To describe the prevalence and form of onset of different causes of binocular diplopia in our setting.
MethodsWe conducted a single-centre, cross-sectional, retrospective study reviewing the medical records of all patients visiting a tertiary-level centre between May 2019 and June 2021 with binocular diplopia as the main symptom. All patients underwent a complete neuro-ophthalmological evaluation and complementary tests for the aetiological diagnosis of diplopia. Data were collected on demographic variables, ocular deviation pattern, complementary test results, and diagnosis.
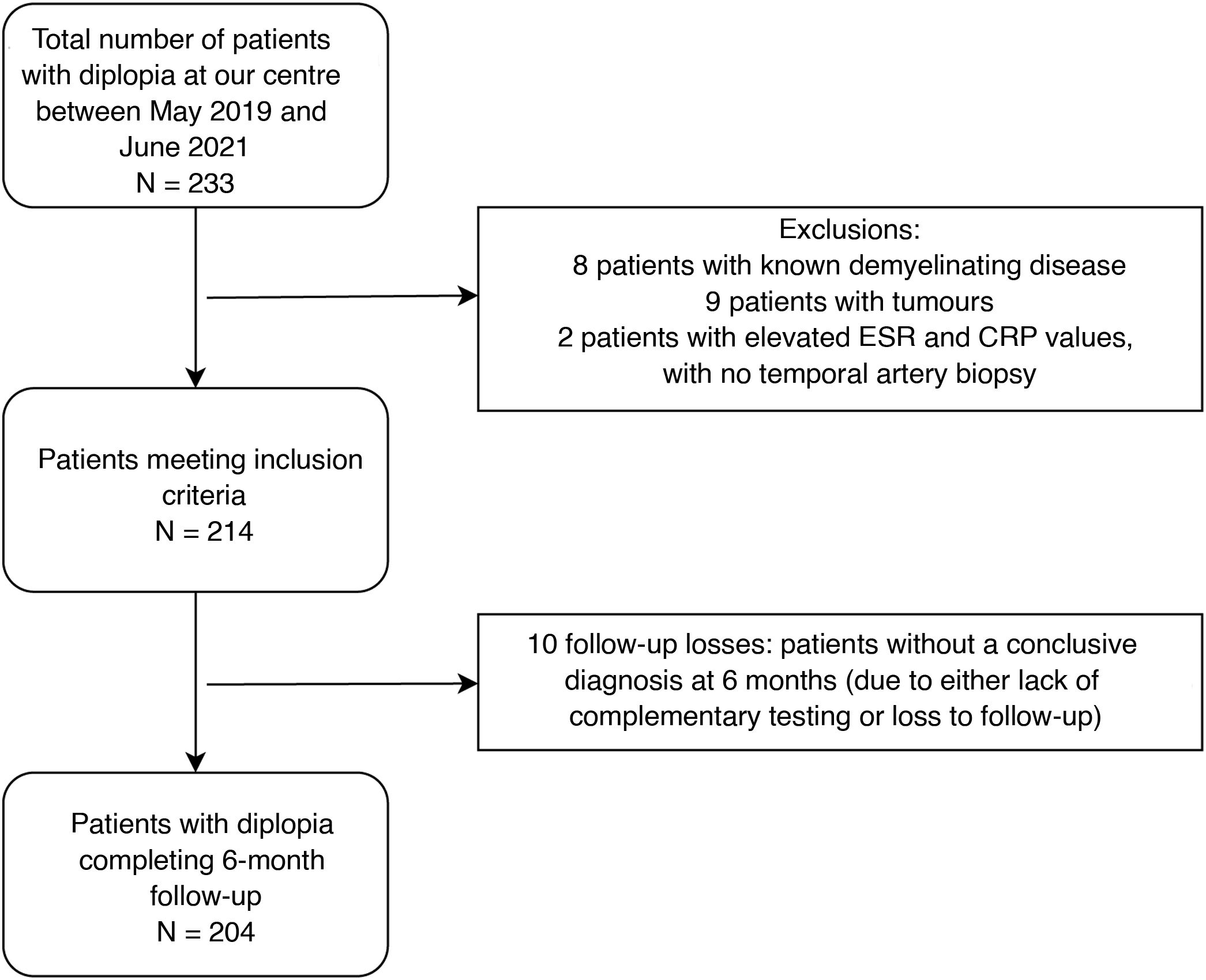
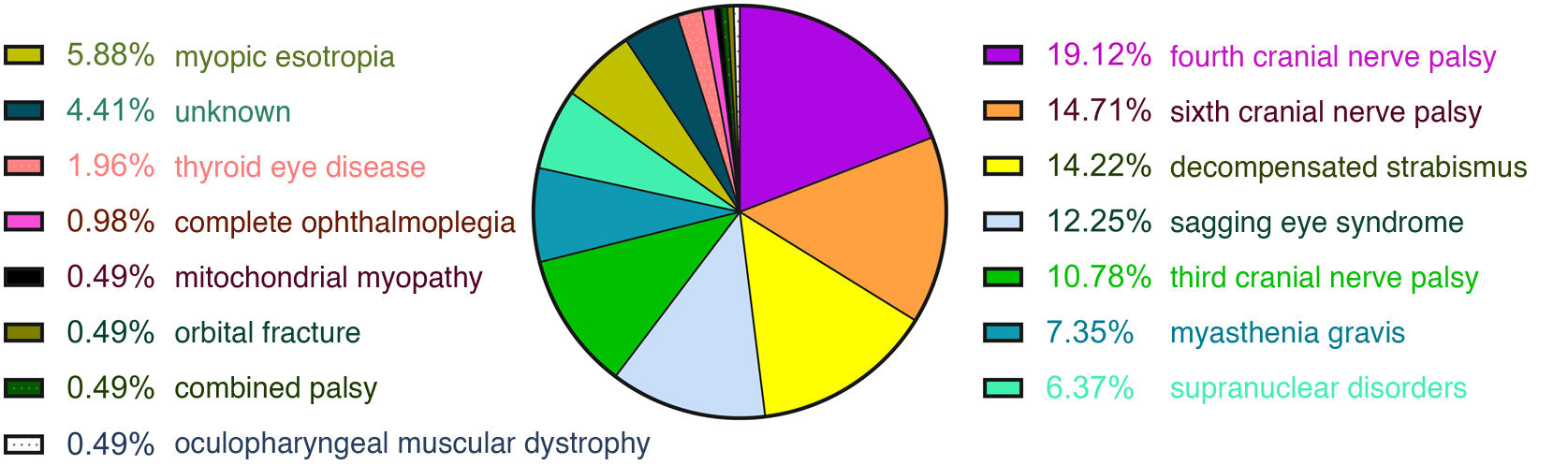
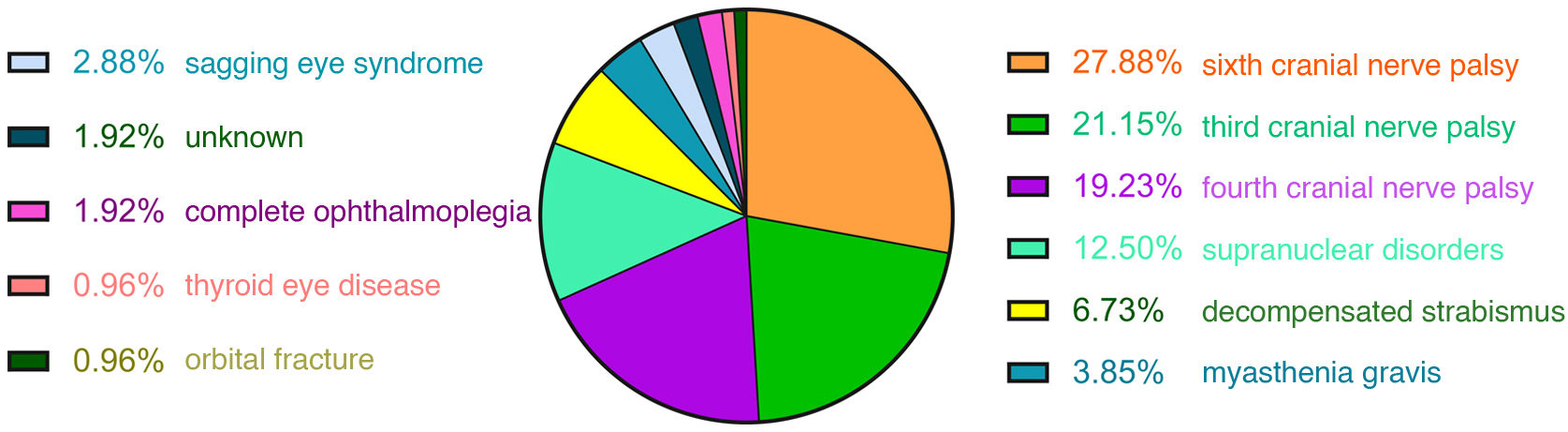
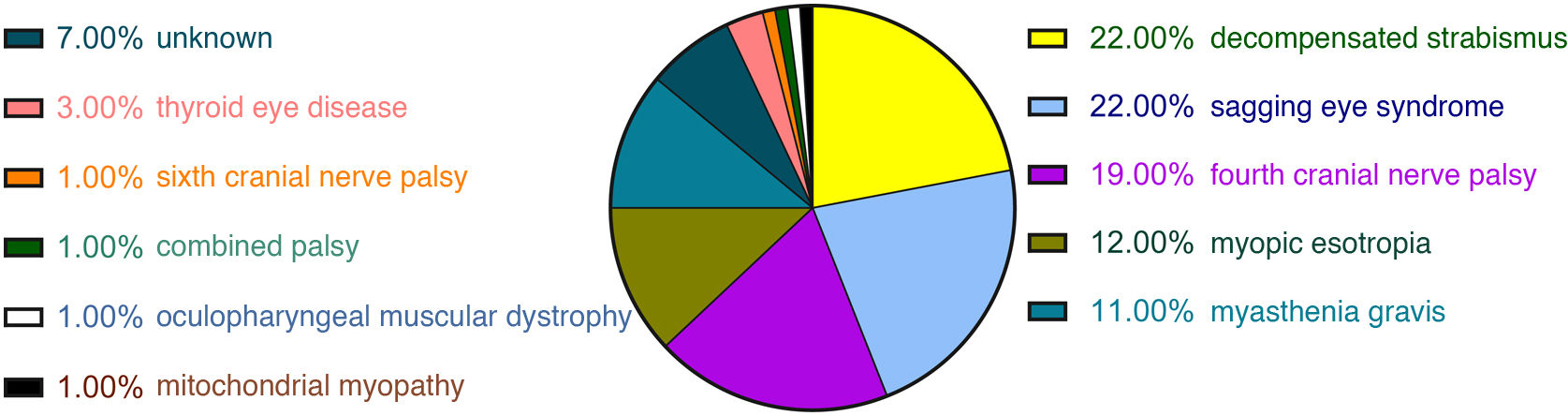
ResultsA total of 204 patients with binocular diplopia were identified during the study period. The most frequent causes of diplopia overall were fourth nerve palsy (19.12%), sixth nerve palsy (14.71%), decompensated strabismus (14.22%), sagging eye syndrome (12.25%), third nerve palsy (10.78%), myasthenia (7.35%), supranuclear disorders (6.37%), and myopic esotropia (5.88%). Presentation was acute (less than 2 weeks’ progression) in 51% of cases. The most frequent causes of acute-onset diplopia were sixth nerve palsy (27.88%), third