Few treatments are currently available for amyotrophic lateral sclerosis (ALS). A combination of lithium carbonate and valproic acid (VPA-Li) was shown to inhibit motor neuron death and delay disease progression.
MethodsOutpatients with a typical ALS presentation were enrolled in a randomized, placebo-controlled trial to assess the efficacy of orally administered VPA-Li. Changes in a functional scale score (ALSFRS-R) and survival rate were chosen as primary outcome variables. Secondary outcome variables included BMI, respiratory monitoring, quality of life, and a global impression of the treatment.
ResultsOut of 42 patients enrolled, 20 individuals receiving VPA-Li and 18 on placebo treatment were included in the final analysis. Forty-five percent of patients receiving VPA-Li completed the trial, whereas only 22.22% of patients in the placebo group attended the final visit 18 months later (P = 0.09). Major changes in the ALSFRS-R score were observed, including a decrease of 1.195 points/month in the placebo group (95% CI: 0.7869–1.6031) and of 0.5085 under VPA-Li treatment (95% CI: 0.2288–0.7882) between months 6 and 14. Adverse events included bad mouth taste, constipation, and anorexia. Survival rate, body weight, and quality of life were positive outcomes by the end of the trial despite a high sample reduction, especially in the placebo group. The inclusion of 212 subjects in each group would confirm these differences.
ConclusionsCombined VPA-Li treatment associated with slower ALS progression and better secondary outcomes. This dual treatment overcame the futility threshold and merits further investigation in ALS.
Contamos actualmente con pocos tratamientos para la esclerosis lateral amiotrófica (ELA), Se demostró que una combinación de carbonato de litio y ácido valproico (VPA-Li) inhibe la muerte de las motoneuronas y enlentece la progresión de la enfermedad.
MétodosPacientes ambulatorios con un cuadro típico de ELA se incluyeron en un ensayo clínico aleatorizado controlado con placebo para evaluar la eficacia de VPA-Li administrado por vía oral. Los cambios en la escala funcional (ALSFRS-R) y la tasa de sobrevida constituyeron los desenlaces primarios. Las variables de desenlace secundario fueron el Índice de masa corporal, el monitoreo de la función respiratoria, la calidad de vida y la impresión global del tratamiento.
ResultadosDe los 42 pacientes incorporados, 20 que recibieron VPA-LI y 18 el placebo, se incluyeron en el análisis final. En total, el 45% de los pacientes tratados con VPA-Li completaron el ensayo, mientras que solo el 22.22% de los del grupo placebo asistieron a la visita final, 18 meses después (p = 0,09). Se observaron como cambios en la ALSFRS-R, una disminución de 1,195 puntos/mes en el grupo de placebo (IC del 95%: 0,7869–1,6031) y de 0,5085 en el tratamiento con VPA-Li (IC del 95%: 0,2288–0,7882), entre los meses 6 y 14. Entre raros eventos adversos se registraron mal sabor de boca, estreñimiento y anorexia. La tasa de supervivencia, el peso corporal y la calidad de vida fueron resultados positivos al final del ensayo a pesar de la reducción de la muestra, especialmente en el grupo placebo. La inclusión de 212 sujetos en cada grupo confirmaría estas diferencias.
ConclusionesUn tratamiento combinado de VPA-Li se vinculó con una progresión más lenta de la ELA y con un mejor desenlace secundario. Este tratamiento dual superó el umbral de futilidad y merece una mayor investigación en ELA.
Between the years 1869 to 1874, Jean-Martin Charcot described and established the clinical-pathological relationships of the progressive and invariably fatal disease that he named amyotrophic lateral sclerosis (ALS). The clinical course can be briefly described as the development of paralysis with rapid muscle emaciation but with no sensory loss, with spastic hypertonia resulting in contractures and permanent deformation, loss of the ability to stand and walk, intermittent clonus in the lower extremities, and preserved of bladder and anal control. Worsened dysarthria, dysphagia, and respiratory symptoms are observed in a latter phase.1 Along with unified clinical criteria,2 several biomarkers support an accurate diagnosis, and current criteria are mostly neurophysiological in nature.3 Additionally, radiological markers were developed in recent years to monitor changes in the corticospinal tract, metabolites, and brain volume, as well as iron deposits.4,5 Fluid markers and genetic tests have revealed a growing number of hereditary forms intricately linked to the underlying processes and led to better understanding of the disease.
For decades, riluzole, a drug acting on glutamatergic transmission, remained the sole approved medication to slow down ALS progression. In 2017, the US Food and Drug Administration approved edaravone, which showed efficacy in delaying the functional decline in ALS patients.6 However, there are still many unmet medical needs for these patients. Lithium (Li) salts showed some promise, but only one early-phase pilot trial reported statistically significant efficacy endpoints, and 3 subsequent large-scale studies failed to reproduce these positive results.7,8 Additionally, a phase 3 trial with valproic acid (VPA) failed to show significant benefits, while an open study using a VPA-Li combined treatment showed good tolerance and clinical stability, making it a promising alternative for ALS patients.9,10 These drugs taken together have previously demonstrated a synergic neuroprotective effect in motor neuron cultures and in transgenic models.11 Thus, a randomized, double-blind, placebo-controlled trial is required to determine the real efficacy of this combination.
