The COVID-19 pandemic has had a disastrous impact on the world's population. Its effects were mainly respiratory, but resulting neurological damage has also been described. In this context, we evaluated the effects of COVID-19 on the subjective perception of neurological and psychiatric symptoms in patients with pre-pandemic neuropsychiatric diseases, as well as the possible association between the evolution of these symptoms and immunological factors.
MethodsA cohort of neurological/psychiatric patients with (n = 99) or without (n = 42) a history of COVID-19 was included. Inclusion took place 7 months after COVID-19 infection, and follow-up was performed 14 months after inclusion. At both assessments, included subjects were asked whether they considered their neurological/psychiatric symptoms to be stable, worsened or improved compared with the situation before COVID-19, or compared with the first assessment. A blood sample of all subjects was taken at both assessments to determine levels of several cytokines.
ResultsA worsening of neurological/psychiatric symptoms was reported by 36.9% of patients, when comparing the situation at follow-up with that prior to COVID-19. Comparing with controls, patients with history of COVID-19 had significantly higher levels of IL-6 and IFN-γ, and patients with a history of symptomatic COVID-19 presented a significant higher level of IL-10. IFN-γ was significantly associated with COVID-19 severity, and its decrease during follow-up was associated with improvement of neurological/psychiatric symptoms in neurological patients with a history of COVID-19, but not in control patients.
ConclusionsMore than 35% of included neuropsychiatric patients have reported worsening of symptoms after non-severe COVID-19. IFN-γ seems to be a marker linked to COVID-19 pathogeny and its evaluation might be useful for monitoring affected patients.
La pandemia de COVID-19 ha tenido un impacto desastroso en la población mundial. Sus efectos fueron principalmente respiratorios, pero también se han descrito daños neurológicos. En este contexto, evaluamos los efectos de la COVID-19 sobre la percepción subjetiva de síntomas neurológicos y psiquiátricos en pacientes con enfermedades neuropsiquiátricas prepandémicas, así como la posible asociación entre la evolución de estos síntomas y factores inmunológicos.
MétodosSe incluyó una cohorte de pacientes neurológicos/psiquiátricos con (n = 99) o sin (n = 42) antecedentes de COVID-19. La inclusión se realizó 7 meses después de la infección por COVID-19 y el seguimiento se realizó a los 14 meses después de la inclusión. En ambas evaluaciones, se preguntó a los sujetos incluidos si consideraban que sus síntomas neurológicos/psiquiátricos eran estables, empeorados o mejorados en comparación con la situación antes de COVID-19, o en comparación con la primera evaluación. En ambas evaluaciones se tomó una muestra de sangre de todos los sujetos para determinar los niveles de varias citocinas.
ResultadosEl 36,9% de los pacientes informó un empeoramiento de sus síntomas neurológicos/psiquiátricos, al comparar la situación en la evaluación de seguimiento con la situación previa a la COVID-19. En comparación con los controles, los pacientes con antecedentes de COVID-19 tenían niveles significativamente más altos de IL-6 e IFN-γ, y los pacientes con antecedentes de COVID-19 sintomático presentaban un nivel significativamente más alto de IL-10. El IFN-γ se asoció significativamente con la gravedad de la COVID-19, y su disminución durante el seguimiento se asoció con la mejora de los síntomas neurológicos/psiquiátricos en pacientes neurológicos con antecedentes de COVID-19, pero no en los pacientes control.
ConclusionesMás del 35% de los pacientes neuropsiquiátricos incluidos han experimentado un empeoramiento de sus síntomas después de una COVID-19 no grave. El IFN-γ parece ser un marcador vinculado a la patogenia de la COVID-19 y su evaluación podría ser útil para el seguimiento de los pacientes afectados.
