Triple-negative tumours are the most aggressive type of breast cancer. We aimed to analyse the main radiologic and histopathologic factors of these tumours to create a risk profile.
Materials and methodsWe analysed data from 140 patients diagnosed with triple-negative breast cancer between January 2007 and December 2016, with follow-up through April 2018. We analysed the following variables in the breast MRI done for staging: size, necrosis, associated findings, adenopathies, and perfusion and diffusion parameters. We analysed the following variables in histopathologic studies of biopsy specimens: histological type, Scarf-Bloom, Ki67, and p53 in the infiltrating component as well as in the in situ component. We analysed the following variables in histopathologic studies of positive lymph nodes and surgical specimens: size, lymphovascular/perineural invasion, and microglandular adenosis. We analysed the relation between the radiologic and histopathologic factors and recurrence and disease-free survival.
ResultsMRI tumour size ≥25mm, non-nodular enhancement, breast oedema, areola-nipple complex retraction, and lymph-node involvement were associated with recurrence and lower disease-free survival. Invasive lobular carcinoma, postsurgical size ≥20mm, and p53<15% were also associated with recurrence and lower disease-free survival. Histologically positive lymph nodes were associated with a greater percentage of recurrence and lymphovascular invasion and with lower disease-free survival. The multivariate analysis found that the variables MRI size ≥25mm, non-nodular enhancement, adenopathies on MRI, and p53 expression <15% were independent predictors of lower disease-free survival.
ConclusionsIn triple-negative breast tumours, factors associated with lower disease-free survival are non-nodular enhancement, size ≥25mm, and adenopathies on MRI, and p53 expression <15% on histopathologic study.
Los tumores triples negativos (TN) constituyen el subgrupo de cáncer de mama (CM) más agresivo. Nuestro objetivo es analizar los principales factores radiopatológicos de estos tumores para crear un perfil de riesgo.
Materiales y métodosEs un estudio constituido por 140 pacientes diagnosticadas de CM TN desde enero del 2007 a diciembre del 2016. Se analizaron los factores radiológicos en resonancia magnética (RM) de estadificación: tamaño, necrosis, hallazgos asociados, adenopatías, parámetros de perfusión y difusión. En biopsias diagnósticas se estudiaron características del componente infiltrante: tipo histológico, Scarf-Bloom, Ki67 y p53 y el componente in situ. Se analizaron las adenopatías histológicamente positivas y en las piezas quirúrgicas: tamaño, invasión linfovascular/perineural y adenosis microglandular. El seguimiento finalizó en abril de 2018. Se evaluó la relación de los factores radiopatológicos con la recidiva y la supervivencia libre de enfermedad (SLE).
ResultadosLos tumores con tamaño igual o superior a 25mm en RM, realce no nodular, edema mamario o retracción del complejo aréola-pezón y adenopatías presentaron más recidivas y menor SLE. Los carcinomas lobulillares infiltrantes, el tamaño posquirúrgico≥20mm y p53<15% también se asociaron con la recidiva y una menor SLE. Las adenopatías histológicamente positivas se relacionaron con un mayor porcentaje de recidivas, y la invasión linfovascular, con una menor SLE. El análisis multivariante ha definido que el tamaño en RM≥25mm, el realce no nodular, las adenopatías en RM, y la expresión menor del 15% de p53 son variables pronósticas independientes.
ConclusionesEl tamaño igual o superior a 25mm, realce no nodular y adenopatías en RM, y una expresión inferior al 15% de p53 condicionan una menor SLE.
Triple-negative (TN) tumours represent 11–20% of breast cancers (BC) and are characterised by the absence of hormonal receptors and of HER2.1 They are the subgroup with the highest risk of recurrence and mortality.2,3 On MRI, they present as unifocal tumours with internal necrosis and “ring” enhancement.4,5 Their aggressiveness and the absence of therapeutic targets has motivated the study of prognostic factors.6,7
The objectives are:
- 1.
To identify which radiological factors assessed in staging and pathological MRIs are associated with tumour recurrence and with a lower disease-free survival (DFS).
- 2.
To create a risk profile through a prognostic score to establish an individualised follow-up of these patients.
It is a retrospective observational study with data collection from January 2007 to December 2016. A total of 140 patients with TN BC diagnosed and treated in our centre were included. The inclusion criteria were: staging MRI, TN subtype by immunohistochemistry, treated or not with neoadjuvant chemotherapy (NCT). The exclusion criteria were: non-TN BC, MRI not assessable or not available. To conduct the study, the approval of the ethics committee was not necessary as it was an observational retrospective study.
The studies were performed on a 1.5T MRI device (GE Healthcare, Milwaukee, USA), with 8-channel surface antenna.8
Radiological factors assessed in staging MRIIn the sequence enhanced in T2, the intralesional signal (hyperintense, isointense or hypointense) was analysed. In the dynamic sequence we have evaluated the tumour size (major axis in millimetres), number of lesions (single, multifocal, multicentric or bilateral), presence of associated findings and tumour enhancement according to the BI-RADS lexicon of the 4th edition because most of the patients were diagnosed before the last edition.9 In tumours with nodular enhancement, morphology, margins and internal enhancement were analysed. And if the enhancement was not nodular, the distribution and internal enhancement was determined.