MethodsThis trial was planned and conducted in a reference center over a 3-year period, from November 2015 to December 2018, to evaluate the combined treatment of valproate and lithium carbonate (VPA-Li) in ALS patients, compared with placebo.
ParticipantsEligible patients were 40 to 70 years old and had a definite diagnosis of ALS according to the revised El Escorial criteria12; neurophysiological studies were verified and completed in our institution, along with a neurological examination and a global evaluation by a specialist in neuromuscular diseases. Only patients with a stable respiratory capacity or forced vital capacity (FVC) higher than 60%, capable of oral feeding, and with a disease duration since the first symptoms of 2 years ± 6 months were eligible. Additional inclusion criteria were having sporadic forms of the disease and a fixed treatment history for at least 3 months, and for the duration of the trial. The patients included were randomly assigned to receive either a combined therapy of valproate (600 mg/day) and lithium carbonate (600 mg/day) divided into three daily doses, or identical placebos, over a period of 18 months. These are fixed doses, established based on the results of the previous clinical trial10 regarding serum levels, safety, and synergy of the 2 drugs. After allocation to a treatment, patients were clinically assessed every 2 months, and laboratory studies were performed at 4-month intervals. Both the patients and the staff involved in this study were blind to treatment assignment. The primary outcome variable was chosen after a joint functional rank and overall analysis of survival, defined as the time elapsed before mechanical ventilation and/or gastrostomy were required, or patient death occurred.
Allocation concealment mechanismRandom allocation was made in blocks. A block comprised 4 treatments (2 placebo and 2 active) to keep the sizes of treatment groups similar. The placebos were indistinguishable from the active treatments in color, size, and weight. They were sent, along with the drugs, to a packaging and labeling service before delivery. The bottles have numbers with the letter V or L followed by 4 digits dispensed by a random system. Each new patient chose an enrollment number for one of the treatments in his or her block until an unassigned label was found.
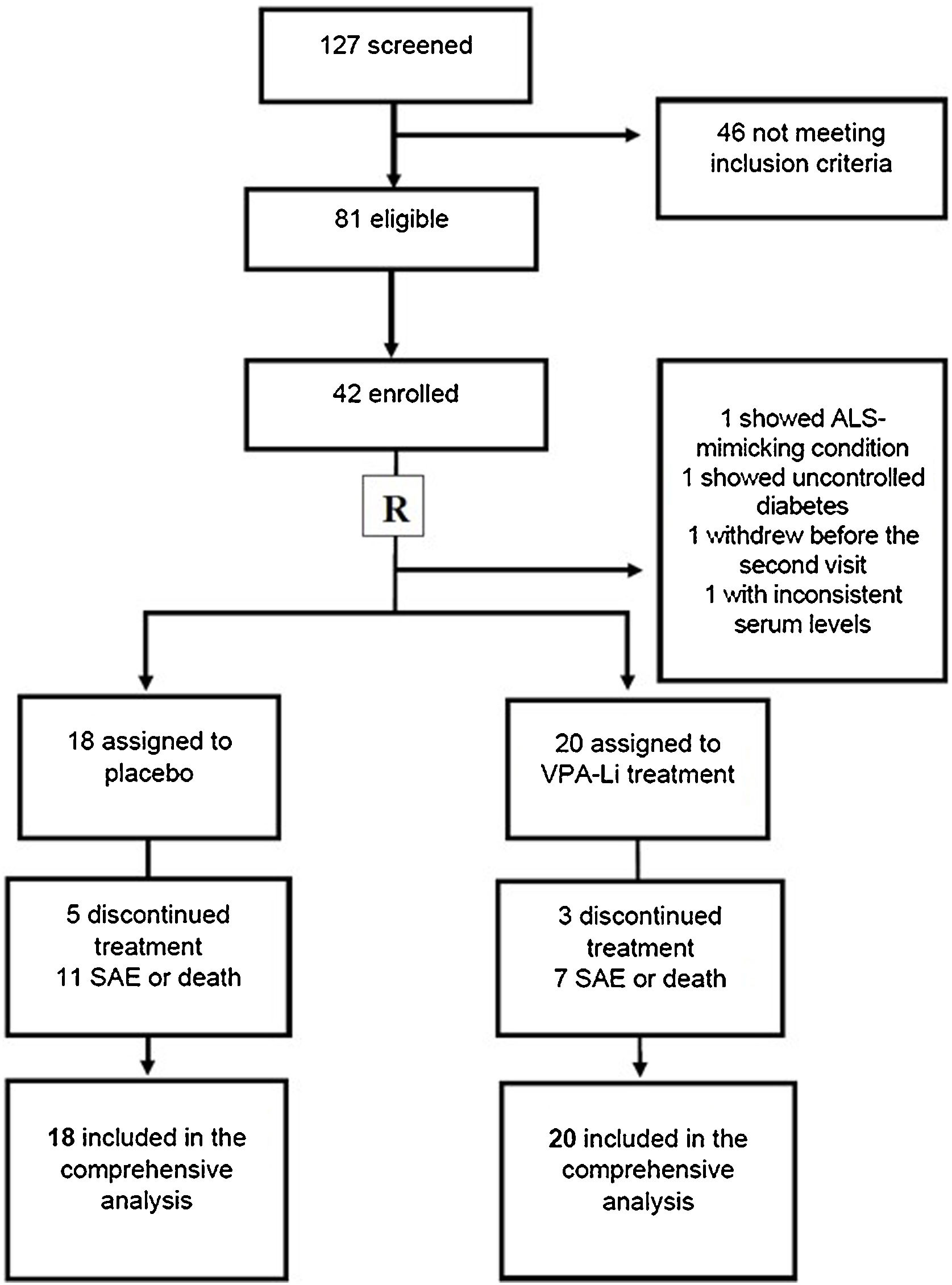
Ethical considerationsBefore enrollment, all patients that met the inclusion criteria provided written informed consent to participate in this 18-month trial. As a safety measure, the contact information of the treating physician was provided in case of deterioration or any severe adverse event. This study was conducted in accordance with the guidelines of good clinical practice, the principles in the Declaration of Helsinki, and all applicable standard operating procedures. For a complete and transparent presentation, the CONSORT statement was followed, including a flowchart. The protocol was previously approved by the institutional Scientific and Ethics Committees, and it was then registered on clinicaltrials.gov, and supported by a governmental grant.
Primary objectiveTo determine whether the combined VPA-Li treatment can slow down the progression of the disease, preserve functional capacities, and improve survival rates.