nerve palsy (21.15%), fourth nerve palsy (19.23%), supranuclear disorders (12.5%), and decompensated strabismus (6.73%). The most frequent causes of subacute/chronic presentation (more than 2 weeks) were decompensated strabismus (22%), sagging eye syndrome (22%), fourth nerve palsy (19%), myopic esotropia (12%), and myasthenia (11%).
ConclusionsThe most frequent aetiology of diplopia in our environment was fourth nerve palsy, followed by sixth nerve palsy, decompensated strabismus, and sagging eye syndrome. Knowing the frequency of each cause of diplopia can help prioritise neuroimaging studies in each case.
Describir la prevalencia y forma de presentación de las distintas causas de diplopía binocular en nuestro medio.
MétodosEstudio unicéntrico de corte transversal, retrospectivo en el que se revisaron a todos los pacientes cuyo síntoma principal fuera el de diplopía binocular sin causa conocida visitados en un hospital terciario entre mayo 2019 y junio 2021. A todos los pacientes se les había realizado una visita neuro-oftalmológica completa y las pruebas complementarias necesarias para el diagnóstico etiológico de diplopía en cada caso. Se recogieron variables demográficas, patrón de desviación ocular, resultados de pruebas complementarias y diagnósticos.
ResultadosEn el período de estudio, 204 pacientes fueron visitados por diplopía binocular. Las causas más frecuentes de diplopía de forma global fueron paresia del IV nervio craneal (19,12%), paresia del VI nervio craneal (14,71%), estrabismos decompensados (14,22%), “sagging eye syndrome” (12,25%), paresia del III nervio craneal (10,78%), miastenia (7,35%), trastornos supranucleares (6,37%) y endotropia miópica (5,88%). El 51% de los casos tuvieron una presentación aguda (menor o igual a 2 semanas). Las causas más frecuentes de diplopía de aparición aguda fueron paresia del VI nervio craneal (27,88%), paresia del III nervio craneal (21,15%), paresia del IV nervio craneal (19,23%), trastornos supranucleares (12,5%) y estrabismos descompensados (6,73%). Las causas más frecuentes de presentación subaguda/crónica (mayor a 2 semanas) fueron estrabismos descompensados (22%), “sagging eye syndrome” (22%), paresia del IV nervio (19%), endotropia miópica (12%) y miastenia (11%).
ConclusiónLas etiologías más frecuentes de diplopía de forma global en nuestro medio fueron las paresias de IV nervio seguidas por las paresias del VI nervio, los estrabismos descompensados y los “sagging eye syndrome”. Conocer la frecuencia de cada causa de diplopía ayuda a priorizar las pruebas en cada caso.
