Patients who have difficulties recognising visual form stimuli are usually labelled as having visual agnosia. However, recent studies let us identify different clinical manifestations corresponding to discrete diagnostic entities which reflect a variety of deficits along the continuum of cortical visual processing.
DevelopmentWe reviewed different clinical cases published in medical literature as well as proposals for classifying deficits in order to provide a global perspective of the subject. Here, we present the main findings on the neuroanatomical basis of visual form processing and discuss the criteria for evaluating processing which may be abnormal. We also include an inclusive diagram of visual form processing deficits which represent the different clinical cases described in the literature. Lastly, we propose a boosted decision tree to serve as a guide in the process of diagnosing such cases.
ConclusionsAlthough the medical community largely agrees on which cortical areas and neuronal circuits are involved in visual processing, future studies making use of new functional neuroimaging techniques will provide more in-depth information. A well-structured and exhaustive assessment of the different stages of visual processing, designed with a global view of the deficit in mind, will give a better idea of the prognosis and serve as a basis for planning personalised psychostimulation and rehabilitation strategies.
Los pacientes que presentan dificultades para el reconocimiento visual de formas estimulares son considerados habitualmente como pacientes con agnosia visual. No obstante, estudios recientes permiten identificar diferentes manifestaciones clínicas que podrían corresponderse con entidades diagnósticas que reflejan déficits diferenciados a lo largo del continuo del procesamiento visual cortical de las formas.
DesarrolloRevisamos diferentes casos clínicos publicados en la literatura científica así como propuestas de clasificación de este déficit con la finalidad de dar una visión integradora del mismo. Exponemos los principales hallazgos en cuanto a las bases neuroanatómicas del procesamiento visual de formas y discutimos acerca de los criterios para evaluar dicho procesamiento cuando pueda estar alterado. Asimismo, presentamos un esquema de los déficits de procesamiento visual de formas que pretende integrar los distintos casos clínicos descritos en la literatura científica. Finalmente, proponemos un árbol de decisión que puede ser útil para guiar el proceso diagnóstico de estos casos.
ConclusionesExiste un amplio consenso en cuanto a las áreas corticales y circuitos neuronales que participan en el procesamiento visual, aunque futuros estudios con las nuevas técnicas de neuroimagen funcional permitirán profundizar en este aspecto. Una evaluación estructurada y exhaustiva de las diferentes etapas del procesamiento visual realizada a partir de una visión integradora del déficit nos facilitara un diagnóstico más objetivo, lo que nos permitirá conocer mejor el pronóstico y será de utilidad para guiar el diseño de estrategias individualizadas de psicoestimulación o rehabilitación.
In general, we can define visual agnosia as difficulty or inability to identify or recognise certain characteristics (shape, colour, movement, category, etc.) of visual stimuli, provided this difficulty is not caused by a peripheral sensory visual deficit.
At the end of the 19th century, Lissauer1 provided a preliminary view of the concept by distinguishing between apperceptive visual agnosia (the inability to receive a complete, conscious visual impression or perception of the stimulus), and associative visual agnosia (the inability to link the visual impression or perception to the meaning of the stimulus). This conceptual schema still provides the reference framework for studying patients who present visual recognition deficits. However, the last 3 decades have witnessed a debate on how to describe clinical cases that suggest new, more specific entities according to the type of deficit the patient may present on the continuum of cortical visual processing.
In this article, we specifically focus on deficits related to cortical perception, recognition, and identification of shapes and outlines, that is, the form traditionally known as shape agnosia. To this end, we will begin by describing different clinical cases and showing how they fit distinct diagnostic classification systems. We will then provide a global scheme for classifying different clinical presentations. We will also review the neuroanatomical basis of visual form processing and examine factors that must be considered when evaluating the different steps in visual processing.