The severe acute respiratory syndrome coronavirus (SARS-CoV-2) was the cause of a pandemic between 2020 and 2022. The associated disease (2019 coronavirus, COVID-19) primarily affects the respiratory system, although other organs may suffer as well, particularly the nervous system.1 The spectrum of central and peripheral neurological manifestations is very wide, from headache, dizziness, sleep disorders, anosmia, dysgeusia, depression, psychological distress to altered consciousness, cerebrovascular disorders, encephalopathies, seizures, and psychosis.2 The mechanisms involved in neurological affectation seem to be mainly inflammatory, hypoxic and thrombotic, as no clear evidence of neurotropism for SARS-CoV-2 have been described.3–5 Another factor that could be involved in some of the neurological symptoms associated with COVID-19 could be the stress experienced by all individuals during the pandemic. For example, the most frequent characteristic of headache associated to COVID-19 was tension-type headache pattern,6 responding well to amitriptyline.7 In addition, it was demonstrated that during pandemic, psychiatric patients experienced a higher increase of symptoms related to stress, compared to subjects without antecedents of psychiatric diseases.8
In a first report of this study, we evaluated the effect of COVID-19 infection in 176 patients affected by neurological/psychiatric diseases. Three groups of neurological/psychiatric patients were included: the first with a history of symptomatic COVID-19 infection, the second with a history of asymptomatic COVID-19 infection, and the third one without any history of COVID-19 infection. Comparing pre and post infection, more than 30% of the included patients considered that their baseline neurological/psychiatric pathology had worsened, the feeling being significantly more frequent in patients with history of symptomatic COVID-19 (51.7%) than in patients of the other 2 groups (30% in asymptomatic COVID-19 group and 21.3% in the control group).9
The factors associated with these observations had not been evaluated, and the aim of the present work was to assess whether immunological factors were correlated with these observations as well as with the evolution of symptomatology 1 year after the first evaluation.
MethodsAs the patients included in this study were a subgroup of those included in the previous paper,7 some of the aspects of the methodology are similar and will be resumed.
PatientsThis study conducted at the Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez (INNNMVS) in Mexico City includes a retrospective and a prospective part (Fig. 1).
141 neurological/psychiatric patients divided in three groups were considered: 80 patients with symptomatic COVID-19 who met the World Health Organization (WHO) case definition (probable and definite cases of COVID-19) (https://apps.who.int/iris/bitstream/handle/10665/ 337834/WHO-2019-nCoV-Surveillance_Case_Definition- 2020.2-eng.pdf), 19 patients with asymptomatic COVID-19 (patients who did not develop symptoms of COVID-19, but in whom the presence of specific serum antibodies was observed in samples taken before the start of vaccination in Mexico), and 42 control subjects (patients at the institute with no history of symptoms consistent with COVID-19, no contact with people affected by COVID-19, and no specific antibodies in serum). Main characteristics of the 3 groups included are presented in Table 1. We excluded from the study patients with acute/subacute neurological or psychiatric pathologies, those in whom a neurosurgical procedure was performed during the study period or 6 months before, and those in whom a complete change in their therapeutic management between the 2 evaluated periods was performed. In addition, all patients included were over 18 years of age, followed at INNMVS and had no decontrolled metabolic diseases. All patients included in the study signed an informed consent prior to blood sample collection.
Main characteristics of the 3 groups of neurologic / psychiatric patients included in the study.