Suspected adenopathies on MRI were those with: diameter >1cm on the short axis, rounded morphology, loss of fatty hilum and eccentric cortical thickening.10–12
By post-processing the dynamic sequence, the maximum intralesional enhancement value was determined, the time to reach the maximum enhancement or TTP (time to peak) of the 1st to the 6th minute, and the functional curves (type 1 or progressive, type 2 or plateau and type 3 or washout).
By analysing the diffusion sequences, we calculated the intratumoural restriction percentages, placing an ROI (4mm2) in the tumour region of maximum restriction and another in normal fibroglandular tissue, to obtain diffusibility percentages, and the restriction percentage is calculated by subtracting the diffusibility percentages of each tissue.
Anatomical pathology factorsThe following characteristics of the infiltrating component were evaluated: histological type, Scarf-Bloom, CK5/6, ki67 and p53 and the presence of carcinoma in situ (CIS) and its grade (high/not high).
The presence of histologically confirmed adenopathies (in biopsy or post-surgical specimens), the size of the anatomopathological (AP) tumour, lymphovascular or perineural invasion and the presence of microglandular adenosis in the post-surgical specimens were studied.
The MRI studies and AP factors were analysed by two radiologists with more than ten years’ experience and an anatomopathologist with more than five years’ experience in the breast section.
Follow-upThe patients were followed-up until the date of death or the final date of follow-up (31/04/2018), and the number and type of recurrence was determined: local, lymph node, visceral, bone, cerebral and multiple.
The data collected were used to calculate the survival curves and disease-free time, defined as the period of time from the date of the MRI until recurrence, death or the last follow-up record.
Statistical analysisWe have analysed the correlation of radiological factors and AP with tumour recurrence and DFS using the programme IBM-SPSS®, Statistics 22.0.
The relationship between the different variables and tumour recurrence was analysed using the χ2 test for categorical variables and the Mann–Whitney U-medians or means comparison tests for quantitative variables.
The significant quantitative variables (tumour size on MRI and AP and p53 value) were dichotomised into two categories by the analysis of ROC curves for inclusion in the multivariate analysis. The comparative analysis was performed with the χ2 test.
Survival measurements were compared with the Kaplan–Meier log-rank test, and the probability of survival at five years using the actuarial method.
Multivariate analysis was performed using the Cox regression model. Using the command for SPSS, UAB All Sets Reg Cox, we selected the set of variables with greater descriptive and predictive power. The Cox regression formula was calculated and a score was assigned to each variable according to its multiplier coefficient B. The value 1 was applied to the lower number and to the rest, their proportional value. Survival curves and times were calculated for each year according to the score.
ResultsDescriptive analysis of radiopathological factorsThe sample consisted of 140 women, with a mean age at diagnosis of 55.5±13.1 years. In the MRI, TN were single lesions, with T2 hypersignal, nodular “ring” enhancement, TTP at 2min and type 2 functional curve.
Infiltrating ductal carcinoma was the most frequent histological type, with a Scarff-Bloom median of 7. CIS was present in 42.9%, the adenopathies were positive in 38.8% and 11% associated lymphovascular invasion.
Follow-upTumour recurrence was observed in 47 (34.7%) patients. The median follow-up in patients with and without recurrence was 18.5 and 82.3 months, respectively. Local recurrence was the most common (11.8%), followed by visceral (7.4%), multiple (8.8%), bone (3.7%), lymph node (1.5%) and cerebral (1.5%). The estimated mean DFS was 88.4 months and the probability of DFS at five and ten years was 66.7% and 54.2%, respectively.