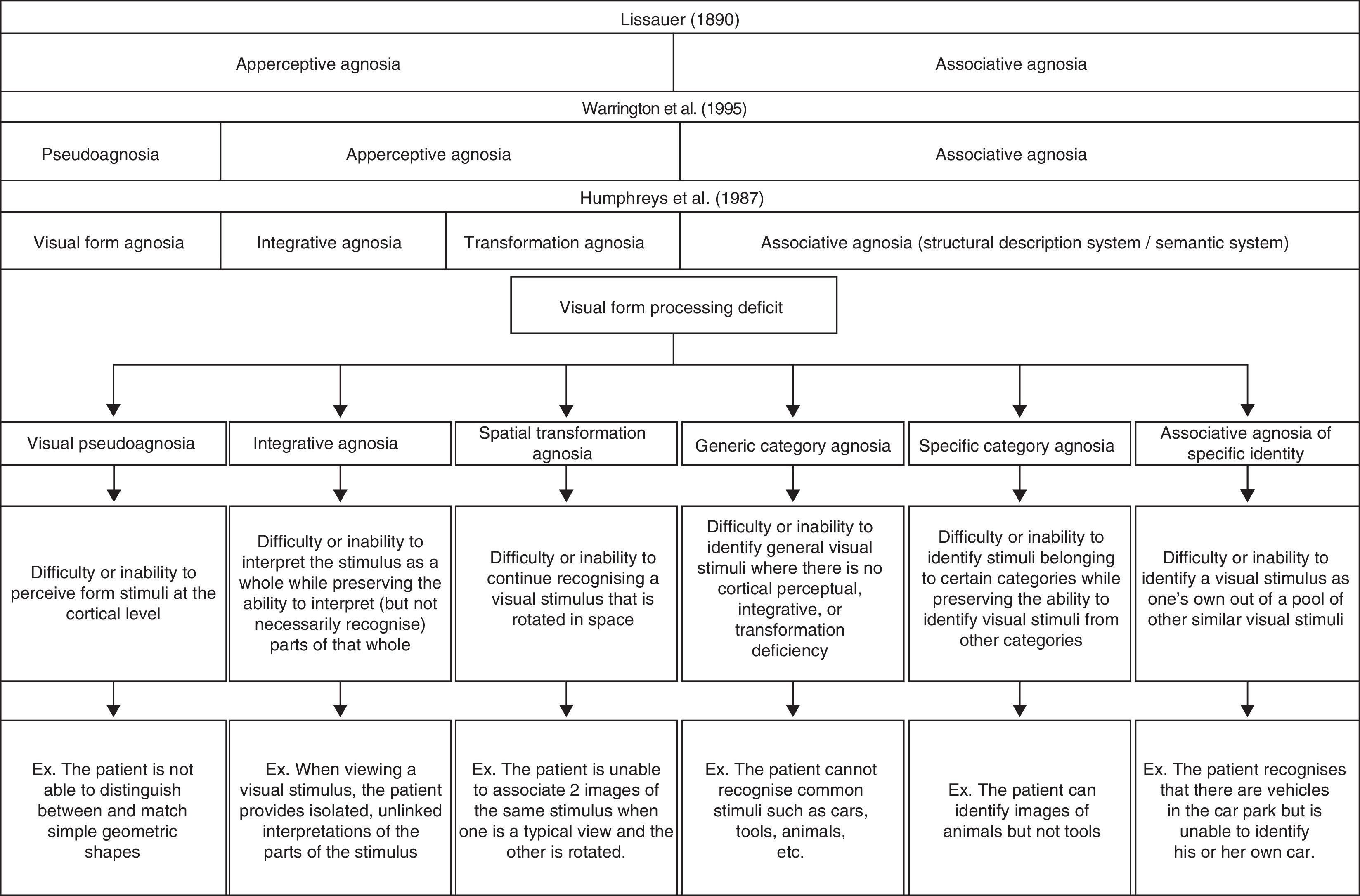
Clinical manifestations and diagnosis of visual form processing deficitsIn 1987, Humphreys and Riddoch2 proposed a classification scheme including 3 distinct types of apperceptive visual agnosia: shape agnosia, integrative agnosia, and transformation agnosia. Shape agnosia is characterised by the patient's difficulty or inability to perceive visual form stimuli correctly.3,4 In line with Warrington and Rudge,5 we believe that the cause of this deficit is an alteration of the cortical perceptive process that is not entirely agnosic, and that it would be more accurate to call this deficit pseudoagnosia.
In contrast, patients with integrative agnosia are able to perceive, quite precisely, the different parts or components making up the visual stimulus, but they cannot integrate them into a coherent shape.6 These patients therefore interpret what they see without being able to recognise the stimulus object, as we deduce from the example of the comments made by patient viewing a picture of a carrot: “I have not even the glimmerings of an idea. The bottom point seems solid and the other bits are feathery. It does not seem to be logical unless it is some sort of a brush.”2
The last type of apperceptive agnosia described by Humphreys and Riddoch2 is transformation agnosia. This deficit is characterised by the patient's inability to recognise an object consistently. This makes it difficult for the patient to link 2 images that show the same stimulus from different perspectives (for example, a normal profile image and a foreshortened image of the same object). Although Bricolo et al.7 refer to this deficit as spatial agnosia, we believe that this term may engender confusion by giving the impression that the error resides in spatial processing and not in inconsistent recognition of the same stimulus.