The main causes of acute diplopia in adults are ocular motor nerve palsies, restrictive causes, supranuclear disorders, and decompensated strabismus.1 In the paediatric population, the most common causes include non-paralytic strabismus, trauma, and ocular motor nerve palsies. Unlike in adults, childhood strabismus does not cause diplopia due to a suppression mechanism that develops after onset of ocular misalignment.2
Decompensated strabismus may present acutely, potentially leading to misdiagnosis of ocular motor nerve palsies or supranuclear disorders; therefore, recognising this condition and understanding its prevalence may help to appropriately prioritise neuroimaging.3
One of the most extensive epidemiological studies conducted to date is that by De Lott et al.,4 which reviewed cases of diplopia in the United States over a 9-year period. A key finding of this study is that approximately 850 000 emergency department visits per year are due to diplopia; however, the study is based on generic diagnoses. As the authors do not differentiate between monocular and binocular diplopia, determining the prevalence of each cause is not straightforward. In a retrospective study by Martinez-Thompson et al.,5 including 753 patients with diplopia, the most frequent cause was paralytic strabismus (including third, fourth, and sixth cranial nerve palsies, supranuclear disorders, and myasthenia gravis), in 44.2% of cases, followed by convergence insufficiency in 15.7%, small-angle hypertropia in 13.3%, and divergence insufficiency in 10.6%.
However, these studies do not analyse the prevalence of each condition based on the form of onset. Therefore, the purpose of this study is to determine the most common aetiologies of binocular diplopia in our setting, distinguishing between acute and chronic presentations.
Material and methodsWe conducted a cross-sectional, single-centre, retrospective study based on a review of clinical records. We included patients older than 18 years whose main reason for consultation was new-onset binocular diplopia not explained by any known disorder. All patients were evaluated at the neuro-ophthalmology unit of Parc de Salut Mar in Barcelona between May 2019 and June 2021. The study was approved by the ethics committee of Parc de Salut Mar (reference no. 2021/10091); exemption from informed consent was granted due to the retrospective nature of the study, the sample size, the scientific relevance of the project, and proper anonymisation of patient data.
All patients were attended at the neurology or ophthalmology departments, either after visiting the emergency department or due to transfer from primary care. We obtained a detailed clinical history from all patients, who also underwent a comprehensive neuro-ophthalmological examination. Patients were followed up for 6 months to collect complementary test results and establish a final diagnosis. The clinical histories were reviewed by the neurologist and the ophthalmologist who had assessed the patients. The following variables were collected: age, sex, pattern of ocular misalignment, form of presentation (acute [≤ 2 weeks] or subacute/chronic [> 2 weeks]), neuroimaging findings, and aetiological diagnosis of diplopia. We excluded patients with a known condition that may explain diplopia.
Diagnosis was established based on the following criteria:
In the case of ocular motor nerve palsy, semiological diagnosis was based on the pattern of ocular misalignment. A laboratory analysis including complete blood count, biochemistry profile, erythrocyte sedimentation rate (ESR), and C-reactive protein was requested for all patients over 45 years of age. Patients with acute diplopia underwent head CT, and those with third cranial nerve palsy also underwent CT angiography. In follow-up consultations, brain and orbit MRI was requested for patients younger than 50 years, those with history of neoplasia or associated neurological symptoms, or cases where paresis did not improve after 2–3 months. Aetiological diagnosis was based on clinical history, examination findings, laboratory test results, and radiological findings. Ischaemic aetiology was established in patients older than 50 years with cardiovascular risk factors who showed complete resolution within 3–6 months, with other causes ruled out by neuroimaging studies, and who presented no other manifestations. In the group of patients with third cranial nerve palsy, we also analysed the aetiology based on the presence of ocular motility impairment and pupillary involvement.
The supranuclear disorders group included patients with internuclear ophthalmoplegia, skew deviation, or upward and downward gaze alterations. These patients underwent an emergency head CT scan. If examination findings were compatible with supranuclear palsy, an emergency outpatient brain MRI study was performed.
Diagnosis of sagging eye syndrome was established in patients with ptosis and small-angle vertical and/or horizontal strabismus. Diagnosis was clinical, although other causes of diplopia were excluded. Patients younger than 80 years underwent a brain and orbit MRI study on an outpatient basis, whereas those older than 80 underwent head CT. Presence of myasthenia gravis was ruled out through laboratory (acetylcholine receptor antibodies) and electrophysiological tests.