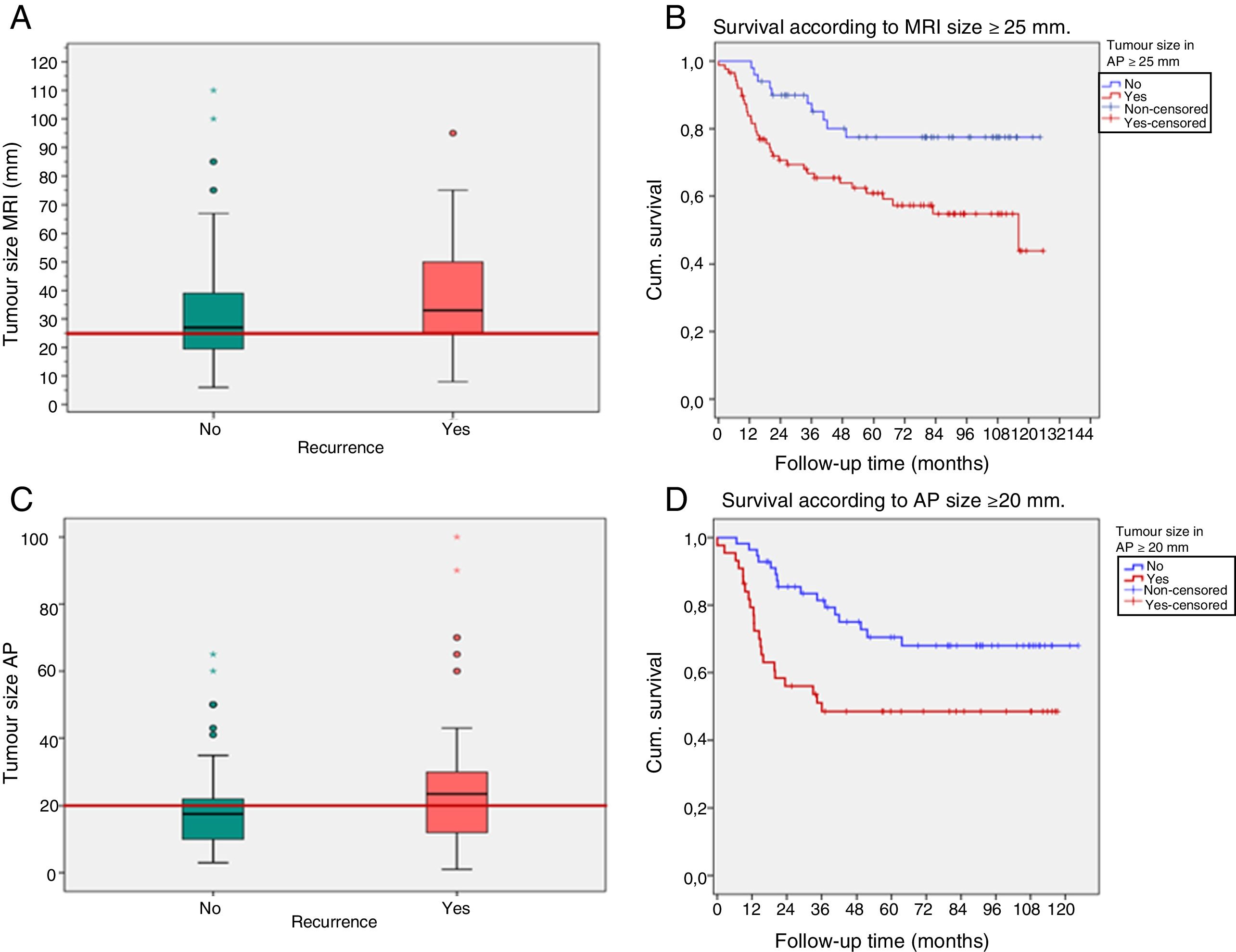
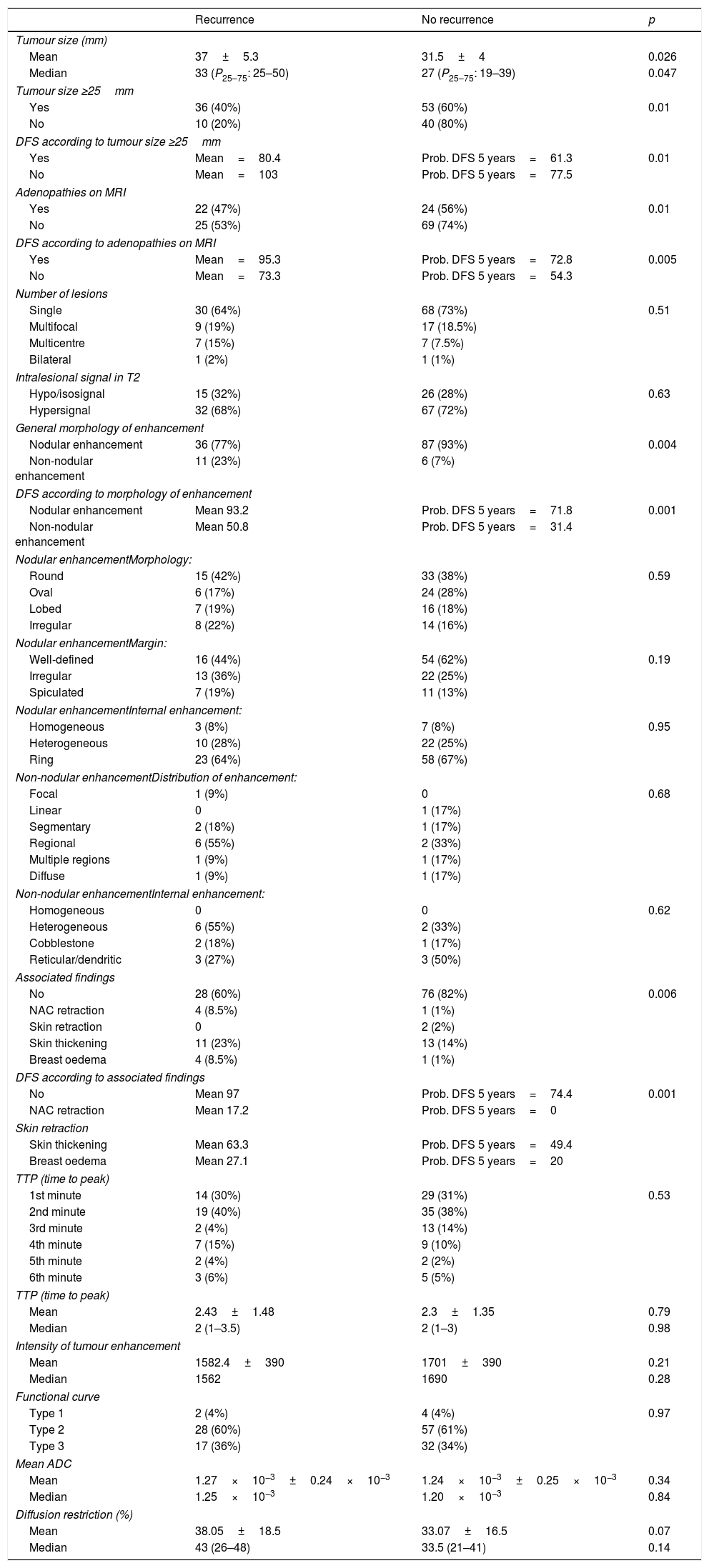
Prognostic correlation of radiopathological factorsThe tumour size on MRI ≥25mm and in AP ≥20mm (cutoff points of ROC curves) was associated with a higher percentage of recurrences (p=0.01 and p=0.03, respectively) and lower DFS (p=0.01 and p=0.007, respectively) (Fig. 1).