Outcome measuresDisease-modifying effects were quantified by scoring the revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) and by measuring FVC using manual spirometry, and the body mass index (BMI). Survival rates were determined by recording the disease’s course throughout the trial until tracheostomy and/or gastrostomy were required, or the occurrence of death. Secondary outcome variables were limb strength loss, weight loss, quality of life according to the ALSAQ-5 scale,13 global impression of the treatment (using a 0–10 visual analogue scale), and the explicit desire of patients to continue treatment. Changes in blood analysis and MRI biomarkers were also recorded.
SafetyIn addition to registering all adverse events and providing patients with continuous access to medical care, respiratory function was monitored and ventilatory support was indicated by a pneumologist when respiratory distress was reported by a patient and confirmed by manual spirometry, low oxygen saturation, and increased arterial PCO2. Treatment adherence was controlled by tablet count every 2 months and measurement of blood levels of lithium and VPA every 4 months, keeping research physicians and patients blind to all results. In addition, laboratory tests scheduled every 4 months include thyroid tests, blood chemistry with electrolytes, blood cell count, and urinalysis.
Statistical analysisTreatment efficacy was defined as a reduction in functional decline of 5 points in the ALSFRS-R score within an 18-month period. Thus, considering a power of 80%, a type I error of 5%, and a 10% loss to follow-up, a minimum of 40 participants (20 for each group) was established, based on the distribution in the previous trial. Statistical differences were evaluated by Pearson’s chi-square or the Fisher exact test for categorical variables, and by either Student’s t test for parametric or the Mann-Whitney test for non-parametric comparison. The repeated-measures ANOVA procedure provided a multivariate analysis for subsequent measurements of ALSFRS-R, FVC, BMI, and other continuous variables. After the overall F test, post hoc analysis was performed to evaluate differences in time measurements. Changes from baseline and pooled differences in ALSFRS-R after 6 months were also calculated.
Survival analysis was performed to estimate time to event and risk in both groups of patients, as well as COX regression, the effect of covariates as deterioration index and basal values. All statistical analyses were performed with the IBM SPSS 22 statistics package.
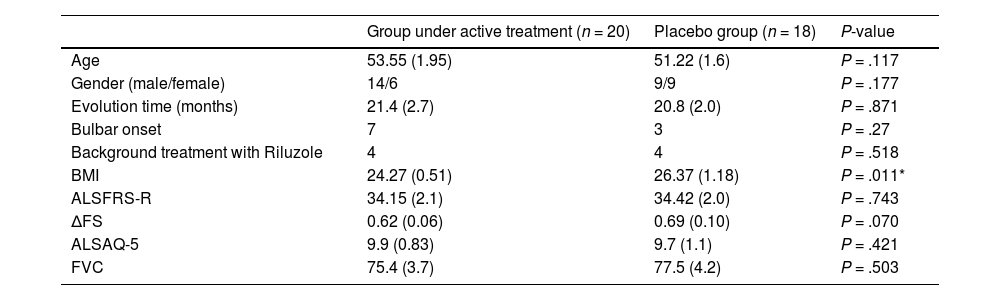
ResultsThe files of 137 patients and incident cases, all with less than 2.5 years of disease progression and with a diagnosis of possible, probable, or definite ALS, were retrieved. In 2015 and 2016, 127 subjects were contacted and invited to participate in the screening phase. From the total list of patients, 31 had died by the time of contact or were not reachable. Only 81 of the remaining subjects attended the selection consultation. Once laboratory analyses and neurophysiology and imaging studies were complete, 58 patients met the inclusion criteria and 44 gave written informed consent to participate (see flowchart, Fig. 1). Two patients died in the period between screening and the next consultation. Two patients did not accept the protocol guidelines, and 2 treated subjects were excluded later, one for presenting with ALS-mimicking condition and the other for violating the protocol by self-medication. The patient database included 38 subjects (23 males and 15 females), with a mean age of 52 years and a disease evolution time of 6 to 36 months. The clinical characteristics of our population at baseline are detailed in Table 1. While no significant differences were observed between both groups in almost all clinical characteristics at baseline, some variables were better in the placebo group, such as a significantly higher BMI (Table 1).