| COVID-19(N = 99) | Controls(N = 42) | P | ||
|---|---|---|---|---|
| Symptomatic(n = 80) | Asymptomatic(N = 19) | |||
| Sex Feminine (N, %) | 57, 71.2% | 13, 68.4% | 24, 57.1% | 0.29 |
| Age | 46.2 ± 13 | 44.4 ± 16.8 | 41.1 ± 16.4 | 0.19 |
| BMI | 27.5 ± 5.9 | 28 ± 4.4 | 25.5 ± 5.2 | 0.11 |
| COVID-19 vaccine before inclusion⁎ | 30, 37.5% | 0, 0% | 9, 21.4% | 0.0001 |
| COVID-19 vaccine between first and second samples⁎ | 53/57, 93% | 7/8, 87.5% | 20/21, 95.2% | 0.76 |
| Main Comorbidities | ||||
| 45 (56.2%) | 11 (57.9%) | 35 (83.3%) | 0.31 |
| 19 (23.7%) | 4 (21.0%) | 2 (4.8%) | 0.03 |
| 5 (6.2%) | 1 (5.3%) | 2 (4.8%) | 0.94 |
| 6 (7.5%) | 0 (0.0%) | 2 (4.8%) | 0.43 |
| Neurologic /psychiatric pathologies | ||||
| 27 (33.7%) | 10 (52.6%) | 16 (38.0%) | 0.31 |
| 12 (15.0%) | 3 (15.7%) | 5 (11.9%) | 0.88 |
| 12 (15%) | 0 (0.0%) | 6 (14.2%) | 0.20 |
| 13 (16.3%) | 1 (5.2%) | 3 (7.14%) | 0.21 |
| 6 (7.5%) | 1 (5.2%) | 7 (16.6%) | 0.21 |
| 6 (7.5%) | 1 (5.2%) | 2 (4.8%) | 0.82 |
| 4 (5%) | 1 (5.3%) | 2 (4.8%) | 0.99 |
| 0 (0.0%) | 1 (10.5%) | 2 (4.8%) | 0.13 |
BMI: Body Mass Index. HBP: High Blood Pressure.
The inclusion of the patients took place approximately 7 months after contracting COVID-19 disease. At inclusion (first evaluation), we asked patients to compare their symptoms between the last 6 months of 2019 (before the onset of the COVID-19 pandemic) and different time periods according to their situation: 6 months after infection for patients with symptomatic COVID-19, 6 months after the determination of specific antibodies for asymptomatic COVID-19 patients, and 6 months before inclusion for control patients.
The questionary used was an ad-hoc questionnaire that collect the subjective impression of the patient regarding its baseline neurological or psychiatric pathology before and after COVID-19. Three categories of neurological/psychiatric evolution between the 2 study periods were considered. Improvement, if the patient refers to a decrease in symptoms due to the neurological/psychiatric condition. Stability, if the patient indicates that his or her symptoms were similar or, in the case of degenerative pathologies, the intensity of the worsening was equal during the 2 periods evaluated. Worsening, if the patient refers to an increase in symptoms related to their baseline pathology or if new neurological/psychiatric symptoms have appeared. Sera of all included patients was also collected at inclusion.
We followed up patients and controls, and a second evaluation was carried out between 8 and 24 months after the first (mean: 14.1 months, SD: 5.5). The same clinical information (subjective impression of the evolution of their baseline neurological or psychiatric pathology comparing with first evaluation and comparing with pre-COVID-19 situation) was collected on this occasion from all 92 participants (60 patients with history of symptomatic COVID-19, 11 patients with history of asymptomatic COVID-19 and 21 uninfected controls), and serum was obtained from 87 of them. The main cause of patient drop-out between interviews was the fact that some patients live far away from Mexico City and were not able to assist to the second appointment.
Immunological evaluationSerum samplesA 10 mL venous blood sample drawn from participants was obtained in tubes with separation gel (BD Vacutainer). The samples were kept at room temperature and processed within 2 h. The samples were centrifuged at 800g for 10 min, sera were obtained, aliquoted, and frozen at −80 °C until their use.