(A–D) Recurrence and survival according to tumour size on magnetic resonance imaging (MRI) and anatomopathology (AP). The box-and-whisker plots (A and C) represent recurrences according to tumour sizes (the red lines show the values of 25 and 20mm, respectively). Survival curves (B and D) according to tumour size ≥25mm on MRI (A and B) and ≥20mm in AP (C and D).
Cum. survival: cumulative survival.
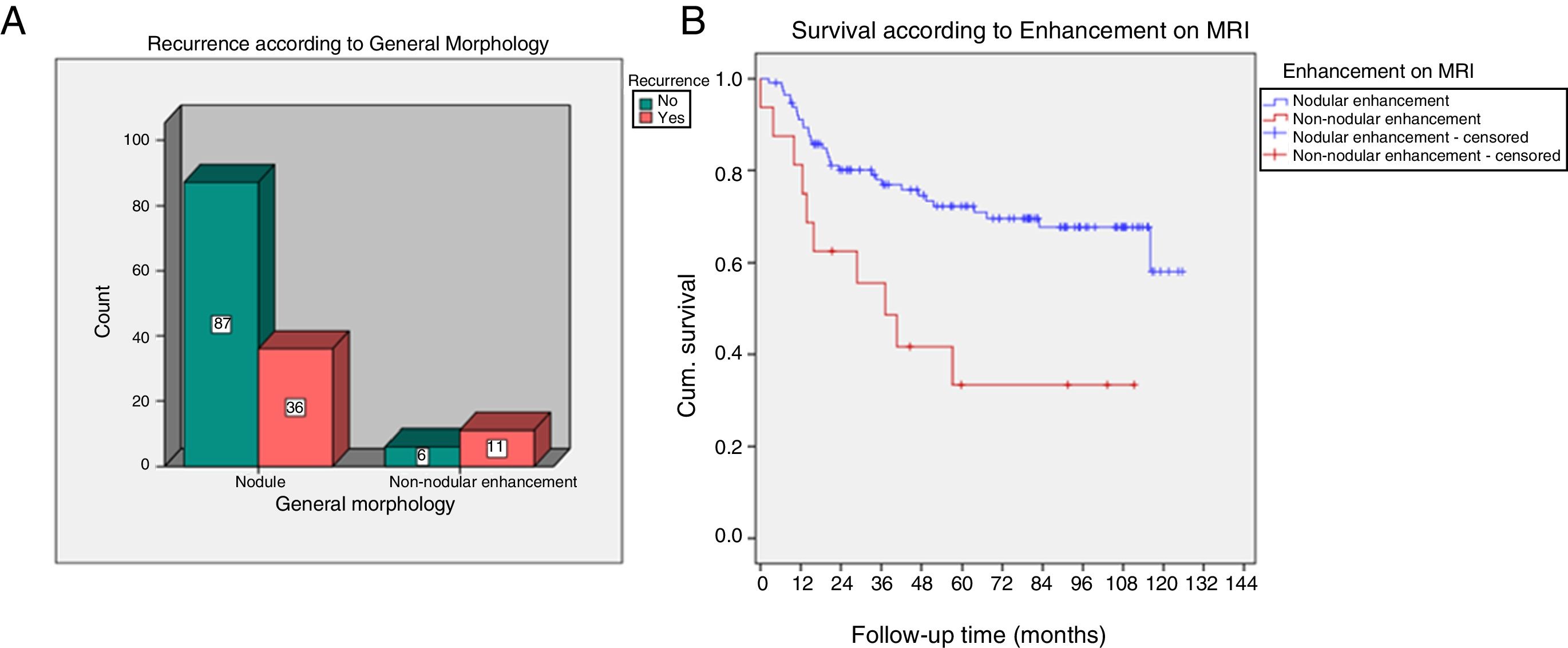
The non-nodular enhancement on MRI was associated significantly with recurrence (p=0.004) and with lower DFS (p=0.001) (Fig. 2).
