Evaluate safety and tolerance levels for intravenous immunoglobulins (IVIG) as treatment for neuromyelitis optica (NMO).
MethodsEight patients meeting Wingerchuk's revised diagnostic criteria were treated with IVIG every 2 months (0.7g/kg body weight per day for 3 days). The primary outcome measure was the occurrence of serious adverse effects, defined according to NIH guidelines for clinical trials. Secondary outcome measures were changes in the yearly rate of attacks and in the degree of neurological disability measured with the Expanded Disability Status Scale (EDSS).
ResultsAll 8 patients were treated; 5 had relapsing optic neuritis with or without myelitis and 3 had recurrent longitudinally extensive transverse myelitis (LETM). The mean age of onset was 20.5 years (range, 7–31 years) and 87.5% were female. The mean duration of the disease before beginning treatment was 9.0 years (range, 3–17 years). Following 83 infusions (range, 4–21 per patient) and a mean follow-up time of 19.3 months (range, 6–39 months), minor adverse events had occurred (headache in 3 patients and a mild cutaneous eruption in a single patient). The relapse rate decreased from 1.8 in the previous year to 0.006 during follow-up (z=−2.5, P=.01). The EDSS score fell from 3.3 [SD 1.3] to 2.6 [SD 1.5] (z=−2.0, P=.04).
ConclusionsTreatment with IVIG is safe and well-tolerated, and it may be used as a treatment alternative for NMO spectrum disorders.
Evaluar la seguridad y tolerancia de las inmunoglobulinas intravenosas (IgIV) para el tratamiento de la neuromielitis óptica (NMO).
MétodosOcho pacientes que cumplían los criterios diagnósticos revisados de Wingerchuk fueron tratados con IgIV cada dos meses (0,7 gr por kg de peso y día durante tres días). Las medidas de resultado principales fueron los eventos adversos graves, definidos de acuerdo con las directrices NIH para los ensayos clínicos. Las medidas de resultado secundarias fueron los cambios en la tasa anualizada de brotes y la discapacidad neurológica medida con la Expanded Disability Status Scale (EDSS).
ResultadosOcho pacientes fueron tratados: 5 con episodios recidivantes de neuritis óptica y/o mielitis, y 3 pacientes con mielitis transversa longitudinal extensa recurrente. La edad media de inicio fue de 20,5 años (rango 7–31), 87,5% mujeres. El tiempo medio de duración de la enfermedad al inicio del tratamiento fue de 9,0 años (rango 3–17). Tras 83 infusiones (rango 4–21), y una media de seguimiento de 19,3 meses (rango 6–39), hubo eventos adversos menores (dolor de cabeza en tres pacientes y erupción cutánea leve en un paciente). La tasa de recaídas se redujo de 1,8 en el año anterior a 0.006 en el seguimiento (z=−2.5, p=0,01). La EDSS se redujo de 3,3 (DE 1,3) y 2,6 (DE 1,5) (z=−2.0, p=0,04).
ConclusionesEl tratamiento con IgIV es seguro y bien tolerado y podría ser una alternativa de tratamiento para los trastornos del espectro de la NMO.
Neuromyelitis optica (NMO or Devic's syndrome) is an idiopathic inflammatory demyelinating disease of the central nervous system which is characterised by exacerbations of optic neuritis and myelitis.1 Doctors once thought that its pathogenesis was related to multiple sclerosis, but studies in the last decade have delivered a growing body of evidence to suggest that alterations in humoral immunity underlie the mechanism in NMO. Deposits of IgG and IgM co-located with products of the complement activation cascade that were discovered around blood vessels initially suggested that the pathogenic agent triggering the humoral immune response was an antigen located in the perivascular space.2,3
At a later point, upon identification in plasma of the specific IgG antibody (NMO-IgG) which travels to the aquaporin-4 water channel, that antibody was proposed as a highly specific marker for NMO.4,5 The decrease in aquaporin-4 at lesion sites6 and in vitro demonstrations of the functional roles of NMO-IgG7,8 have provided additional information about the pathophysiological role of these antibodies. Intravenous immunoglobulin treatment (IVIG) is commonly used for neurological immune deficiencies.9 IVIG has various theoretical action mechanisms, including blocking Fc receptors10; activating complement components in the fluid phase of the cellular surface11; inducing the production of anti-idiotypic antibodies with a block effect12; increasing the catabolism of pathogenic IgGs; reducing the expression and differentiation of B lymphocytes in the bone marrow; and modulating cytokine production. This explains the multiple action points involved in the complex process of humoral immune response.9 Furthermore, IVIGs reduce proliferation of T-cells and various pro-inflammatory cytokines including interleukin 1, tumour necrosis factor alpha, and interferon-gamma. Earlier reports on clinical cases have pointed to a possible beneficial effect of IVIG in preventing NMO relapses.13,14 We therefore designed an open observational prospective uncontrolled study based on the hypothesis that IVIG may be a safe and effective treatment for this disorder. We present our experience with IVIG treatment in 8 patients with either NMO or NMO spectrum disorders.