Clinical characteristics of patients at baseline.
| Group under active treatment (n = 20) | Placebo group (n = 18) | P-value | |
|---|---|---|---|
| Age | 53.55 (1.95) | 51.22 (1.6) | P = .117 |
| Gender (male/female) | 14/6 | 9/9 | P = .177 |
| Evolution time (months) | 21.4 (2.7) | 20.8 (2.0) | P = .871 |
| Bulbar onset | 7 | 3 | P = .27 |
| Background treatment with Riluzole | 4 | 4 | P = .518 |
| BMI | 24.27 (0.51) | 26.37 (1.18) | P = .011* |
| ALSFRS-R | 34.15 (2.1) | 34.42 (2.0) | P = .743 |
| ΔFS | 0.62 (0.06) | 0.69 (0.10) | P = .070 |
| ALSAQ-5 | 9.9 (0.83) | 9.7 (1.1) | P = .421 |
| FVC | 75.4 (3.7) | 77.5 (4.2) | P = .503 |
Data are reported as mean (SEM).
ALSAQ-5: Quality of life scale score; ALSFRS-R: Revised ALS functional rating scale score; BMI: Body mass index; ΔFS: progression rate at time of diagnosis; FVC: Forced vital capacity.
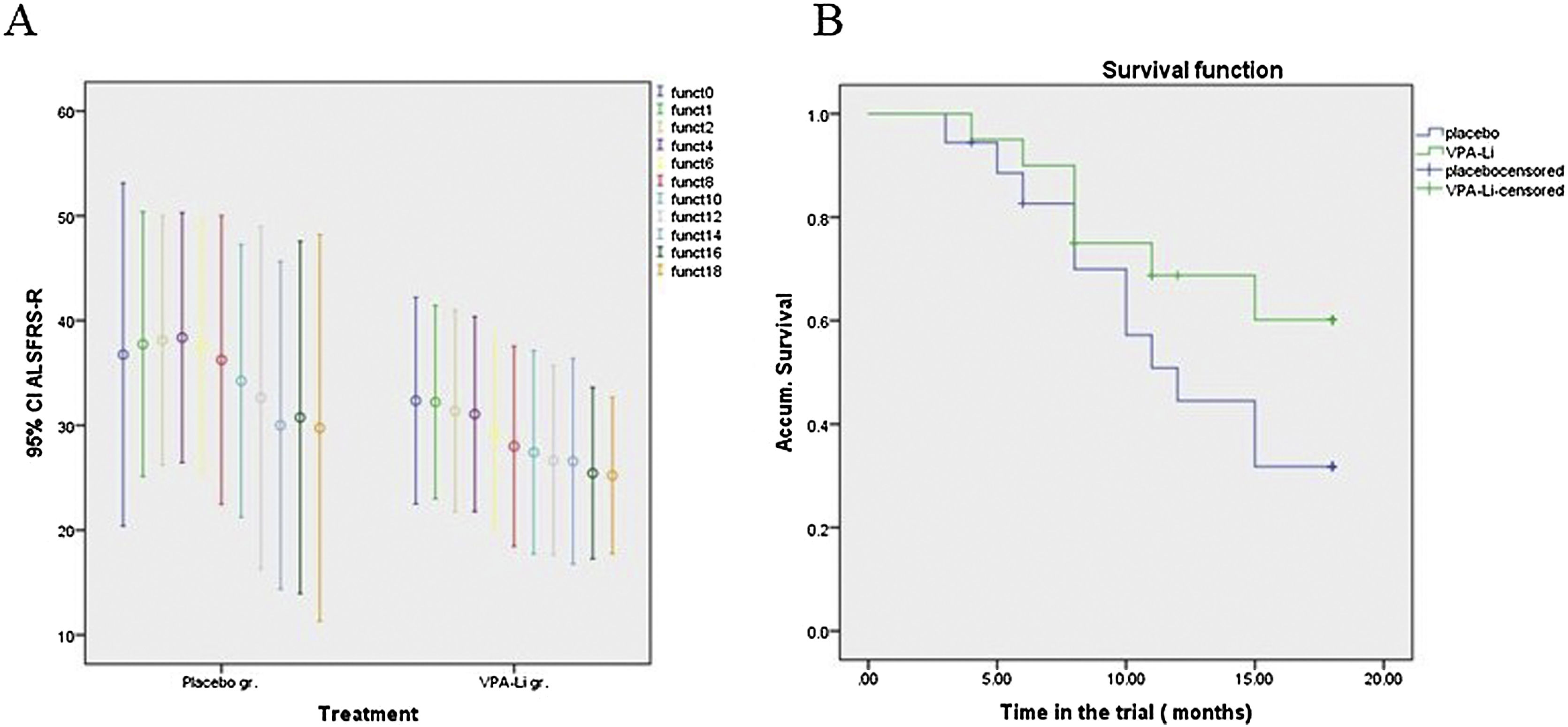
Throughout the trial, no significant differences were observed between groups in the primary outcome variable, changes in scores on the ALSFRS-R, and our patients failed to reach the expected 5-point difference during follow-up. Additionally, no significant differences were observed between groups when analyzing only limb-onset ALS cases, even though each serial evaluation showed lower deterioration indices in patients under active treatment. Excluding bulbar ALS forms, a decrease of 0 to 4 points per month was observed in the functional score; a multivariate analysis showed a mean average decrease of 0.92 points in the treated group, while a decrease of 1.174 points was observed in the placebo group (P = .128, Fig. 2A). Even after calculating z-score and logarithmic transformation of all consecutive measurements of ALSFRS-R, we observed no difference between the groups throughout the duration of the trial. A marked placebo effect was observed during the first 6 months, with a notable functional improvement in the placebo group. In addition, the last 6 months of the study were characterized by a severe decrease in patient sample size, which became too low to be analyzed statistically. Subsequently, observed changes from month 6 to month 14 were calculated; this analysis showed a mean decrease of 1.195 points per month in ALSFRS-R score for the placebo group (95% CI: 0.7869–1.6031), whereas the group receiving the VPA-Li treatment showed a mean decrease of only 0.5085 points per month (95% CI: 0.2288–0.7882). An ANOVA test of these differences in these time-intervals yielded an F = 4.574, P = 0.036. On the other hand, a shallower slope in score deterioration was found both for general functionality and ventilatory capacity in treated patients in the same period (Fig. 2B and 3A).
Primary outcome variables: Functional score and survival.
