected, this association was affected by education in that risk was higher in patients with higher educational levels. Researchers considered patients with low educational levels and no cognitive complaints as the reference group. Subjects with low educational levels but with cognitive complaints had a 1.53 times higher risk of developing AD than the reference group, and 2.33 times in the case of the group with a high educational level.
On the contrary, Wang et al.49 found no relationship between SCI and dementia. They studied 543 subjects from a rural area who underwent 2 evaluations 3 years apart. In both evaluations, cognitive complaints were correlated with poorer performance on neuropsychological tests, but no longitudinal decline was detected. If we compare these results with those of the Rotterdam study, we find that the rural and less educated population in the latter case may have reduced the predictive value of cognitive complaints.
In general, most longitudinal studies showed a positive association, although discreet, between cognitive complaints and future decline (Table 2).
The role of depressionCertain personality traits and depression have been postulated as causes of cognitive complaints.2 Depression is associated with cognitive complaints.29,32 Depression itself reduces cognitive performance and is a risk factor for future impairment52; it acts as both a risk factor and a prodromal symptom of AD.53 Moreover, depressed patients older than 65 display a rapid rate of decline which no longer runs parallel to that of healthy controls.54
The impairment profile in cases of depression, mainly affecting attention span and executive function and entailing predominantly frontal atrophy, does not correspond to the memory impairment profile of AD. However, hippocampal changes have been described in patients with depression,55 which is a risk factor for AD.56 Kramberger et al.57 assessed CSF AD markers in patients with SCI and AD, and correlated findings for those markers with patients’ results on the Cornell scale for depression. Patients with AD and depression had a significantly lower total Tau protein (T-tau) level than those without depression, although no differences were observed in levels of phosphorylated tau (P-tau) or β-amyloid. In the SCI group, T-tau and P-tau levels were lower in the group with depression, but no differences were observed in β-amyloid. These findings appear to be contrary to the hypothesis that associates AD with depression.
The 7-year longitudinal study by Jorm et al.30 described a relationship between negative affect and cognitive complaints at the beginning of the study, but they observed that cognitive complaints were an independent factor for cognitive impairment during follow-up once depression was controlled. Amariglio et al.41 also adjusted their results for depressive symptoms and found an association between cognitive complaints and poorer cognitive performance. Other studies confirm that depression acts as a confounding factor in the assessment of cognitive complaints.29,33,46
Studies of patients with SCI should include a statistical adjustment for this factor.
Imaging studiesImaging study findings in the SCI group differ from those in the general population.58,59 Van der Flier et al.59 compared 20 patients with SCI to 28 control subjects and found a statistically significant reduction in the left-side hippocampal volume in the first group. Saykin et al.58 describe reduced grey matter density in the medial temporal, frontotemporal, and other neocortical regions; findings were similar to those seen in amnestic MCI, but less pronounced (n=40). Degree of atrophy was related to the degree of memory impairment and cognitive complaints.
White matter lesions affect the learning curve of SCI patients, but not those of MCI patients or healthy controls,35 and the lesions increase risk of cognitive impairment when SCI is also present.46
Grambaite et al.60 analysed 45 patients and compared hippocampal volume and white matter diffusivity between patients with SCI and MCI with high levels of T-tau in CSF and patients with SCI and MCI with normal levels of T-tau. These authors found decreases in visual memory and hippocampal volume in the first group and a correlation between memory performance and white matter diffusion in subjects with normal levels of T-tau.
Functional neuroimagingSeveral functional MRI (fMRI) studies have shown alterations in the coding of episodic memory in dementia with AD and MCI, both in the medial temporal cortex and neocortical structures. Cognitively normal subjects who are homozygous for ApoE*4 display increased brain activation of the left hippocampal, parietal, and prefrontal regions compared to carriers of the ApoE*3.61,62 Furthermore, subjects with increased activation presented a steeper decline in the 2 following years.61 In cases of MCI, authors have observed both increased and decreased activation of prefrontal and medial temporal regions. These inconsistencies could be due to the heterogeneity of the group, or to inclusion of subjects in different stages of the disease. The process begins with compensatory hyperactivation which subsequently fails and gives rise to hypoactivation in more advanced stages of the disease.38,63 This correlates with the theory that suggests cortical thickening in the MCI phase previous to atrophy in AD, a sequence also described by Spanish authors in the Molinuevo group36 from a structural point of view.