MethodOpen prospective uncontrolled observational study designed to evaluate the safety, tolerability, and clinical effect of IVIG for NMO spectrum disorders. Adverse drug events were defined according to NIH criteria for clinical trials as any unwanted effect of a medicine.15 Patients diagnosed with NMO16,17 and/or NMO spectrum disorders1 volunteered for treatment with IGIV during active disease. We define active disease as at least 1 relapse in the previous year in a patient who did not respond to conventional treatment or immunosuppressant drugs. Patients gave their informed consent before being included in the study. Data recorded at the beginning of the study were as follows: age at the time of the first clinical event; sex; neurological disability measured by the Expanded Disability Status Scale (EDSS), and the number and dates of relapses or exacerbations in the past year and previous years.
Prior clinical and paraclinical examinations included serology testing for Epstein–Barr, measles, rubella, herpes simplex virus 1, and HIV; polymerase chain reaction for JC virus and tuberculosis; treponemal tests; and a blood test to rule out other causes of myelitis and/or optic neuritis.
Cerebral and spinal cord MRIs were taken at the beginning of the study. We also analysed visual and somatosensory evoked potentials; antinuclear antibodies and NMO-IgG antibodies in serum; and CSF with IgG and IgM oligoclonal bands (OCGB, OCMB. OCGB was measured in 7 patients and OCMB in 6 patients).
Appointments were scheduled every 2 months. Patients were assessed by a neurologist before IVIG was administered in order to record EDSS, appearance of relapses, and possible adverse effects of the treatment.
The main outcome variables were adverse effects and serious adverse effects; secondary outcome variables were appearance of new exacerbations and increases in disability after IVIG treatment. All patients had previously been treated with high doses of IV methylprednisolone for exacerbations of myelitis or optic neuritis. We used an avidin–biotin method with paraformaldehyde fixation and frozen rat brain sections, performed in Hospital Clínic in Barcelona, to detect NMO-IgG as described in the study cited here.16 Oligoclonal bands in CSF were analysed at Hospital Ramón y Cajal in Madrid. OCGB and OCMB were measured using isoelectric focusing (IEF) and immunodetection techniques which have also been described previously.18,19
Patients were treated with IVIG (Grifols Flebogamma® 5%) dosed at 0.7g/kg body weight over 3 days during a treatment period of 8 weeks. Patients with a history of failed maintenance treatments (immunosuppressant drugs of any type) did not complete a washout period. Flebogamma® 5% is a highly purified human antibody complex (≥99% IgG) that does not modify human IgG containing specific antibodies in the donor population. Subclasses of IgG may be broken down as approximate percentages of total IgG as follows: IgG1, 70.3%; IgG2, 24.7%; IgG3, 3.1%; and IgG4, 1.9%. IgA content is less than 0.05mg/mL and low levels of IgM were also present.
All patients had normal IgA serum levels prior to beginning treatment. Each infusion was administered in outpatient care; treatment was supervised by specialist nurses and overseen by a neurologist. Initial infusion rate was 0.5mg/kg/min. If the patient did not experience discomfort during the first 30minutes, the infusion rate was gradually raised to a maximum of 5mg/kg/min. Mean total perfusion time was 6hours. Any adverse events were recorded during infusion sessions or the patient's scheduled appointments.