Decompensated strabismus included patients with the same type of misalignment (esotropia, exotropia, vertical strabismus) in all gaze positions (comitant strabismus) without limitation of eye movement, presenting intermittently or gradually. Patients were asked about presence of strabismus in childhood. Among the patients without a clear history of strabismus, those aged under 80 years underwent brain and orbit MRI on an outpatient basis, whereas those older than 80 underwent head CT.
Esotropia associated with myopia included cases of limited abduction, esotropia with or without hypotropia, and myopia greater than 6 dioptres. Brain and orbit MRI was performed to rule out structural causes of sixth cranial nerve palsy, and to plan for corrective surgery.
Diagnosis of myasthenia gravis was established in patients with fluctuating diplopia and positivity for acetylcholine receptor antibodies and/or pathological jitter as assessed with single-fibre EMG.
Diagnosis of giant cell arteritis was based on the following criteria: age over 50 years, compatible symptoms, ESR ≥ 50 mm/h and/or C-reactive protein > 0.5 mg/dL, and compatible temporal artery biopsy findings.
Thyroid eye disease was diagnosed in patients with compatible clinical, laboratory, and neuroimaging findings.
Complete ophthalmoplegia was diagnosed in 2 patients who were unable to move one eye.
Oculopharyngeal muscular dystrophy was diagnosed when genetic testing for PABPN1 (> 11 GCG repeats) was positive, and mitochondrial myopathy when biopsy results were compatible.
Finally, cases were classified as unknown diplopia when the cause could not be determined despite neuroimaging, laboratory, and neurophysiology testing, and even muscle biopsy in some cases.
Data were analysed using the SPSS statistical software, version 22.0. A descriptive analysis was performed, with categorical variables expressed as percentages and absolute frequencies, and continuous variables as means (with standard deviations [SD]), medians, and ranges.
ResultsOf the 233 patients with diplopia assessed at Parc de Salut Mar between May 2019 and June 2021, 204 met the inclusion criteria (Fig. 1). Mean age (SD) was 66 (18) years and median age was 68 years (range, 21–98). The sample included 98 women and 106 men. The most frequent causes of diplopia in our study were fourth cranial nerve palsy (19.12%), sixth cranial nerve palsy (14.71%), decompensated strabismus (14.22%), sagging eye syndrome (12.25%), and third cranial nerve palsy (10.78%) (Fig. 2). Onset was acute in 104 patients and subacute/chronic in 100. Figs. 3 and 4 display the frequencies of each diagnosis in patients with acute and subacute/chronic diplopia.
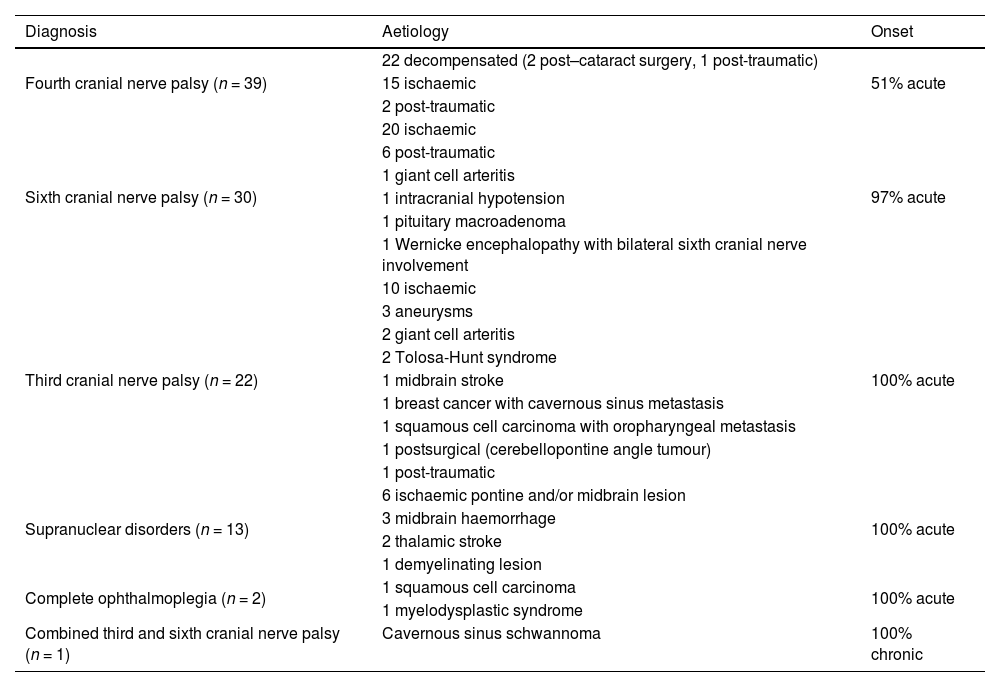
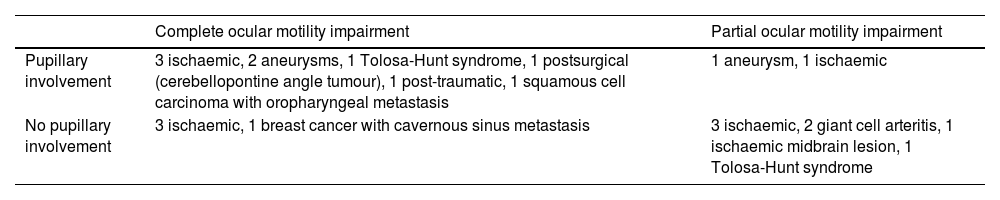
Table 1 presents the aetiology of ocular motor nerve palsies, supranuclear disorders, combined palsy, and complete ophthalmoplegia in our sample. Table 2 describes the aetiology of third cranial nerve palsy according to the presence of pupillary involvement and ocular motility impairment.