A) Mean ALSFRS-R scores (and confidence interval), measured bimonthly. The placebo group (left) showed a placebo effect in the first semester. Thereafter, deterioration was faster for this group. B) Survival outcome for patients in active treatment (green) vs. placebo (blue); while the differences were not statistically significant, they show a trend, clearly seen in the plot.
Survival rate was defined as the time before either enteral feeding and/or invasive mechanical ventilation was required, or death occurred. Seven patients died during the trial: 3 from the treated group (15%), and 4 from the placebo group (22.22%). On the other hand, 5 subjects in the placebo group (27.77%) and 2 in the treated group (10%) opted to leave the study while another one was taken by family to another country during the month 11. From the 23 remaining subjects, 5 required a gastrostomy in the follow-up period, 3 of whom were in the placebo group.
Respiratory complications like oxygen desaturation, decreased FVC in manual spirometry, need for ventilatory support, and respiratory tract infection that required measures like hospitalization for tracheostomy led to termination of the trial for 6 patients, 3 from each treatment group. Twelve patients attended all visits and completed the trial: 8 in the treated group (40%) and 4 in the placebo group (22.2%). Another patient from the treated group attended all scheduled visits, although he was excluded from the final analysis after 1 year, due to the need for a tracheostomy. This patient chose non-invasive ventilation and wanted to remain in the study. The follow-up time until an event occurred allowed us to evaluate true permanence in the study, survival time to the end point, and the cumulative survival since disease onset. Survival during the trial was 11.53 months (95% C.I.: 8.769–14.305) for the placebo group and 14.918 months (12.212–17.623) for the VPA-Li treated group. Thus, the time in the trial was 18.82% lower in the placebo group. While no statistically significant differences were found between groups, even after correcting for deterioration index or comparing brachial- against limb-onset disease, all curves showed a higher survival rate in the active treatment group. A Mantel Cox analysis between the 2 groups plus a third group, composed of 39 typical ALS subjects followed up as historical controls (HC), showed a significantly higher survival rate in patients in the trial vs. HC patients (unpublished result). While the differences between both enrolled groups were not statistically significant, the survival curve suggests a protective effect of the dual treatment (Fig. 2B). At the end of the 18-month period, 53.2% (12.1 SE) of subjects in the treated group were alive and without complications requiring hospitalization, compared to 25.5% (10.7 SE) of patients in the placebo group. The hazard ratio of reaching an unfavorable event was of 0.472 under the active treatment (95% CI: 0.182–1.225; p = 0.123).