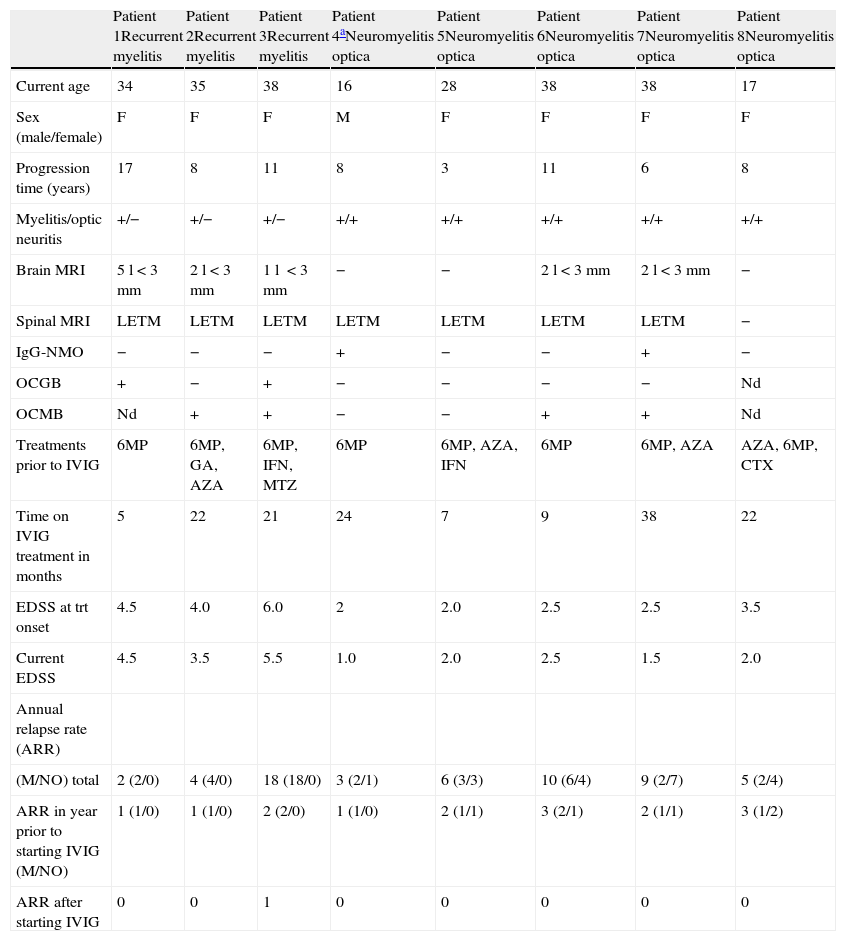
ResultsPatient characteristicsBeginning in February 2006, 5 patients with relapsing forms of NMO and 3 patients with recurrent longitudinally extensive transverse myelitis (LETM) were included in the study. Patients comprised 7 women (87.5%) and 1 man. Mean age at disease onset was 20.5 years (range, 8–31 years). Table 1 lists patients’ clinical and demographic characteristics.
Clinical and demographic characteristics of patients.