Cytokine titration (ELISA)Commercial sandwich enzyme linked immunosorbent assay (ELISA) kits were employed to quantify, in the serum, the pro- and anti-inflammatory human cytokines IL-1β, IL-6, IL-10, IL-12 P40, IL-12 P70, IL-17A, INF-γ (all from BioLegend, San Diego, CA, USA), and adhesion molecules ICAM-1, VCAM-1 and E. Selectin (R&D Systems, bio-techne, USA), following the supplier's instructions. Briefly, sandwich ELISAs were performed in 96-well, flat-bottom microtiter plates (Nunc-Immuno Plate Maxisorp). The detection limits were 3.9 pg/mL for IL-1β, IL-10, and IL-17A, 7.8 pg/mL for IFN-γ, 15.6 pg/mL for IL-6 and IL-12 P70, 62.5 pg/mL for IL-12 P40, 93.8 pg/mL for E- selectin, 15.5 pg/mL for VCAM-1, and 31.3 pg/mL for ICAM-1. All analyses were run in duplicate.
Statistical analysisData were compiled in an Excel sheet and analyzed using SPSS and GraphPad prism software. As results exhibited a non-normal distribution, non-parametrical analysis was used. Chi-squared test was used to evaluate differences between percentage or proportion, Mann–Whitney test was used to evaluate differences between the medians of 2 groups, while Kruskall-Wallis test was used to evaluate differences between the medians of several groups. Wilcoxon matched pairs signed rank test was used to assess clinical and immunological changes between first and second visits.
ResultsClinical evolution of the patientsOf the 141 patients included in the initial clinical and inflammatory assessments, fifty-six (39.7%) felt that their baseline neurological/psychiatric condition had worsened compared with the pre-COVID-19 situation. Those were 43 patients with a history of symptomatic COVID-19 (53.7%), 6 patients with a history of asymptomatic COVID-19 (31.6%) and 7 uninfected controls (16.7%) (Supplementary Fig. 1). Worsening was significantly more frequent in patients with a history of symptomatic COVID-19 compared with controls and compared with the groups of controls + asymptomatic COVID-19 patients (p < 0.0001 for both comparisons).
From the 92 patients included in the second evaluation, worsening of neurological/psychiatric symptoms since the first evaluation was reported by 25 (27.2%) (Supplementary Fig. 1). These included 19 patients with a history of symptomatic COVID-19 (31.7%), one patient with a history of asymptomatic COVID-19 (9.0%) and five uninfected controls (23.8%). The differences in the proportion of worsened patient condition between the three groups were not statistically significant. When we assessed the worsening between before COVID-19 and the second evaluation, 34 (36.9%) patients reported affirmatively. These included 26 patients with a history of symptomatic COVID-19 (43.3%, i.e. the 19 patients who reported an aggravation at the second assessment (compared with the first), plus 7 patients who considered themselves worsened since the first assessment and in whom this aggravation persisted at the second assessment (stability of symptoms between the first and second), three patients with a history of asymptomatic COVID-19 (27.3%) and five uninfected controls (23.8%). Here too, although the proportion of worsened patients was higher in those having a history of symptomatic COVID-19, differences between groups were not statistically significant.
Inflammatory parameters evaluated at inclusion141 sera were evaluated corresponding to 80 patients with antecedents of symptomatic COVID-19, 19 with antecedents of asymptomatic COVID-19, and 42 controls. In the cases of symptomatic COVID-19 patients, the sample was taken in a mean of 6.7 months after the disease (SD: 4.2 months).
Comparison between symptomatic COVID-19, asymptomatic COVID-19 and control patientsTable 2 presents a resume of the differences in inflammatory parameters between the three groups of included patients, while complete results are stated in Supplementary table 1. IL-6 and IFN-γ concentrations were significantly higher in patients with history of COVID-19 compared with controls, considering either only symptomatic COVID-19 patients (p = 0.002 and p = 0.01, respectively) or only asymptomatic patients (p = 0.004 and p = 0.01, respectively). IL-10 concentration was higher in patients with a history of symptomatic COVID compared with controls (p = 0.01). On the other hand, IL-17A concentration was higher in controls compared with symptomatic and asymptomatic COVID-19 patients (p < 0.0001 for both comparisons), IL12P40 and IL12P70 concentrations were higher in asymptomatic vs. symptomatic COVID-19 patients (p = 0.03), and IL12P70 concentration was also higher in control compared with symptomatic COVID-19 patients (p = 0.001). Also, VCAM-1 concentration was higher in asymptomatic COVID-19, followed by controls and finally by symptomatic COVID-19. The differences between the 3 groups were all significant (COVID-19 symptomatic vs. controls, p < 0.001; COVID asymptomatic vs. controls, p = 0.005; COVID symptomatic vs. asymptomatic, p = 0.003). Absence of significant differences between groups was observed for IL-1β, ICAM-1 and E-selectin.