Although integrative agnosia and rotation agnosia have both been categorised as apperceptive agnosia, we find this classification to be misleading as well; a pure basic deficit of cortical perception does not appear to exist in either case. We support the idea that both integration and mental rotation of visual stimuli are processes arising from working with information that has already been perceived. These processes may be influenced by such cognitive processes as attention or peripheral visual processes that involve perception of visual fields or eye movements. In this sense, a global model of shape agnosia should maintain the concept of integrative agnosia and incorporate the concept of spatial transformation agnosia in such a way as to merge the concepts of transformation agnosia proposed by Humphreys and Riddoch2 and spatial agnosia as described by Bricolo et al.7
The second major type described by Lissauer is associative visual agnosia.1 In this case, patients have difficulty retrieving previously stored semantic information, although the sequence of processes prior to that step remain intact: perceptual processing, structuring, and access to the structural description of the visual stimulus.8 Some authors mention the concept of category-specific agnosia in conjunction with associative visual agnosia. Nevertheless, this term may also be confusing, given that some patients have difficulty identifying stimuli belonging to specific categories (for example, stating “this is a car” or “this is a house”). This inability to identify the category defining the stimulus may be called generic category associative agnosia. As we see in cases studied by Damasio,9 other patients are unable to identify stimuli belonging to certain specific categories although they can identify stimuli from other categories. This is known as category-specific agnosia (for example, the patient recognises different types of fruit but not tools). This deficit tends to present a peculiar dichotomy; many patients are able to identify stimuli within the ‘living things’ category but not the ‘non-living things’ category or vice versa. The Farah and McClelland10 model suggests that identifying living things is more closely linked to their visual representation, whereas identifying non-living things is more closely linked to the idea of how they are used. However, scholars including Barbeau and Giusiano11 have also presented cases of a recognition differential between more specific categories (for example, recognising tools but not musical instruments). Other patients have demonstrated inability to recognise the specific characteristics of a visual stimulus that would enable them to distinguish it from other stimuli within the same category (for example, this is my car, this is my house). This type can be called associative agnosia of specific identity. This last condition is easy to detect in cases of prosopagnosia in which patients are usually able to recognise that a face is a face, but they cannot identify the person to whom it belongs. ‘Shape agnosia’ is the term habitually used to refer to difficulty identifying objects in different semantic categories when the visual stimuli are not faces; however, only a few cases in the medical literature9 describe tasks of recognising a visual stimulus as one's own when the stimulus in question is not a face.
In Fig. 1, we present a global classification of visual form processing deficit compared to other classic proposals described in medical literature.
It seems there is no consensus regarding the neuroanatomical localisation of different clinical manifestations of visual form processing deficits, which may be explained in part by the lack of universally accepted criteria for both classifying and evaluating cases. The next section will present the most significant contributions to this topic in medical literature.
Neuroanatomical basis of visual form processingVisual form processing involves complex participation between different cortical structures and systems. These systems work along a continuum that begins at a basic perceptual stage and continues along different stages of information processing and association using different cerebral routes that become extremely complex in their final stages. This complicated process begins in the occipital cortex. The primary visual cortex (V1) provides a cortical map of visual fields and includes the structural characteristics of visual stimuli by combining information from both eyes.12 V2, the largest visual association area in the occipital lobe, responds to factors including orientation, depth, and colour, and it is used in analysing the profiles of visual stimuli. In turn, the posterior ventral area (PV) and V3 are responsible for visual processing during the basic and intermediate stages. Respectively, they participate in basic shape perception and in analysing movement and depth.13,14 Cases in which the patient presents impairment in the initial phases of visual form processing seem to be related to changes at the level of V1, V2, PV, and V4.15 A recent study suggests that lesions in the medial fusiform gyrus in the right hemisphere may also contribute to changes in the initial phases of visual form processing.16