| Patient 1Recurrent myelitis | Patient 2Recurrent myelitis | Patient 3Recurrent myelitis | Patient 4aNeuromyelitis optica | Patient 5Neuromyelitis optica | Patient 6Neuromyelitis optica | Patient 7Neuromyelitis optica | Patient 8Neuromyelitis optica | |
| Current age | 34 | 35 | 38 | 16 | 28 | 38 | 38 | 17 |
| Sex (male/female) | F | F | F | M | F | F | F | F |
| Progression time (years) | 17 | 8 | 11 | 8 | 3 | 11 | 6 | 8 |
| Myelitis/optic neuritis | +/− | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ |
| Brain MRI | 5l<3mm | 2l<3mm | 1l <3mm | − | − | 2l<3mm | 2l<3mm | − |
| Spinal MRI | LETM | LETM | LETM | LETM | LETM | LETM | LETM | − |
| IgG-NMO | − | − | − | + | − | − | + | − |
| OCGB | + | − | + | − | − | − | − | Nd |
| OCMB | Nd | + | + | − | − | + | + | Nd |
| Treatments prior to IVIG | 6MP | 6MP, GA, AZA | 6MP, IFN, MTZ | 6MP | 6MP, AZA, IFN | 6MP | 6MP, AZA | AZA, 6MP, CTX |
| Time on IVIG treatment in months | 5 | 22 | 21 | 24 | 7 | 9 | 38 | 22 |
| EDSS at trt onset | 4.5 | 4.0 | 6.0 | 2 | 2.0 | 2.5 | 2.5 | 3.5 |
| Current EDSS | 4.5 | 3.5 | 5.5 | 1.0 | 2.0 | 2.5 | 1.5 | 2.0 |
| Annual relapse rate (ARR) | ||||||||
| (M/NO) total | 2 (2/0) | 4 (4/0) | 18 (18/0) | 3 (2/1) | 6 (3/3) | 10 (6/4) | 9 (2/7) | 5 (2/4) |
| ARR in year prior to starting IVIG (M/NO) | 1 (1/0) | 1 (1/0) | 2 (2/0) | 1 (1/0) | 2 (1/1) | 3 (2/1) | 2 (1/1) | 3 (1/2) |
| ARR after starting IVIG | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
LETM: longitudinally extensive transversal myelitis; IGIV: intravenous immunoglobulins; 6MP: 6-methylprednisolone; GA: glatiramer acetate; IFN-β: interferon beta; AZA: azathioprine; CTX: cyclophosphamide; M/NO: myelitis/optic neuritis; trt: treatment; Nd: not determined.
Patient 1: 34-year-old female who had experienced an initial episode of myelitis 17 years before and a second episode with moderate sequelae (EDSS 4.5) in 2008. An NMO-IgG test was performed during a stable phase of the disease; results were negative. The patient refused treatment with azathioprine and started IVIG treatment in November 2008.
Patient 2: 35-year-old female who had experienced an initial episode of myelitis 8 years prior and a second exacerbation 1 year after that. She was initially diagnosed with multiple sclerosis and treated with interferon beta 1a between 2002 and 2004 (discontinued due to adverse effects) and with glatiramer acetate during 4 months in 2005. She later suffered a new episode of myelitis. At that point, doctors diagnosed recurrent myelitis and changed her treatment to azathioprine. Results from an NMO-IgG test taken during a stable phase of the disease, while the patient was being treated with azathioprine, were negative. The patient began IVIG treatment in June 2007 after having experienced a new relapse of myelitis.
Patient 3: 38-year-old woman with 18 relapses of acute myelitis in the past 11 years and a diagnosis of RRMS based on the presence of oligoclonal IgG bands in CSF. She was initially treated with interferon beta 1b between 2004 and 2006, after which treatment was changed to 10mg/m2 mitoxantrone (MTX) monthly during 3 months, followed by quarterly infusions after the detection of increases in neurological disability with each relapse. Despite the MTX, she suffered 2 additional relapses and was offered IVIG treatment. An NMO-IgG test taken during a stable phase of the disease and after treatment with MTX was negative. IVIG treatment was started in July 2007. After 15 months of treatment, the patient suffered an additional episode of myelitis. Her treatment was changed to rituximab in January 2009.
Patient 4: 16-year-old male who suffered an initial attack of myelitis at the age of 8 (in 2000) and an episode of optic neuritis in 2001. He was started on low doses of steroids at that time. In February 2007, he suffered a second attack of optic neuritis. An NMO-IgG test was performed after the patient had received high doses of methylprednisolone for the optic neuritis. The result was positive. IVIG treatment was started in May 2007.
Patient 5: 28-year-old female patient whose first episode of myelitis occurred in 2004. Optic neuritis appeared 1 year later. She was initially diagnosed with multiple sclerosis and started on treatment with interferon beta-1a. She suffered 2 additional relapses and treatment was changed to oral steroids and azathioprine a year later. Despite azathioprine treatment, she suffered 2 relapses (myelitis and NO) and IVIG treatment was started in September 2008. An NMO-IgG test was performed after the latest episode while the patient was being treated with azathioprine. Results were negative.