Resume of the differences of inflammatory markers between the 3 groups of patients in the first evaluation.
| Cytokine / marquers* | COVID symptomatic(n = 80) | COVID asymptomatic(n = 19) | Controls(n = 42) |
|---|---|---|---|
| IL-1 (pg/mL) | + | + | + |
| IL-6 (pg/mL) | ++ | ++ | + |
| IL-10 (pg/mL) | ++ | + /++ | + |
| IL-17A (pg/mL) | + | + | ++ |
| IL-12 P40 (pg/mL) | + | ++ | + / ++ |
| IL12-P70 (pg/mL) | + | ++ | ++ |
| IFN-g (pg/mL) | ++ | ++ | + |
| ICAM (ng/mL) | + | + | + |
| VCAM (ng/mL) | + | +++ | ++ |
| E-selectina (ng/mL) | + | + | + |
Same symbols (+, ++, or +++) in the boxes of a same line indicate that there are no significant differences in the marker between the groups of patients evaluated. Different symbols in boxes of a same line indicate that significant differences exist. The boxes where the number of crosses is higher indicates in which group a higher level of each marker was found.
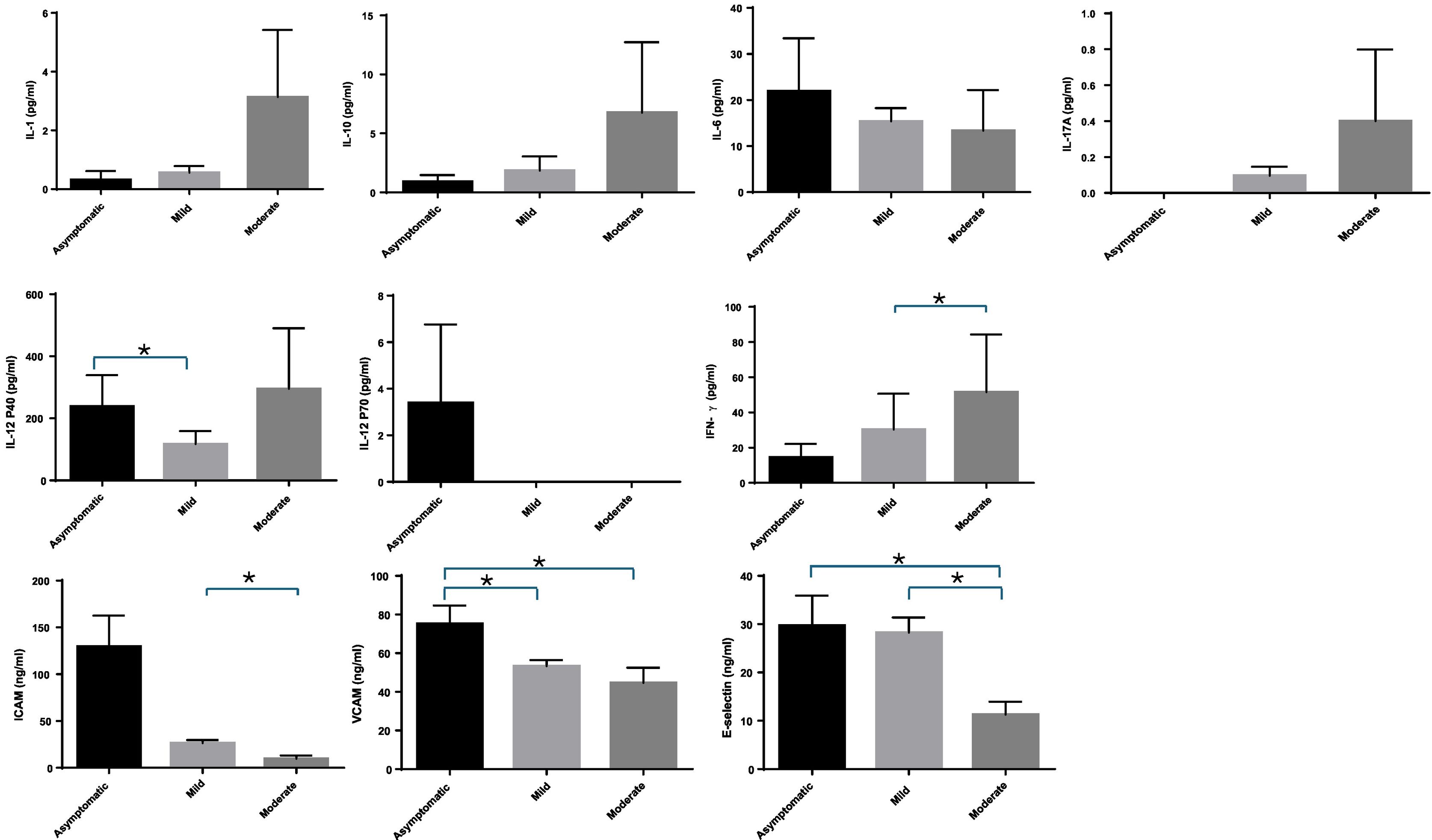
We evaluated differences in inflammatory markers regarding the severity of COVID-19 [asymptomatic (n = 19) vs. mild (n = 67) vs. moderate (n = 8) + severe (n = 3) + critical (n = 2), (total n = 13), [Fig. 2]. The very different numbers of patients between the groups meant that no further comparisons could be made. Concentrations of IL-1β, IL-10, IL-17A and IFN-γ were higher in more severe forms of the disease. But these tendances were only significant for IFN-γ, when comparing mild and more severe patients (p = 0.019). On the other hand, some markers showed a lower concentration with increased severity of the disease. This was the case of IL-6, IL-12P70, ICAM-1, VCAM-1 and E-selectin. The tendencies were statistically significant when comparing VCAM-1 