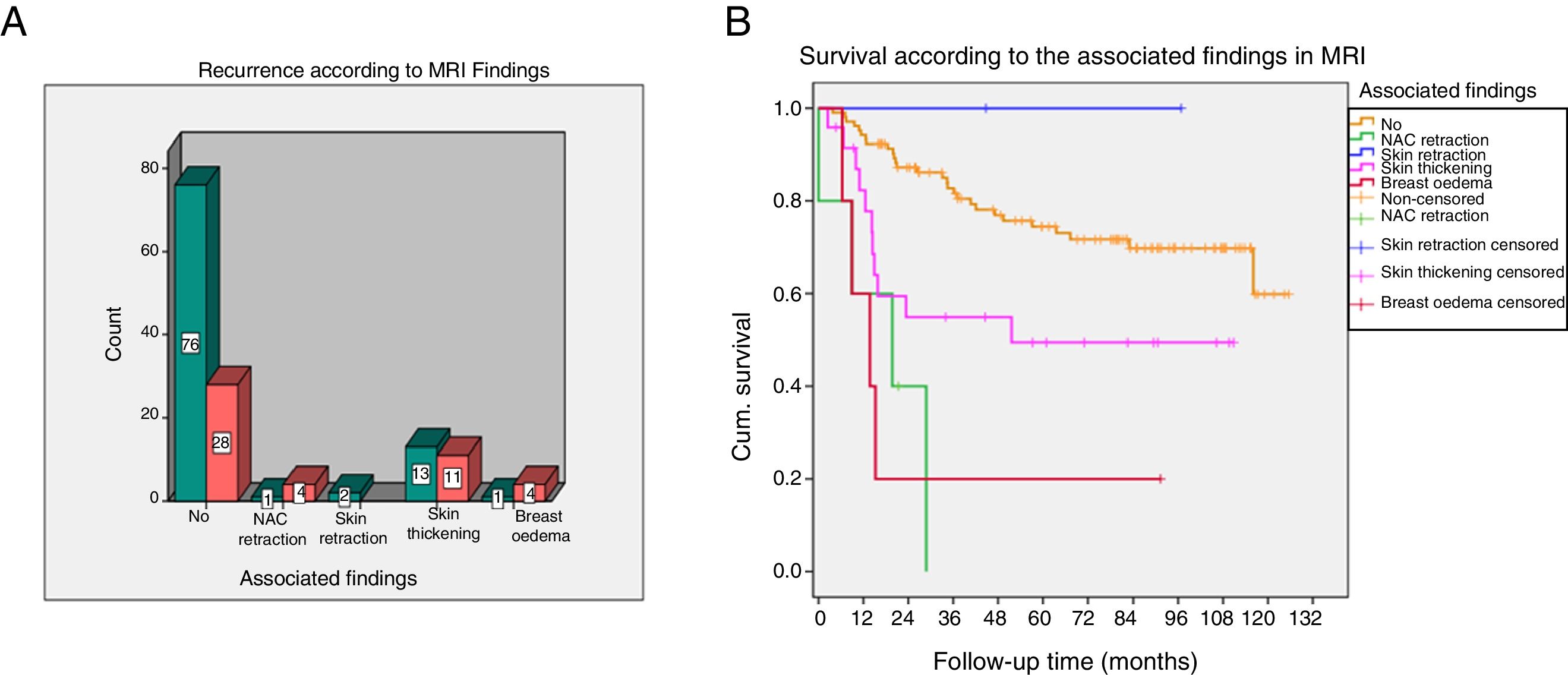
The nipple areola-complex (NAC) retraction and breast oedema associated more recurrences (p=0.006) and lower DFS (p<0.001) (Fig. 3).
(A and B) Recurrence and survival according to the associated findings on magnetic resonance imaging (MRI). The box-and-whisker plot (A) represents recurrences according to the presence of associated findings on MRI. Survival graph (B) of the associated findings.
Cum. survival: cumulative survival.