Patient 6: 38-year-old woman previously diagnosed with Crohn's disease. The patient was referred to the MS unit in 2008 after numerous episodes of myelitis and optic neuritis dating back to 1997. She had experienced a myelitis relapse and 2 NO relapses in the same year. Until then, she had been treated exclusively with high doses of steroids and only during relapses. An NMO-IgG test taken during a stable phase of the disease, with no baseline treatment, was negative. The patient refused conventional immunosuppressant drugs and began IVIG treatment in June 2008.
Patient 7: 38-year-old woman referred to our MS unit in 2005 with a diagnosis of NMO. She presented optic neuritis followed by transverse myelitis in 2002 and was treated with steroids and oral azathioprine. Nevertheless, the patient continued suffering relapses and was referred for an evaluation of non-conventional treatments. An NMO-IgG test taken during a stable phase of the disease, after azathioprine had been discontinued, was negative. IVIG treatment was started in July 2006. The patient was stable throughout a follow-up period of 39 months.
Patient 8: 17-year-old female with a family history of Behçet's disease who experienced an initial episode of optic neuritis at the age of 8. In the following year, she suffered from aseptic meningoencephalitis that caused convulsions and coma. Brain and spinal MR images were normal. Based on her history of mouth ulcers, she was then assigned a suspected diagnosis of Behçet's disease. Doctors started treatment with oral steroids, azathioprine and valproic acid. The patient recovered from the aseptic meningoencephalitis and remained stable until March 2006, when she suffered a second episode of optic neuritis. The following year, she experienced 2 new attacks of optic neuritis and an episode of acute transverse myelitis, based on which she was diagnosed with NMO. An NMO-IgG test was carried out during a stable phase of the disease, when the patient was being treated with azathioprine; results were negative. Doctors started IVIG treatment in September 2007 after interrupting azathioprine; steroids were maintained but decreased gradually until February 2008.
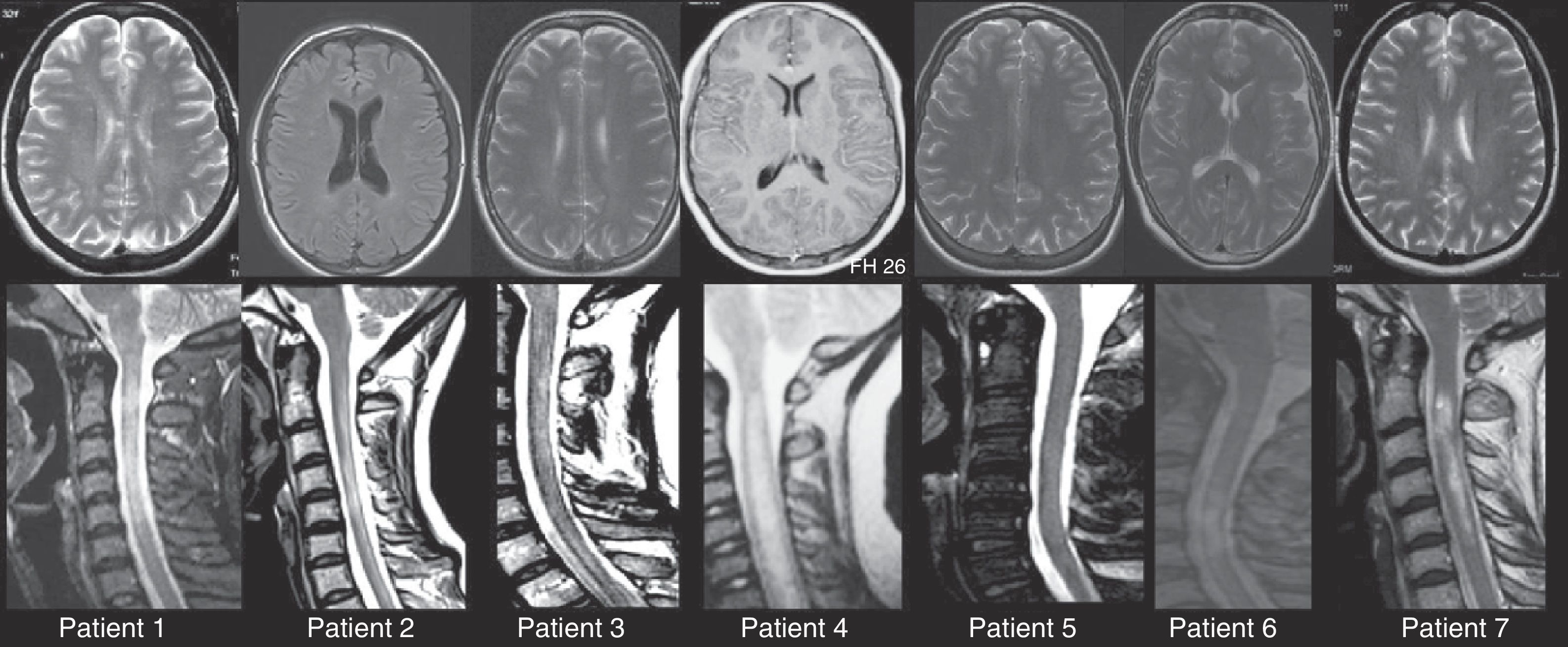
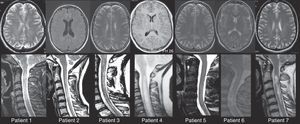