and E-selectin between asymptomatic vs. moderate COVID-19 patients (p = 0.046 and p = 0.0026, respectively); ICAM-1 and E-selectin between mild and moderate COVID-19 patients (p = 0.012 and p = 0.009, respectively), and when comparing VCAM-1 between asymptomatic and mild COVID-19 patients (p = 0.003).
Comparison regarding type of neurological/psychiatric pathologies of included patientsThe pathologies of the included patients were epilepsy (n = 53), nerves & muscles diseases (n = 20), demyelinating diseases (n = 18), psychiatric pathologies (n = 17), neurodegenerative disease (n = 14), headache (n = 9), vascular (n = 7), and autoimmune encephalitis (n = 3). We found no significant differences in the markers assessed between the different pathologies (Supplementary table 2).
Association between Inflammatory parameters and evolution of neurological / psychiatric symptomsSupplementary Fig. 2 shows, when all the participants were included, the levels of inflammatory markers in patients who reported stable or improved neurological/psychiatric symptoms between the first sampling compared with the situation before COVID-19 (n = 85, 60.3%), and those who experienced worsening neurological/psychiatric symptoms over the same period (n = 56, 39.7%). Only one comparison was significant: the higher level of IL-10 in patients who fell worsening of their neurological symptoms compared with those presenting stable or improving symptoms (p = 0.016). When only the patients with COVID-19 antecedents were considered, no significant differences associated with clinical evolution appears, while when only controls were considered, IL-17A was significantly higher in worsened participants (data not shown, p = 0.041).