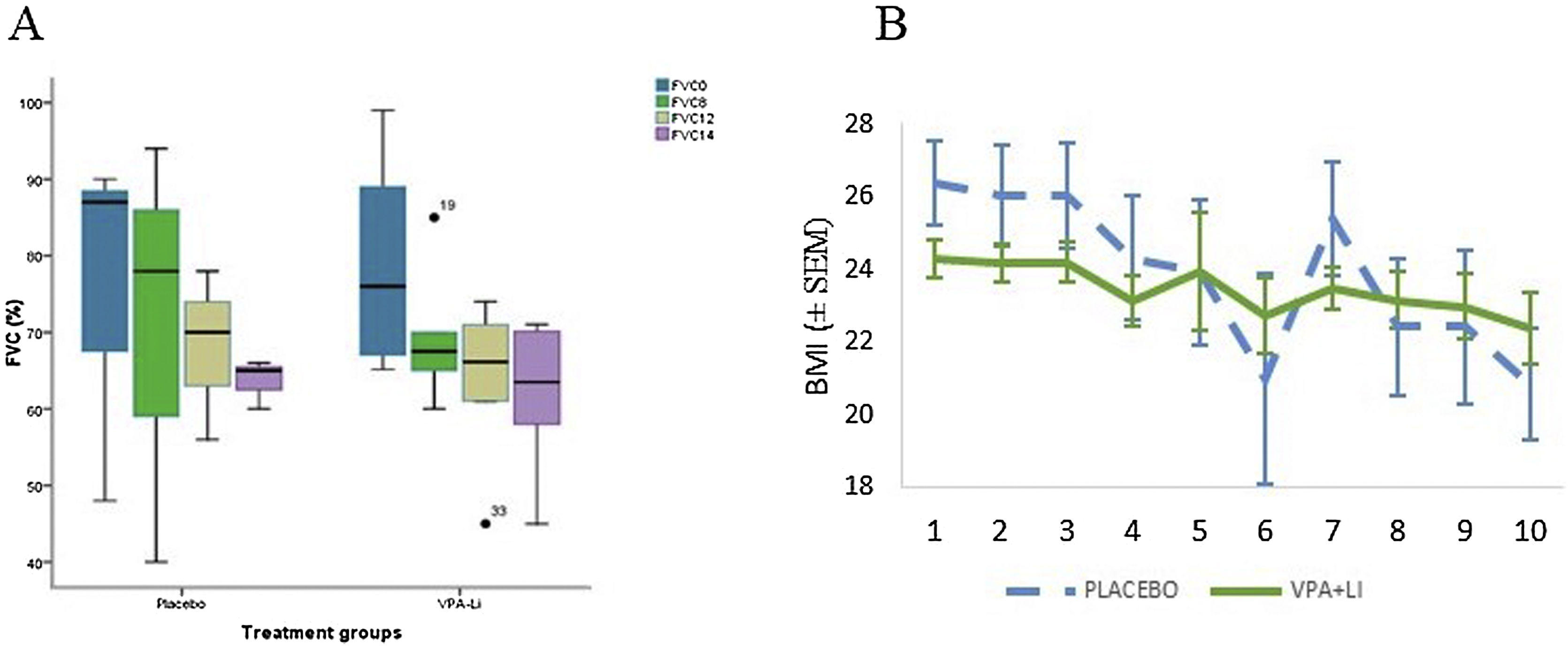
Secondary outcome measuresSpirometry data were obtained from 22 patients (14 in the treatment group and 8 in the placebo group) over a 14-month period. No significant differences between groups were observed, although deterioration rate was higher in the placebo group (Fig. 3A). A significant difference in the change of this parameter was observed on month 14 (Mann-Whitney test, P = 0.048). Additionally, a Cox regression analysis using baseline measurements as a covariable, showed a significant difference in FVC between both groups (Wald index: 4.169; P = 0.041). Among other secondary outcome variables, BMI was significantly higher in the placebo group at baseline, but it was similar in both groups after 12 months, and higher in the treated group by the end of the trial (Fig. 3).
Forced vital capacity and body mass index.
(A) FVC at baseline and at 8, 12, and 14 months; deterioration slope was more pronounced in the placebo group (left). (B) BMI bimonthly measurements. An abrupt change in the placebo group was observed after 12 months due to the loss of 3 subjects. A repeated measures ANOVA showed a significant difference in changes from baseline (P = 0.026).