Aetiologies of ocular motor nerve palsies, supranuclear disorders, combined palsy, and complete ophthalmoplegia.
| Diagnosis | Aetiology | Onset |
|---|---|---|
| Fourth cranial nerve palsy (n = 39) | 22 decompensated (2 post–cataract surgery, 1 post-traumatic) | 51% acute |
| 15 ischaemic | ||
| 2 post-traumatic | ||
| Sixth cranial nerve palsy (n = 30) | 20 ischaemic | 97% acute |
| 6 post-traumatic | ||
| 1 giant cell arteritis | ||
| 1 intracranial hypotension | ||
| 1 pituitary macroadenoma | ||
| 1 Wernicke encephalopathy with bilateral sixth cranial nerve involvement | ||
| Third cranial nerve palsy (n = 22) | 10 ischaemic | 100% acute |
| 3 aneurysms | ||
| 2 giant cell arteritis | ||
| 2 Tolosa-Hunt syndrome | ||
| 1 midbrain stroke | ||
| 1 breast cancer with cavernous sinus metastasis | ||
| 1 squamous cell carcinoma with oropharyngeal metastasis | ||
| 1 postsurgical (cerebellopontine angle tumour) | ||
| 1 post-traumatic | ||
| Supranuclear disorders (n = 13) | 6 ischaemic pontine and/or midbrain lesion | 100% acute |
| 3 midbrain haemorrhage | ||
| 2 thalamic stroke | ||
| 1 demyelinating lesion | ||
| Complete ophthalmoplegia (n = 2) | 1 squamous cell carcinoma | 100% acute |
| 1 myelodysplastic syndrome | ||
| Combined third and sixth cranial nerve palsy (n = 1) | Cavernous sinus schwannoma | 100% chronic |
Aetiologies of third cranial nerve palsy, according to presence of pupillary involvement and/or ocular motility impairment.
| Complete ocular motility impairment | Partial ocular motility impairment | |
|---|---|---|
| Pupillary involvement | 3 ischaemic, 2 aneurysms, 1 Tolosa-Hunt syndrome, 1 postsurgical (cerebellopontine angle tumour), 1 post-traumatic, 1 squamous cell carcinoma with oropharyngeal metastasis | 1 aneurysm, 1 ischaemic |
| No pupillary involvement | 3 ischaemic, 1 breast cancer with cavernous sinus metastasis | 3 ischaemic, 2 giant cell arteritis, 1 ischaemic midbrain lesion, 1 Tolosa-Hunt syndrome |
Of the 29 cases of decompensated strabismus, 2 cases appeared following cataract surgery and one was due to presbyopia. Three patients did not undergo neuroimaging studies due to a clear history of strabismus before the onset of diplopia. Diplopia presented with esotropia in 16 patients, exotropia in 2, vertical misalignment in 4, esotropia plus vertical misalignment in one, and exotropia plus vertical misalignment in 5.
In the subgroup of 25 patients with sagging eye syndrome, diplopia appeared after cataract surgery in 4 and after YAG laser capsulotomy in one. Five patients presented the following radiological findings: slight asymmetry in extraocular muscle thickness, slight descent of the lateral rectus muscle, mild elongation of the band connecting the superior rectus and the lateral rectus (LR-SR band), and poor visualisation of the LR-SR band. Diplopia presented as small-angle esotropia in 14 patients, small-angle vertical deviation in 9, and with mixed components in primary gaze position in 2.
Among the 15 patients with myasthenia gravis, diplopia appeared following cataract surgery in one patient and in the context of lung adenocarcinoma in another. Four cases presented with esotropia, 4 with vertical diplopia, 2 with exotropia, 2 with ptosis and limited abduction, and 3 with ptosis and vertical diplopia. Eight patients tested positive for acetylcholine receptor antibodies and 14 presented pathological jitter on single-fibre EMG.