Rodda et al.38 compared the fMRI profiles from 10 subjects with cognitive complaints to those of 10 healthy controls during a memory encoding task. No differences were observed between groups in the baseline cognitive study. The SCI group obtained similar results in memory encoding and made the same number of errors as controls while also displaying more activation of the left dorsolateral prefrontal and premotor cortices. This finding in the SCI group could be indicative of compensatory hyperactivation, similar to that typically occurring in MCI.
In a similar study assessing divided attention tasks, Rodda et al.39 reported increased activation of the thalamus, posterior cingulate, and bilateral caudate as well as the left hippocampal and parahippocampal formation. As in their previous study, they did not find any differences in performance between the 2 groups.
Single-photon emission computed tomography perfusion imaging distinguishes between amnestic and non-amnestic MCI, but not between SCI and controls.64
Positron-emission tomography with fludeoxyglucose (F-18) provides interesting results. Patients with SCI showed slower metabolism in the parahippocampal, temporoparietal, and frontal inferior regions, the fusiform gyrus, and thalamus compared to control subjects. These findings replicate those found in healthy subjects with genetic risk factors for AD (autosomal dominant form of familial AD, high family load, or patients homozygous for ApoE*4) and exhibiting MCI.15
Anatomical pathologyMemory complaints proximate to death have been linked to AD anatomical pathology findings, as in one study forming part of the Rush Memory and Aging Project.65 The study assessed 90 brains of deceased patients and the correlation remained after adjusting for age, sex, state of health, depression, and the interval between last evaluation and autopsy. In patients without dementia, AD-type pathology (assessed histochemically) and amyloid and T-tau load (assessed by antibody immunohistochemistry) were correlated with cognitive complaints. In patients with dementia, only T-tau level was significantly correlated with the level of complaints. In both groups, the level of cognitive complaints correlated with the results from neuropsychological tests. These results corroborate the findings obtained by a previous study.66
BiomarkersThe combination of low β-amyloid 1-42 (Aβ42) levels and high levels of T-tau and P-tau in CSF helps distinguish patients with AD from controls. In AD, Aβ42 in CSF will decrease initially, while T-tau and P-tau will increase at a later point.37 Antonell et al.37 did not find differences in CSF biomarkers between subjects with SCI and controls, although their sample was small. The DESCRIPA study, which included more patients, did find a higher frequency of AD-type biomarkers in the SCI group compared to control subjects.67
Furthermore, in the DESCRIPA study, levels of Aβ42 predicted performance on semantic and working memory tests in controls and subjects with SCI, while T-tau levels were a better predictor of performance for patients with MCI.68 Another study supported these findings69 but also pointed out that an early decrease in Aβ42 would be linked to the first cognitive changes, whereas different Tau-related mechanisms would better explain the decline in intermediate stages of the disease. Interestingly, cognitive performance became independent of biomarker levels in the dementia stage.69
In a healthy elderly population, increased amyloid deposition shown by PiB-PET in the right prefrontal region, anterior cingulate cortex, right precuneus, and posterior cingulate cortex was correlated with less confidence by subjects in their memory skills. These brain regions are the same ones showing early amyloid deposition in AD. Although cognitive performance in this study was the same between subjects with high and low PiB uptake, authors conclude that the participants’ subjective perception was valid and may predict incipient AD since it correlates with an early indicator of that disease.70
Chételat et al.71 found a correlation between PiB uptake and atrophy in subjects with SCI but not in healthy controls. A corollary study showed that healthy subjects with high PiB uptake presented higher medial temporal volume than subjects with SCI and high PiB uptake.72 Healthy subjects with high PiB uptake levels had larger temporal volumes than healthy subjects with low PiB uptake levels; therefore, this finding cannot be explained by atrophy in subjects with SCI. These enlarged temporal lobes are most probably due to transient oedema or a reaction to amyloid deposition. They may also represent a higher brain reserve and only those subjects with a larger temporal volume would exhibit cognitive integrity with such a high amyloid load. The study by Fortea et al.36 found an inverted-U relationship between Aβ42 levels and cortical volume. However, further longitudinal studies will be necessary to elucidate whether this inverted U is due to disease progression or to the behaviour of groups of different subjects.