In summary, the 8 patients described above met the criteria for NMO spectrum disorders. Visually evoked potentials were severely and bilaterally altered in all cases. Brain MRIs revealed no abnormalities in 3 patients, while 5 patients had lesions smaller than 3mm that did not meet Paty's magnetic resonance imaging criteria20 for a diagnosis of MS. In 7 patients, spinal MRI revealed extensive spinal cord lesions that longitudinally affected more than 3 vertebral segments (Fig. 1).
Brain and spinal MRIs for patients 1 through 7. In all cases, we observe extensive myelitis affecting more than 3 vertebral segments with only minimal, non-specific changes in the brain MRI. Spinal MRI studies are T2-weighted sequences. Morphological and hyperintensity characteristics vary because studies were performed at different points in time using different MR units. Brain MRI studies show T2-weighted sequences except for Images 1 and 4, which are T1-weighted sequences with and without gadolinium contrast, respectively.
NMO-IgG tests were performed for all patients; only 2 patients tested positive (25%). Paired plasma/CSF samples were analysed in 7 patients and OCGB was present in 2 cases (28.5% of that sample). OCMB was screened in 6 patients and present in 4 of them (66.6%). Plasma screenings for antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-SSA, and anti-SSB were negative in all cases.
Administration of treatment and recorded adverse effectsA total of 83 cycles of IVIG were administered (range, 4–21) during a mean follow-up period of 19.3 months (range, 6–39). One patient (case 6) experienced a mild dermatological reaction with cutaneous erythema and skin eruption but no systemic signs. Grifols Flebogamma® 5% was substituted in that case with KIOVIG®, another plasma-derived product consisting of a preparation of highly purified human IgG with an immunogenic potential lower than that of conventional immunoglobulin preparations. Four patients experienced headache during IVIG administration and one continued to have headaches weeks after the last session of a year-long course of treatment. Another patient experienced dysgeusia associated with treatment. Patient 8 suffered from avascular necrosis of the head of the femur 3 months after starting IVIG. This adverse event was considered to be secondary to long-term steroid use, given that the patient had been on oral steroids for 7 years. He underwent bilateral femoral head replacement surgery in 2008. No other serious adverse events were reported.