Some patients may perceive the parts of the whole stimulus correctly, but since they cannot integrate them, they are unable to identify the stimulus. Researchers have observed that lesions near the parieto-temporo-occipital junction may cause deficient integration of the different parts making up a visual stimulus.4
Another factor we should emphasise in visual form processing is mental rotation of the visual stimulus. By mentally rotating images, we can predict how the object will look from a different spatial perspective.17 This rotation process involves activating different areas in the occipital and temporal visual cortex comprising both the ventral and dorsal visual pathways.18 Furthermore, the rotation process also involves certain areas of the frontal lobe – Brodmann areas 9 and 46 – involved in working memory for visuospatial information.19
The dorsal visual pathway is involved in visuospatial processing in that it indicates ‘where’ the stimulus is located. The posterior ventral visual pathway is more linked to identifying the stimulus (the ‘what’ pathway), and it is therefore of crucial importance in identifying form stimuli. Research suggests that following the posterior ventral pathway from the occipital to the temporal lobe reveals a specific series of modules that specialise in recognising specific categories of visual stimuli. These modules are the fusiform face area, which is active in face recognition; the parahippocampal place area, for locations; and the extrastriate cortex for recognising parts of the body.20,21 The rest of the posterior ventral cortex seems to be active in general recognition of other visual stimuli.22 As we follow the ventral pathway, other traits describing visual stimuli are added, such as shape, texture, brightness, and colour. Integrating these traits will facilitate identification of the object even in those cases in which the outlines or contours of the object are incompletely defined, or even illusory, as in the case of Kanizsa's Triangle.23
Regarding the participation of both hemispheres in visual processing, numerous studies have linked associative visual agnosia to bilateral temporo-occipital lesions,24 although there have also been cases in which patients exhibited lesions in the right or left hemisphere only.25 Clinical cases of different lesions to brain structures are frequently described, but we must not underestimate the importance of lesions to pathways, especially the temporo-limbic pathway, in visual agnosia. On this subject, Damasio et al.26 observed that the inferior longitudinal fasciculus was often damaged in patients with visual agnosia. This prevents communication between occipital visual association areas and the medial-temporal memory area, thereby limiting recognition of previously familiar visual stimuli.
On the other hand, studies have also observed that different lesions in areas of the right or left ventral pathway may give rise to failure to recover conceptual information about specific generic categories. There have been clinical cases in which patients had difficulty identifying visual stimuli within one category (for example, ‘animals’) but not those from another (for example, ‘tools’). This type of dissociation has led some authors to posit the existence of partially segregated neural systems that process stimuli belonging to different conceptual categories.9,27 For example, Tranel et al.28 observed that animal recognition requires activation of the mesial occipital/ventral regions of the right temporal lobe and the mesial occipital region of the left hemisphere, whereas tool recognition activates the left parieto-temporo-occipital junction. Researchers have also observed significant differences in the activation of brain hemispheres in patients processing visual stimuli in the same semantic category and in those processing stimuli pertaining to different categories.29 These authors provide data that point to the left hemisphere's involvement in recognising objects when they must be selected among different categories, whereas the right hemisphere selects and recognises objects within the same category.
Assessment strategiesAccording to the apperceptive-associative model, patients with difficulty identifying and copying shapes experience apperceptive visual agnosia, whereas those that can perform those tasks but do not recognise the stimuli are said to have associative visual agnosia.30 Based on the many studies we revised, we observe that patients’ clinical manifestations are more complex than this and must be fitted to a model that employs a continuum for visual form processing. We also observe that due to the considerable variety of non-standardised assessment methods, results are difficult to interpret objectively, which is an obstacle to diagnosing the deficits presented by the different subjects in these studies. Devinsky et al.14 have listed various standardised tests used to evaluate visual agnosias, but since manifestations are patient-specific, both researchers and clinicians tend to use creative and innovative methods of assigning specific diagnoses to their patients.