ConclusionsMost of the studies found a poorer baseline cognitive performance among patients with cognitive complaints. The sole exception is the Hughes Hall Project,32 although only patients with a MMSE below 25 underwent complete neuropsychological studies and therefore no deficits could be detected in patients with largely normal MMSE scores.
Most of the longitudinal population-based studies show an association between cognitive complaints and cognitive impairment during follow-up, although the risk seems to be only slightly increased.28,30,40,46 Clinical cohorts of patients who seek medical advice due to memory concerns may possibly show a stronger association with AD than do random samples of the general population. It is interesting that a high educational level increases the strength of association; this finding might reflect a ceiling effect in the neuropsychological tests. Subjects with high educational levels would be able to evaluate their own cognitive performance better than tests.40 Coincidentally, the only longitudinal study in our review that found an association between SCI and dementia was the one by Wang et al.,49 conducted in an illiterate rural population.
The role of depression in cognitive performance, cognitive complaint, and dementia remains to be determined. It seems to display a multifactorial mechanism by directly causing poorer performance due to attention and motivation deficit, eliciting cognitive complaints, participating in the genesis of impairment as a risk factor for dementia, and presenting as an accompanying symptom in patients with established dementia.
Imaging findings from patients with SCI are different from those of the general population; they present a decreased hippocampal volume and an atrophy pattern similar to that of subjects with amnestic MCI.58,59 Furthermore, these patients present anatomical pathology findings compatible with AD.65,66
Functional neuroimaging studies are more difficult to interpret but suggest that the SCI group differs from healthy controls in brain activation patterns during cognitive tasks. Heterogeneous aetiopathological traits and different stages of progression in each group could explain the differences between studies; however, this increase in activation probably corresponds to a compensation mechanism for loss of function.
Aβ42, T-tau, and P-tau levels are interesting markers in the SCI group, since these subjects are more likely than controls to present a typical AD pattern. Aβ42 seems to decrease early and be linked to the earlier cognitive disorders, while T-tau levels are involved in intermediate stages of AD. Follow-up periods of more than 2 years may be necessary to observe how these parameters evolve.37,67,68,73
Subjective cognitive complaints could correspond to a preclinical stage of AD that even precedes MCI. SCI is of interest for both its similarities to and differences with established AD. Some of these patients present an unstable equilibrium in which compensatory mechanisms and cognitive reserve start to falter as the disease progresses.
Based on these findings, we might propose a pathophysiological hypothesis that would include SCI in the AD spectrum. Aβ42 level drop and amyloid deposition would constitute the initial steps and be accompanied by an increased cerebral activity during memory tasks to compensate for poorer performance. This may be the moment when the first cognitive complaints are registered, most frequently in subjects with high functional requirements. Temporal lobes appear to go through a stage in which their volume increases before they present atrophy. Increased t-tau levels appear at the same time as apparent impairment, representing the change to MCI.
SCI groups are heterogeneous. Methodological differences between trials make it difficult to define the characteristics and progression of these patients. While the future may bring drugs able to slow AD in its early stages, our first task will be to identify the candidates for early intervention.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Garcia-Ptacek S, Eriksdotter M, Jelic V, Porta-Etessam J, Kåreholt I, Manzano Palomo S. Quejas cognitivas subjetivas: hacia una identificación precoz de la enfermedad de Alzheimer. Neurología. 2016;31:562–571.