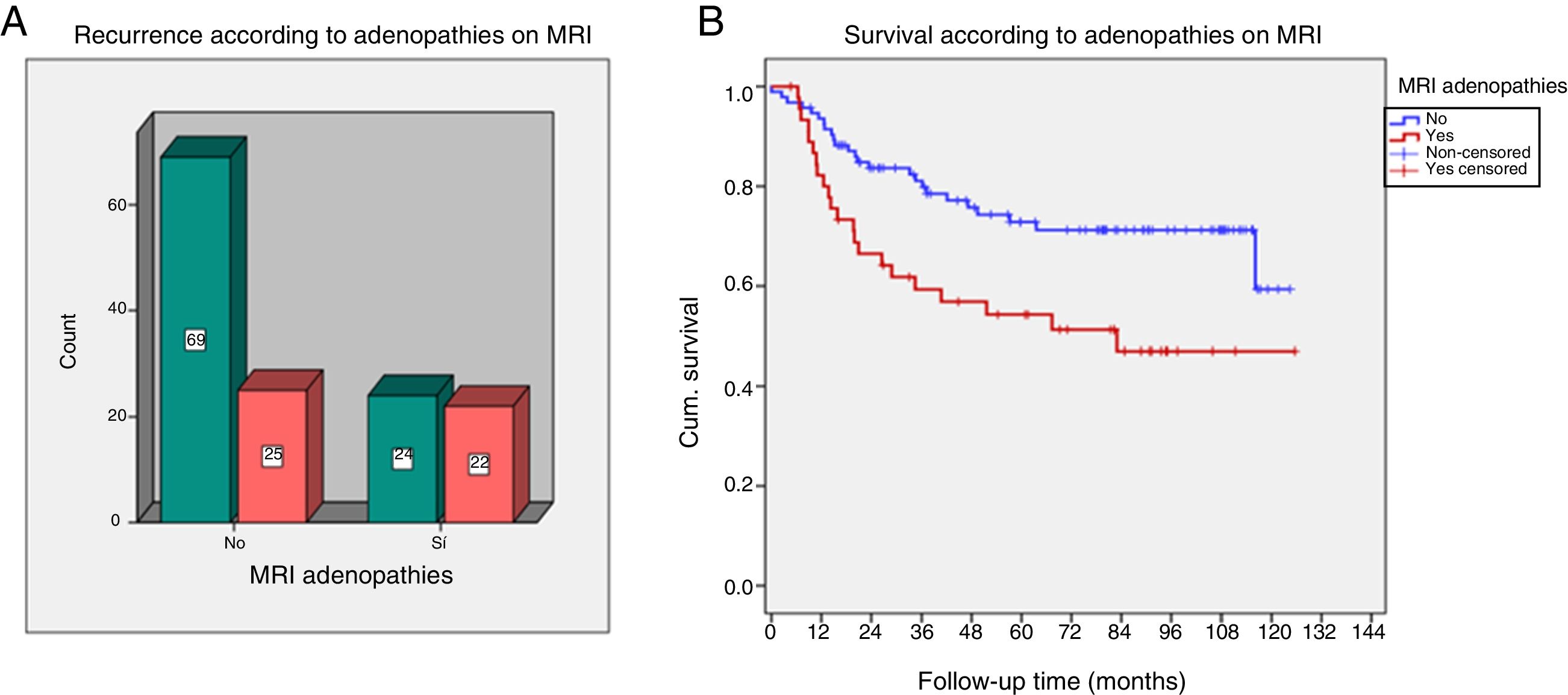
Patients with adenopathies on MRI and AP associated a higher proportion of recurrences (p=0.01 and p=0.03, respectively). A lower DFS was demonstrated in patients with adenopathies on MRI (p=0.005) (Fig. 4).
(A and B) Recurrence and survival in the presence of suspicious adenopathies on magnetic resonance imaging (MRI). The box-and-whisker plot (A) represents recurrences according to the presence of adenopathies on MRI. Survival chart (B) according to the presence of adenopathies.