Of the 13 cases of supranuclear palsy, 3 were secondary to haemorrhage detected by CT and 10 were diagnosed using MRI. Elevation restriction was observed in 3 patients, internuclear ophthalmoplegia in 3, skew deviation in 2, comitant esotropia in 2, internuclear ophthalmoplegia plus sixth cranial nerve palsy in 2, and internuclear ophthalmoplegia plus skew deviation in one. In 12 patients, diplopia was accompanied by other neurological symptoms, including altered level of consciousness, dizziness, gait alterations, dysarthria, headache, hemiparesis, and language impairment; a single patient presented diplopia with no other neurological symptoms.
Of the 12 patients with esotropia associated with myopia, 4 presented the following radiological findings: displacement of lateral rectus muscles, muscle atrophy, and poor visualisation of the LR-SR band. Ten patients presented horizontal diplopia in primary gaze position, and the remaining 2 presented both horizontal and vertical diplopia.
Among the 9 cases of unknown aetiology, one patient presented acute esotropia. This was a case of comitant esotropia with no apparent cause, despite the lack of pathological findings in complementary tests (MRI, laboratory tests, single-fibre EMG). Another patient presented monocular elevation deficiency with negative forced duction test results. One patient underwent muscle biopsy, with inconclusive results, and another patient responded to corticosteroids but presented no markers of myasthenia gravis.
Our sample included 2 patients with complete ophthalmoplegia. One of these presented maxillary sinus squamous cell carcinoma, with CT showing extraocular muscle infiltration into one eye. The other patient presented a myelodysplastic syndrome, with MRI displaying extraocular muscle enlargement.
DiscussionOverall, the most common causes of binocular diplopia in our setting are fourth cranial nerve palsy, sixth cranial nerve palsy, and decompensated strabismus. The most frequent aetiologies of acute diplopia are ocular motor nerve palsies (affecting the sixth, third, or fourth cranial nerves) and supranuclear palsy. On the other hand, the most frequent causes of chronic diplopia include decompensated strabismus, sagging eye syndrome, and fourth cranial nerve palsy. Together, decompensated strabismus and sagging eye syndrome account for 9.61% of all cases of acute diplopia.
The prevalence of different causes of diplopia varies considerably according to the form of presentation. In a retrospective study by Goseki et al.,6 including 945 patients with diplopia, the most frequent cause was sagging eye syndrome (31.4%), followed by fourth cranial nerve palsy (10.3%). However, this study did not differentiate between acute and chronic presentations. In the retrospective study by O’Colmain et al.,1 which included 149 patients with acute diplopia (< 4 weeks), 53.7% of the sample presented isolated third, fourth, or sixth cranial nerve palsy; 10.7% had diplopia due to orbital mechanical causes; 10.1% had a supranuclear disorder; and 8.1% presented decompensation of pre-existing heterophoria. These findings are consistent with those observed in our study. From a clinical viewpoint, they underscore the importance of including decompensated strabismus and sagging eye syndrome in the aetiological diagnosis of patients with acute diplopia.
Supranuclear palsy was the cause of diplopia most frequently associated with pathological MRI findings (100%). The 13 patients with supranuclear disorders underwent emergency CT and urgent outpatient MRI. In patients with midbrain haemorrhage, both CT and MRI revealed bleeding; however, only MRI detected the causal anatomical lesion in the remaining patients. In cases of posterior circulation stroke of less than 24 hours’ progression, MRI is reported to offer higher sensitivity than CT (80%–95% vs 16%).7
Of the 22 cases of third cranial nerve palsy, 10 (45.45%) were of ischaemic origin: 3 due to aneurysms, 2 due to giant cell arteritis, 2 due to metastasis, 2 due to Tolosa-Hunt syndrome, one due to a midbrain ischaemic lesion, and one occurring after surgery to treat a cerebellopontine angle tumour. In the study by O’Colmain et al.,1 22.1% of cases of isolated third cranial nerve palsy were of microvascular aetiology. Aneurysms constitute a severe potential cause of third cranial nerve palsy. When classified according to clinical examination findings (pupillary involvement and ocular motility impairment), ischaemic cases in our series presented with highly variable features, whereas all cases associated with aneurysm showed pupillary involvement. The management of third cranial nerve palsy is a controversial subject. Several protocols have been proposed, according to which the need for neuroimaging is established based on clinical examination results. Some studies recommend brain MRI and MR angiography or CT angiography, depending on the clinical findings.8,9 However, recent publications support the use of these neuroimaging studies regardless of pupillary involvement or ocular motility impairment, as compressive causes (including aneurysms) may present without pupillary involvement.10,11
In cases of fourth cranial nerve palsy, it is important to distinguish between acquired and congenital causes; congenital cases are secondary to dysgenesis of the fourth cranial nerve or the superior oblique muscle, which may cause decompensation.12 Among our cases of acute fourth cranial nerve palsy, 2 were post-traumatic, 15 were ischaemic, and 3 were decompensated. Acute fourth cranial nerve palsy is rarely caused by tumours; in a study by Hesselink et al.,13 tumoural causes were identified in only 2 of 131 patients with fourth cranial nerve palsy. In our series, 49% of cases were chronic, resulting from decompensation of congenital fourth cranial nerve palsy. Our findings are in line with those of a retrospective study of 73 cases of fourth cranial nerve palsy, 49% of which were congenital.14 In a 2009 review, Brazis9 concluded that neuroimaging is not necessary when physical examination findings are suggestive of long-standing fourth cranial nerve palsy (large vertical fusional amplitudes, head tilt, facial asymmetry, overacting inferior oblique muscle).