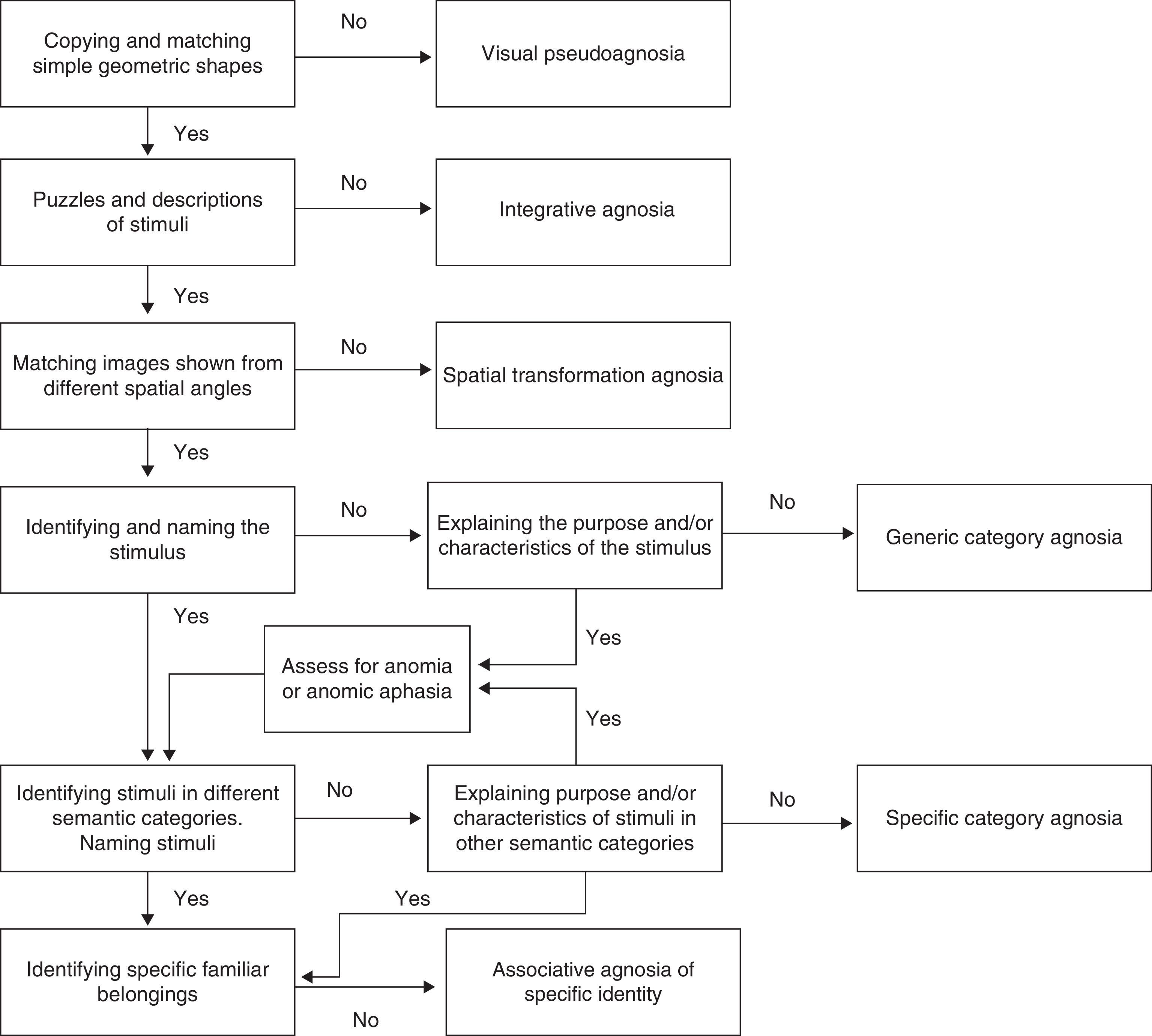
The inability to recognise shapes due to impaired visual processing at basic levels, that is, due to apperceptive agnosia or pseudoagnosia, is mainly characterised by loss of the ability to identify and match simple figures.31 Instruments such as the Efron test,32 the Visual Object and Space Perception battery (VOSP),33 and the form perception/evaluation section of the Birmingham Object Recognition Battery (BORB)34 are useful for performing assessments. Other useful tools are tasks requiring detection of basic geometric shapes against a blurry background, such as the figure detection test35 and the Gollin incomplete figure test.36 Another ability that is affected in these patients is copying shapes or objects presented to them visually; it seems that copying requires intact representation of the perceived stimuli. This being the case, tasks involving copying geometric shapes, letters, or simple figures is useful for evaluating this type of agnosia.3 Lastly, it may be necessary to evaluate the patient's conceptual understanding of those stimuli which he or she cannot recognise. As suggested by Riddoch et al.,4 this will confirm whether the patient's inability to recognise the stimuli is due only to a perception deficit.
The inability to identify more complex stimuli may be the result of inability to combine the parts making up the object, even though the patient may conserve the ability to distinguish between stimuli that are more structurally simple. Assessing integrative agnosia involves using puzzles with images of simple objects such as fruits, tools, or furniture followed by a verbal description of the objects. The purpose is to detect impaired integration and check whether the patient remains unable to extract information, even incomplete information, about the image that is presented. Aviezer et al.37 have also evaluated this deficit by using Gestalt completion tests such as Kanitzsa figures. We feel that the Ghent and Poppelreuter overlapping figure tests38,39 are not useful for evaluating this deficit. The models employed in the first test study the basic perception process; the second test evaluates associative visual processing since it requires a structured description of each of the overlapping figures.