Cum. survival: cumulative survival.
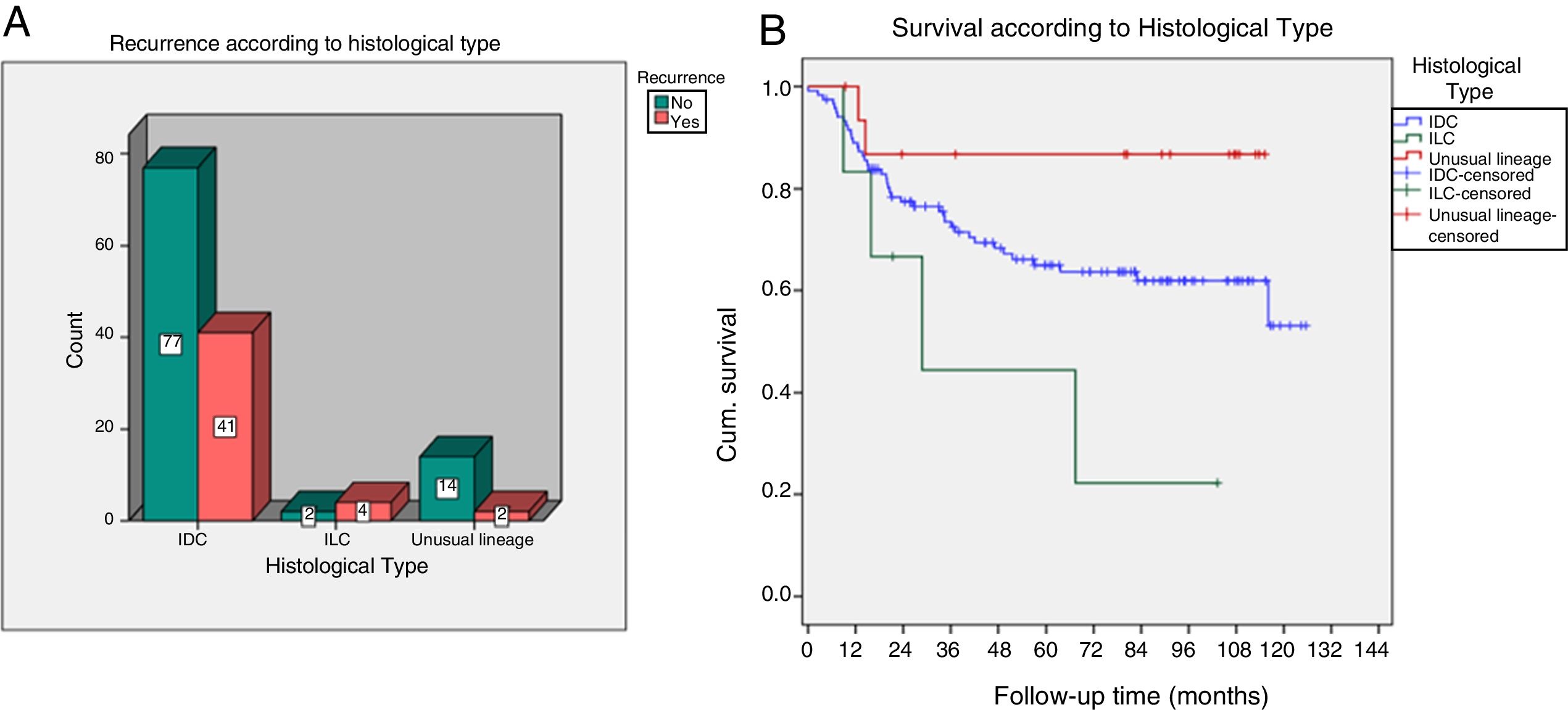
Infiltrating lobular carcinoma had a higher proportion of recurrences (p=0.04) and lower DFS (p=0.048), with a five-year survival probability of 44.4%, compared to 65% in ductals and 86.7% in unusual lineage (Fig. 5).
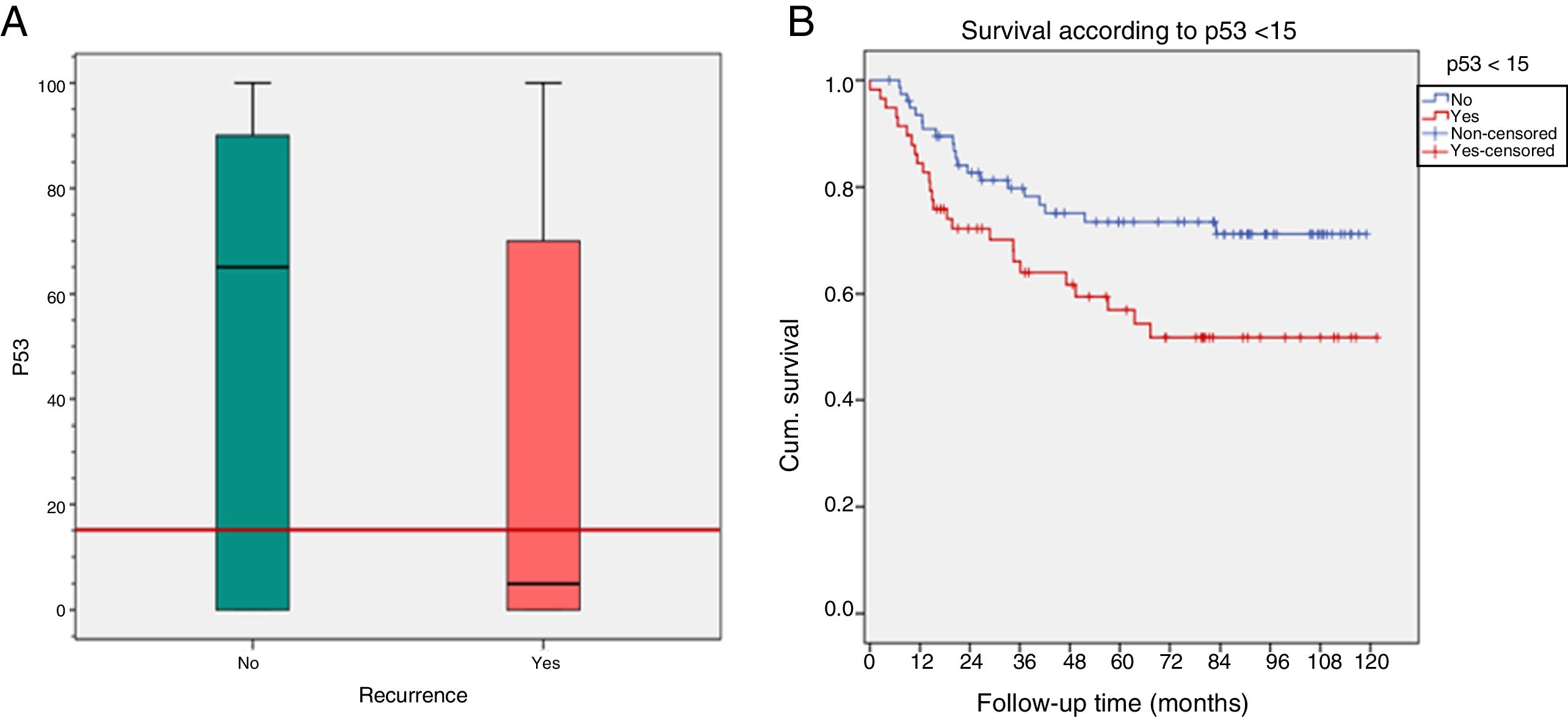
The expression of p53 <15% (cutoff point of ROC curves) was related to recurrence (p=0.03) and with a lower DFS (p=0.024) (Fig. 6).