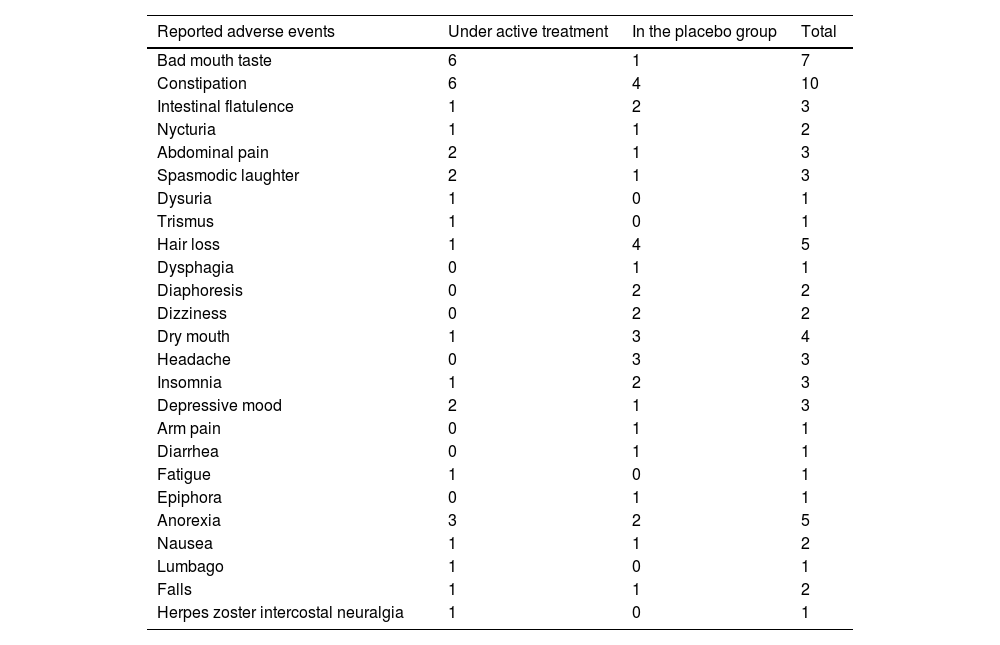
Adherence based on tablet count was of 86.9%, and attendance at the scheduled visits (380) was 65.53%. Serum drug levels were regularly checked by the external treating physician and only one case had to be excluded from the final analysis due to inconsistent results. The average blood lithium values were of 0.4562 mEq/L (0.0345 SEM) and VPA serum levels of 38.6312 mcg/mL (4.1309 SEM) in the treated group. As shown in Table 2, the main non-severe adverse events (AEs) were bad mouth taste, constipation, and anorexia. The incidence of severe AEs was of 61.1% (11/18) in the placebo group and 35% (7/20) in the treated group.
Non-severe adverse events in subsequent visits.
| Reported adverse events | Under active treatment | In the placebo group | Total |
|---|---|---|---|
| Bad mouth taste | 6 | 1 | 7 |
| Constipation | 6 | 4 | 10 |
| Intestinal flatulence | 1 | 2 | 3 |
| Nycturia | 1 | 1 | 2 |
| Abdominal pain | 2 | 1 | 3 |
| Spasmodic laughter | 2 | 1 | 3 |
| Dysuria | 1 | 0 | 1 |
| Trismus | 1 | 0 | 1 |
| Hair loss | 1 | 4 | 5 |
| Dysphagia | 0 | 1 | 1 |
| Diaphoresis | 0 | 2 | 2 |
| Dizziness | 0 | 2 | 2 |
| Dry mouth | 1 | 3 | 4 |
| Headache | 0 | 3 | 3 |
| Insomnia | 1 | 2 | 3 |
| Depressive mood | 2 | 1 | 3 |
| Arm pain | 0 | 1 | 1 |
| Diarrhea | 0 | 1 | 1 |
| Fatigue | 1 | 0 | 1 |
| Epiphora | 0 | 1 | 1 |
| Anorexia | 3 | 2 | 5 |
| Nausea | 1 | 1 | 2 |
| Lumbago | 1 | 0 | 1 |
| Falls | 1 | 1 | 2 |
| Herpes zoster intercostal neuralgia | 1 | 0 | 1 |
All these adverse events were dealt with.
Quality of life was assessed for every patient who finished or left the trial alive and was able to complete the ALSAQ-5 test, either by themselves or aided by a family member. Using the formula [(final score − initial score)/trial time (months)], 8 patients in the placebo group were compared with 12 patients under active treatment; a mean decrease of 0.6434 points per month and of 0.2052 points was calculated, respectively. The difference was significant according to the Mann-Whitney test, P = 0.048. The global impression on the treatment, was rated as 4.8 in the placebo group and as 6.75 in patients receiving the VPA-Li treatment, a significantly higher result (P = 0.039).
DiscussionThis trial offers promising results and valuable clinical experience with the two drugs administered. Our study reflects the outstanding progress made to plan a robust clinical program aiming to improve the lives of patients with this devastating disease. A design with futility rules was not strictly used, but we had to conduct a partial (interim) study after 2 years, where the slope of the regression line of decrease in ALSFRS-R scores was notably higher in the placebo group. By that time, the sample included 14 patients in the treatment group and 12 in the placebo group, and the results were encouraging. However, the difference between groups was not statistically significant by the end of the planned trial.
A prolonged placebo effect was observed during the first 6 months. This could be due to the texture and pleasant taste of the placebo tablets, in addition to possible positive effects of starch, methylthionine chloride, or other components assumed to be inert. This effect improved functional scores, especially in the placebo group. While analyzing the follow-up curves, we considered measurements performed during the following eight months, since the later phase was marked by an insufficient sample size. Thus, functional deterioration was found to be significantly higher in the placebo group.