Evolution of cytokines between first and second samples regarding clinical status at the moment of second sampleResults comparing neurological/psychiatric symptoms between the second and first assessments are presented in Fig. 3.
Evolution of cytokines between first and second samples in patients with COVID-19 antecedents (Fig. 3A and B, n = 66) and without (control, Fig. 3C and D, n = 21). Worsening or stability/improvement of neurological/psychiatric symptoms is assessed by comparing the second vs. the first evaluation.
In patients with antecedents of COVID-19 (Fig. 3 A, B), worsening symptoms (Fig. 3-A) were associated with significant decrease of IL-6 and increase of ICAM, VCAM and E-selectin, while improving symptoms (Fig. 3-B) were associated with decrease of IFN-γ (p = 0.0012) and increase of IL-10 (p = 0.0016), ICAM, VCAM and E-selectin (p < 0.0001 for the 3 markers).
In controls, improving symptoms (Fig. 3-D) were associated with decrease of IL-17A (p = 0.0010) and increase of IL-10 (p = 0.0005), ICAM-1, VCAM-1 and E-selectin (p = 0.0001, p = 0.0026 and p = 0.041 respectively). No significant changes were associated with worsening symptoms, but the number of patients was low (n = 5, Fig. 3-C).
It is interesting to note that the same results were obtained when comparing symptoms before COVID-19 and the situation at the second sampling (Supplementary Fig. 3).
DiscussionCOVID-19 was a pandemic that evolved in different waves between early 2020 and mid-2022, profoundly affecting the wellbeing of the population. Firstly, due to its direct impact on health, secondly due to its indirect effects on health (deficient medical attention, impossibility to attend the normal therapies) and thirdly due to its social impact (decrease of social interactions, stress). In this context it was interesting to evaluate the impact of COVID-19 on neurological and psychiatric patients, with the objective to evaluate the evolution of their symptoms and decipher whether immunological factors were involved.
We find that affected COVID-19 patients present persistent significant immunological changes compared with controls. Indeed, although the first samples were taken in a mean of 6.7 months after the disease, significantly higher levels of two inflammatory cytokines (IL-6 and IFN-γ) persisted in patients with antecedents of asymptomatic or symptomatic COVID-19 compared with controls, while IL-10 increased in symptomatic COVID-19 patients compared with controls. The increase of IL-6 and IFN-γ show the activation of humoral and cellular immune mediators in COVID-19 patients. In the same form, IL-10 increase in symptomatic COVID-19 patients show a secondary regulatory immune response in individuals exposed to virus.
These results are in line with the few published studies evaluating the cytokine signature associated with COVID-19 several months after infection. Particularly, around two months after COVID-19 diagnosis it was found that IFN-γ and IL-6 levels in specifically stimulated whole blood supernatants were significantly higher in individuals with COVID-19 antecedents compared with non-infected individuals.10 In this same study IFN-γ, IL-6 and IL-10 were three of the eight biomarkers that stood out as the main inflammatory mediators with discriminative potential between the COVID-19 and healthy unexposed individuals.
It was also interesting to note that cytokines profiles were quite similar between symptomatic and asymptomatic individuals; indeed, only IL-12 P40, IL-12 P70 and VCAM-1 were significantly different between the two groups, being higher in the asymptomatic group. Very few studies have evaluated the immune response of asymptomatic individuals, but one demonstrated that asymptomatic individuals mount a virus-specific T cell response indistinguishable from symptomatic patients in magnitude, but functionally more fit with an increase of Th1, proinflammatory and anti-inflammatory cytokines.11 Our results probably reflect this observation.