In our study, 60% of cases of sixth cranial nerve palsy were of ischaemic origin. Margolin15 recommends a period of observation before requesting neuroimaging studies in patients over the age of 50 with sixth cranial nerve palsy who present cardiovascular risk factors and no other relevant symptoms or comorbidities. However, other authors recommend early neuroimaging assessment. Several studies have attempted to resolve this controversy.16–19 In a study of 109 patients older than 50 years with third, fourth, and sixth cranial nerve palsies, Tamhankar et al.20 reported that 16.5% of patients (95% CI, 10.7%–24.6%) presented non-ischaemic aetiologies. Based on these results, the authors recommend early neuroimaging assessment.
Contrary to expectations, 73% of cases of myasthenia gravis in our sample were subacute/chronic. Some patients may present a paucisymptomatic form of the disease, with small-angle deviations that do not prompt them to seek urgent medical attention.
In patients with sagging eye syndrome, MRI typically reveals LR-SR band thinning, leading to elongation, rupture, or disappearance of said band; however, these changes are not specific to sagging eye syndrome, but rather can also be observed in older adults without strabismus.21 In our series, 20% of patients with sagging eye syndrome presented some of these neuroimaging findings. Indication of neuroimaging assessment in these patients is not clearly established: although diagnosis is clinical, other causes of diplopia must also be ruled out.
High myopia may be associated with globe subluxation at the supratentorial level, with inferior displacement of the lateral rectus muscle and medial displacement of the superior rectus muscle.22 These patients present esotropia, occasionally associated with hypotropia, which can mimic sixth cranial nerve palsy. Twelve patients in our series presented esotropia associated with myopia, 33.3% of whom displayed findings compatible with extraocular muscle displacement.
In the patients with thyroid eye disease and orbital fracture, symptoms were diagnostic. In thyroid eye disease, both STIR and contrast T1-weighted MRI sequences may help to assess the degree of disease activity.23
Decompensated strabismus accounts for 14.22% of all cases of diplopia. Guidelines for the management of patients with long-standing childhood strabismus recommend clinical follow-up without neuroimaging.24 However, strabismus may decompensate when ocular misalignment worsens or refractive conditions change (eg, following cataract surgery), potentially leading to diplopia.
Three patients were diagnosed with giant cell arteritis. Two presented incomplete third cranial nerve palsy without pupillary involvement, and one presented sixth cranial nerve palsy with limited abduction and a vertical component. Diplopia rarely presents as the initial manifestation of giant cell arteritis, occurring in 2%–15% of patients. The cause of diplopia is multifactorial; one potential cause is vasculitic occlusion, which causes ischaemia of the ocular motor cranial nerves, extraocular muscles, or both.25 Therefore, it is advisable to request ESR and C-reactive protein tests in patients over the age of 50 years who present diplopia.15
The main limitation of our study is the sample size, which prevents us from accurately establishing the prevalence of rarer diseases. Furthermore, its retrospective design inherently introduces diagnostic bias. All cases of diplopia attended at the emergency department, through in-hospital interconsultations, at outpatient consultations, or at hospital neurology or ophthalmology consultations are centralised in our hospital’s neuro-ophthalmology unit. To obtain a representative sample of patients with diplopia in our setting, we gathered data from patients attended at this unit. However, there may be a selection bias in the proportion of patients with diplopia included, as some patients may not have sought medical attention or been referred to hospital.
ConclusionOverall, the most frequent cause of binocular diplopia is fourth cranial nerve palsy, followed by sixth cranial nerve palsy and decompensated strabismus. Diplopia secondary to ocular motor nerve palsies typically presents acutely, whereas diplopia secondary to strabismus is usually chronic. However, exceptions exist, as strabismus may decompensate acutely. Understanding the frequency of each aetiology of diplopia in our setting can help prioritise neuroimaging assessment in clinical practice.