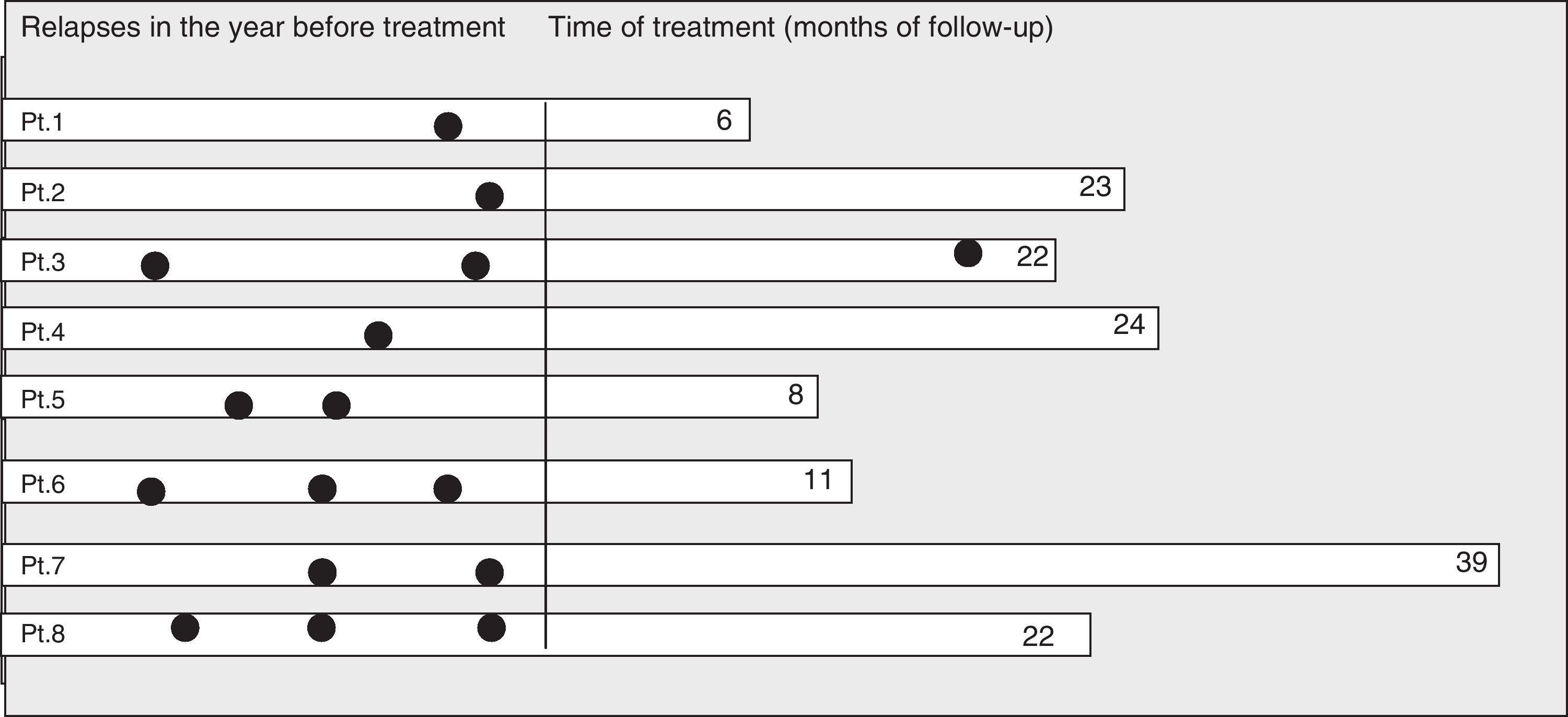
Response to treatment with intravenous immunoglobulins: relapses and disabilityThe mean number of relapses in the year prior to treatment was 1.8 (range, 1–3, SD 0.8). The mean number of total relapses was 7.1 (range, 2–18, SD 5.2). After a mean follow-up period of 19.3 months, only one patient (patient 3) suffered a relapse, which took place after 15 months of IVIG treatment. The patient's EDSS worsened from 4.5 to 5.5; she did not respond to high doses of methylprednisolone and her treatment was changed to rituximab (Fig. 2). The reduction in disease activity was statistically significant; z=−2.5, P=.01 (Wilcoxon test).
EDSS at the beginning of the study was 3.3 (range 2.0–6.0, SD 1.3) and improved to 2.6 (range 1.0 to 5.5, SD 1.5). These differences were also statistically significant; z=−2.0, P=.04. EDSS improved in 5 cases and remained unchanged in the other 3. Improvement in the EDSS score was generally seen after the second IVIG cycle.
DiscussionResults of this study suggest that IVIG treatment is safe and well-tolerated in NMO spectrum disorders.