Phenotypic diversity, a nonlinear disease progression, the need of high-cost resources, and a rapid reduction of sample size in time must be considered when planning ALS-related clinical trials. Even after applying strict diagnostic criteria and selecting patients with similar sociodemographic traits, the high interindividual variability prevented us from finding significant differences in outcome variables like functional scale scores, which showed unusually high dispersion rates. An extraordinarily faster progression was observed in some cases, like a subject under active treatment, whilst long periods of stability (6 and 8 months) and even some improvements were observed in two other patients. As described elsewhere, plateaus and reversals are common in time-progression plots over semesterly periods.14
Another limitation of the trial is the impediment to follow an intention-to-treat protocol by monitoring a functional outcome, because the disease endpoint occurred during the trial period in most cases. Protocol violations like one desertion case and one patient in the placebo group with detectable levels of active pharmaceutical ingredients were excluded from the analysis. Additionally, another patient was treated with Edaravone after the reports were published.15,16 Several events are known to hasten or delay ALS development; for instance, the progression of bulbar forms is faster, and the functional scale widely used in clinical trials may not be well suited to rate patients with relatively preserved limb strength. A longitudinal analysis showed that bulbar forms, more frequent in women, are diagnosed earlier than spinal cases; lower limb-onset ALS also shows a significant delay in the deterioration of the ALSFRS-R motor subscale scores, while bulbar ALS cases usually deteriorate earlier and faster than spinal-onset cases. In future studies, it would be advisable to separate bulbar cases from limb-onset ALS, at least when the disease progression is measured with ALSFRS-R, as previous findings of differential functioning between patients with limb- versus bulbar-onset have suggested.17 We tried to perform a separate analysis of both disease forms and only study spinal-onset cases but were unable to find significant differences due to the low sample size at that time. An alternative approach could be to report ALSFRS-R results not as a single combined score, but rather as domain specific subscores: bulbar, motor, and respiratory.18 Assessment of the respiratory function with a handheld spirometer during the trial led to a decrease in the number of measurements, due to several patients being unable to seal their lips to perform an optimal evaluation. However, taking into account patient safety and the need to detect the need for mechanical ventilation, our evaluations were complemented with arterial bood gases and a consultation with a pulmonologist.
This trial gave us crucial information for future studies. On one hand, the combined VPA-Li treatment was inexpensive, well-tolerated and well-rated by the patients. Although the baseline levels of variables like BMI—a recognized protective factor19—were better in the placebo group, the final analysis clearly showed a better outcome in the treated group. When compared with efficacy studies of drugs highly relevant to ALS, our results are not so different from those of the edaravone trial, which showed, after 6 months, a decrease of 5.01 points (SE 0.64) versus 7.50 in the placebo group. No effect of edaravone was reported on quality of life.20 While most studies, showed no effect or only a trend toward functional improvement,21–23 the recent study with masitinib in combination with riluzole showed a significant difference of 3.4 points (p = 0.016), corresponding to a 27% slowing in the rate of functional decline, compared with riluzole plus placebo. This significant result was obtained only in subjects with a normal or slow progression rate from disease-onset to baseline.24 In our trial, an effect on functional decline was only observed between months 8 and 14. Survival undergoes the same discrete modifications with modest but significant effect with riluzole that was 56.8% of subjects alive without tracheostomy at 18 months vs. 50.4% in the placebo group.23 Including cases requiring gastrostomy, we obtained a survival of 53.2% in patients on VPA-Li at the end of the trial.
Finally, taking the standard deviation determined in this trial and a difference of 5 points in the ALSFRS-R score in a one-sided null hypothesis for a power of 80%, a sample size of 212 patients in each group was calculated as required.25,26 Considering the time-to-event endpoints, we determined an even smaller sample size using parametric models that include prior knowledge of survival patterns.27 These data indicate that a prospective, phase 3, randomized trial is suitable to determine the effect of the combined treatment on patient functional performance and survival.
ConclusionDuring the therapeutic intervention a clear trend toward protection was demonstrated in all the variables studied. Although the ALSFRS-R score failed to show statistically significant differences in the whole follow-up between both groups, functional changes observed on the second semester were significantly better in patients under active treatment; additionally, FVC and weight were more stable in treated patients. Concerns that the functional scale may not be suitable to monitor bulbar forms have been expressed previously. In addition to the changes in survival rates and functional scores, secondary endpoints such as global impression and quality of life, showed the superiority of the combined treatment.
Conflict of interestThe authors report no conflict of interest.
AuthorshipAll authors made substantial contributions to all the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
The study received no specific grant funding from commercial entities, such as pharmaceutical companies.
FundingFunded by CONACYT, grant No. 234154. Clinicaltrials.gov identifier NCT03204500. GILA association also provided help and support with patients’ transportation.
We thank all patients and accompanying persons, seriousness and dedication of implicated fellows and trainees, Dr. Yara de Alba, treating physician, Nurse Guisety, Dr. Burgos, neurophysiologist, and Lic. Vianey Mancilla Dominguez, respiratory therapist.