In our first assessment, IFN-γ was the only cytokine whose increase was associated with the severity of COVID-19 (mild vs. moderate). IFN-γ in the acute phase of COVID-19 had been showed to be an independent risk factor associated with mortality,12 However, in one study its level did not vary between mild and moderate patients in plasma samples taken 8 to 12 days after symptoms onset.13 We were unable to find any studies evaluating IFN-γ longer after infection, as we did in our study.
At the first evaluation (around 6 months after infection), no association between evolution of neurological/psychiatric symptoms and cytokines levels was found in patients with COVID-19 antecedents. At the second evaluation (around 1 year after first evaluation), improvement of neurological and psychiatric symptoms in patients with a history of COVID-19 was associated with a decrease of IFN-γ and an increase of IL-10. Regarding controls, at the first evaluation IL-17A was associated with perception of worsening symptoms while at second evaluation improving symptoms were associated with decrease of IL-17 and increase of IL-10. It is interesting to note the differences between patients with and without a history of COVID-19 infection. Of particular note is the association of IL-17 with clinical worsening and its decrease with clinical improvement in controls. Indeed, many data have established a link between IL-17A and depression, which may have been present in these neurological patients without a history of COVID-19, due to the stressful period represented by the pandemic.14
Our study aimed to assess the immunological factors involved in the worsening of neurological/psychiatric patients during COVID-19 pandemic, and to explore if these factors were stress-related or directly linked to infection. In this context, our results are consistent with the relevance of IFN-γ in case of previous COVID-19 infection. Indeed, we showed that this cytokine was significantly higher in COVID-19 patients than in controls, that it was significantly associated with disease severity, and that its decrease during follow-up was associated with improvement of neurological/psychiatric symptoms in neurological patients with a history of COVID-19 but not in neurological control patients. Although IFN-γ was found to be associated with depression post COVID-1915 and with post-traumatic stress disorder,16 the fact that we found no relevant features regarding IFN-γ in controls patients (that were also affected by the stress of the pandemic) orient to a direct relation between COVID-19 infection and presence of IFN-γ. It is also interesting to note that IFN-γ was implicated in the presence of long COVID syndrome. Indeed, an increase of CD4+ and CD8+ T-cells secreting IFN-γ was found to be more present in patients affected compared with patients that did not develop such syndrome, and IFN-γ was describe as one of 4 biomarkers associated with the occurrence of long-COVID.17,18
IFN-γ is the type II IFN produced by NK cells and T lymphocytes and is important in all phases of the immune response.12,19 IFN-γ is essential for antiviral defense as it downregulate virus replication and activates cytokine production by T cells, augmenting the cytotoxic T lymphocyte killing activity.12,20 However, persistent high levels of IFN-γ worsens the systemic inflammation, increasing tissue injury and organ failure.12,21 Our results - higher levels in patients vs. controls, association with COVID-19 severity, decrease associated with improvement of neurological/psychiatric symptoms in patients but not in controls- are in accordance with these observations, favoring the hypothesis of the main role of COVID-19 vs. stress factors in the worsening of neurological symptoms in neurological/psychiatric patients during the COVID-19 pandemic.
ConclusionOur results show the association between IFN-γ and evolution of neurological symptoms in neurological/psychiatric patients after COVID-19 non-severe infection. The concentration of IFN-γ appears to be directly related to COVID-19 infection and not to stress-related factors. This result is of interest; Indeed, this could be an argument for promoting the early use of antiviral agents22 in the event of infection of neurological/psychiatric patients, in order to avoid aggravation of their symptoms.
Ethical approvalThe authors declare that they have followed their center's protocols on the publication of patient data and have obtained the corresponding permissions.
Funding informationThis work was funded by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT, DGAPA, UNAM), Project number: IN206921.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
We gratefully acknowledge the neurologists and psychiatrists at INNNMVS who assisted us in including patients in this study by referring their patients with a history of SARS-Cov-2 infection. We acknowledge Mario Contreras Fleury for English copyediting.