After 83 infusions and a mean of 19 months of follow-up (minimum period 6 months), only a few minor adverse events had been reported. Although we had a limited number of cases and follow-up times were short, the decreases in both relapse rate and degree of disability indicate that IVIG may constitute an effective maintenance treatment for NMO and NMO spectrum disorders. NMO spectrum disorders are uncommon and frequently difficult to diagnose. Several of our patients were initially diagnosed with multiple sclerosis and treated with immunomodulatory drugs. The 2 cases with paediatric onset met the clinical criteria for NMO since they had experienced recurrent episodes of myelitis and optic neuritis. One of those cases also suffered an episode of encephalopathy, which has been reported as a possible symptom in children with NMO.21 The very few available NMO studies are non-randomised and include low numbers of patients, and all such studies employ broad-spectrum immunosuppressants and monoclonal antibodies.22–25 While these treatments have been shown to be partially effective, they are associated with the possibility of serious adverse events, such as potentially fatal infections or malignant illness, which do not occur with IVIG.23,26 Adverse events associated with IVIG treatment are generally mild and transient, such as headaches, erythema, and dysgeusia. This was supported by our study. Potentially serious adverse events are uncommon, and include acute tubular necrosis, which is often temporary and reversible; aseptic meningitis; or thrombotic complications. Our series included 1 case of avascular necrosis of the femoral head, considered to be secondary to long-term steroid treatment. There are 2 possible confounders that affect the analysis of IVIG effectiveness for NMO in our study. The first is regression toward the mean. This phenomenon may have affected the decrease in the relapse rate. NMO naturally manifests in clusters of clinical relapses or exacerbations which alternate with periods of low disease activity. It is therefore possible to think that IVIG administration may have coincided with remission periods, although it would be highly unlikely for that coincidence to occur in all of the cases. On the other hand, 66.6% of the patients had been treated previously with immunomodulatory or immunosuppressant drugs, and this could have induced a persistent state of remission. The decision to start IVIG treatment was made based on the presence of relapses despite other treatments, and all patients remained stable during follow-up. In addition, we found steady improvement in EDSS in 5 patients and sustained stability in 3 patients (5 patients of the total were monitored for more than 22 months). These two facts support IVIG treatment being effective for NMO-spectrum disorders. Another important finding from our study was the high frequency of OCMB (66.6%). This is more than double the rate published for series of patients with MS.27 The presence of OCMB in CSF may be indicative of intrathecal IgM synthesis. This possibility correlates with findings from anatomical pathology studies: Ig deposits (mainly IgM) that are co-localised with initial products of the complement activation cascade are found near blood vessels in early lesions in NMO.2,3
NMO-IgG was measured during a stable phase of the disease in 8 cases, either during or shortly after immunosuppressant treatment in 5 cases. This may explain the low frequency of positive NMO-IgG tests in our patients (25%).28,29 The effectiveness of IVIG in NMO is supported by the mechanisms that these immunoglobulins are known to possess.4–7 IVIGs act upon the main mechanisms involved in NMO pathogenesis,2,3 and some positive results of this treatment have been previously reported in case series.13,14,22 We decided to administer treatment dosed as a fortnightly infusion of 0.7g/kg body weight per day during 3 days. This dosage has been shown to be well-tolerated and effective in other neurological diseases, including CIDP30 and myasthenia gravis.31 Optimal doses, whether for inducing remission or as maintenance treatment, remain a topic of debate and further studies on this subject are needed.32,33 In summary, IVIG treatment is safe and well-tolerated. It appears to be effective in preventing relapses in NMO and related diseases, at least during the first 2 years of treatment. Controlled trials including larger patient populations and covering longer follow-up periods are required in order to confirm these results and determine appropriate doses and infusion periodicity.
Conflicts of interestM.J. Magraner served as a member of the board of advisors to Biogen Idec.
F. Coret has served as a member of the board of advisors to TEVA and Sanofi-Aventis.
B. Casanova has served on the board of advisors to Biogen Idec, Merck-Serono, TEVA, Sanofi-Aventis, and Bayer-Schering.
Please cite this article as: Magraner MJ, et al. Estudio del efecto del tratamiento con inmunoglobulinas por vía intravenosa en la neuromielitis óptica. Neurología. 2013;28:65–72.