Another clinical entity that might go unnoticed or be confused with another type is transformation agnosia. According to Bricolo et al.,7 these patients have no difficulty distinguishing, matching, copying, recognising, or naming simple objects when they are shown in profile or in typical views, meaning that they are able to complete image matching and discriminating tasks. Results from such tests would indicate correct visual processing and recognition. Nevertheless, certain patients would continue having difficulty performing mental transformation of the visual information provided, which translates to an inability to recognise visual stimuli if they are presented from different angles. In general, useful tasks would be those involving matching and discriminating visual stimuli shown from different perspectives, and selecting matching objects shown with unusual views that would require the patient to compare objects depicted with different degrees of spatial rotation. This deficit can mainly be observed using the following tasks: the Kohs block-design test,40 the manikin test,41 the flags test,42 and the mental rotation test by Shepard and Metzler.43
Patients who do not experience difficulty analysing the primary sensory information of the stimulus, but who are unable to integrate the structural information from the stimulus with semantic memory to assign a term to the stimulus, display associative agnosia. According to Charnallet et al.,8 shape and object recognition, as evaluated using the names and descriptions of images of the objects in question, is severely impaired in such cases. Patients with generic category agnosia fail at the stage of identifying the name, purpose, or category corresponding to the object. We observe that different studies have made use of the BORB34 and the Pyramids and Palm Trees tests44 to screen for this deficit. They have also suggested using recognition tasks for images of objects pertaining to different semantic categories and contrasting that result with recognition of the same stimuli presented using a different sensory channel (for example, animal sounds, tactile recognition of objects, etc.). According to Grossman et al.,45 images presented should include black-and-white drawings like those used in the Boston naming test,46 as well as colour photographs of real objects. The latter are perceptually more complex and place a higher demand on cognitive resources. One study describes the case of a patient with agnosia for drawings who has difficulty recognising sketched images and silhouettes but is able to recognise photographs of real objects accurately.47
Meanwhile, using stimuli pertaining to different semantic categories also responds to the requirement of evaluating specific category recognition deficits. As a result, the stimulus array should include images of both living and non-living things (animals and tools, for example).48 If it is unclear whether difficulty naming objects is the result of an agnosic deficit rather than anomic aphasia or optical aphasia, doctors can ask the patient to describe what the object is used for, and in what context, to determine whether or not the deficit is due to a problem identifying the object. One formal evaluation tool allowing us to distinguish a basic naming deficit from associative agnosia is the Boston Naming Test with verbal cues.46
Lastly, we feel that an exhaustive assessment of the visual form processing continuum must include an evaluation of the ability to recognise and identify one's own belongings. According to Damasio,9 some patients are unable to recognise their own belongings but have no difficulty recognising other stimuli within general or specific categories. These patients experience problems recognising their own cars, clothing, watches, etc. For this reason, assessing such a clinical profile would require creating a battery of images of objects belonging to the patient. These images would be presented alongside other similar objects and the patient would then be asked to identify, for example, which car was his own.
To better place this deficit on the visual processing continuum, Fig. 2 shows a decision algorithm that will be useful for evaluating and diagnosing visual form processing deficit.
ConclusionsAn integrated model for visual form processing must include all possible manifestations of visuoperceptual impairment and use terminology describing the processes that are affected in each patient. This will let us discriminate easily between the different clinical entities described to date.
Researchers largely agree on the delimitation of the cortical areas and neural circuits responsible for visual form processing. However, we believe that future studies with more precise neuroimaging techniques accompanied by more standard and objective assessments will be better able to distinguish between different clinical types of visual form processing deficit.
Numerous assessment strategies are currently available and may be very useful for evaluating visual processing deficits, even though they are still used in an arbitrary way. We believe that an exhaustive assessment of visual form processing must include tests that evaluate all processes from the most basic (perception) to the most complex (identifying objects within a category). In this way, it will be possible to establish a correct diagnosis and therefore gain a better understanding of the patient's prognosis. This will also guide the design of personalised strategies in psychostimulation or rehabilitation programmes for each case, resulting in better quality of life for patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Unzueta-Arce J, García-García R, Ladera-Fernández V, Perea-Bartolomé MV, Mora-Simón S, Cacho-Gutiérrez J. Alteraciones en el procesamiento visual de formas: clasificación clínica integradora. Neurología. 2014;29:482‐489.