A lower DFS (p=0.028) was shown in patients with lymphovascular invasion (probability of DFS at five years of 40.7%) compared to those who did not present invasion (69.8%).
No prognostic differences were found in the rest of the radiopathological factors analysed.
The radiopathological results are shown in Tables 1 and 2.
Results of factors assessed in staging MRI.
| Recurrence | No recurrence | p | |
|---|---|---|---|
| Tumour size (mm) | |||
| Mean | 37±5.3 | 31.5±4 | 0.026 |
| Median | 33 (P25–75: 25–50) | 27 (P25–75: 19–39) | 0.047 |
| Tumour size ≥25mm | |||
| Yes | 36 (40%) | 53 (60%) | 0.01 |
| No | 10 (20%) | 40 (80%) | |
| DFS according to tumour size ≥25mm | |||
| Yes | Mean=80.4 | Prob. DFS 5 years=61.3 | 0.01 |
| No | Mean=103 | Prob. DFS 5 years=77.5 | |
| Adenopathies on MRI | |||
| Yes | 22 (47%) | 24 (56%) | 0.01 |
| No | 25 (53%) | 69 (74%) | |
| DFS according to adenopathies on MRI | |||
| Yes | Mean=95.3 | Prob. DFS 5 years=72.8 | 0.005 |
| No | Mean=73.3 | Prob. DFS 5 years=54.3 | |
| Number of lesions | |||
| Single | 30 (64%) | 68 (73%) | 0.51 |
| Multifocal | 9 (19%) | 17 (18.5%) | |
| Multicentre | 7 (15%) | 7 (7.5%) | |
| Bilateral | 1 (2%) | 1 (1%) | |
| Intralesional signal in T2 | |||
| Hypo/isosignal | 15 (32%) | 26 (28%) | 0.63 |
| Hypersignal | 32 (68%) | 67 (72%) | |
| General morphology of enhancement | |||
| Nodular enhancement | 36 (77%) | 87 (93%) | 0.004 |
| Non-nodular enhancement | 11 (23%) | 6 (7%) | |
| DFS according to morphology of enhancement | |||
| Nodular enhancement | Mean 93.2 | Prob. DFS 5 years=71.8 | 0.001 |
| Non-nodular enhancement | Mean 50.8 | Prob. DFS 5 years=31.4 | |
| Nodular enhancementMorphology: | |||
| Round | 15 (42%) | 33 (38%) | 0.59 |
| Oval | 6 (17%) | 24 (28%) | |
| Lobed | 7 (19%) | 16 (18%) | |
| Irregular | 8 (22%) | 14 (16%) | |
| Nodular enhancementMargin: | |||
| Well-defined | 16 (44%) | 54 (62%) | 0.19 |
| Irregular | 13 (36%) | 22 (25%) | |
| Spiculated | 7 (19%) | 11 (13%) | |
| Nodular enhancementInternal enhancement: | |||
| Homogeneous | 3 (8%) | 7 (8%) | 0.95 |
| Heterogeneous | 10 (28%) | 22 (25%) | |
| Ring | 23 (64%) | 58 (67%) | |
| Non-nodular enhancementDistribution of enhancement: | |||
| Focal | 1 (9%) | 0 | 0.68 |
| Linear | 0 | 1 (17%) | |
| Segmentary | 2 (18%) | 1 (17%) | |
| Regional | 6 (55%) | 2 (33%) | |
| Multiple regions | 1 (9%) | 1 (17%) | |
| Diffuse | 1 (9%) | 1 (17%) | |
| Non-nodular enhancementInternal enhancement: | |||
| Homogeneous | 0 | 0 | 0.62 |
| Heterogeneous | 6 (55%) | 2 (33%) | |
| Cobblestone | 2 (18%) | 1 (17%) | |
| Reticular/dendritic | 3 (27%) | 3 (50%) | |
| Associated findings | |||
| No | 28 (60%) | 76 (82%) | 0.006 |
| NAC retraction | 4 (8.5%) | 1 (1%) | |
| Skin retraction | 0 | 2 (2%) | |
| Skin thickening | 11 (23%) | 13 (14%) | |
| Breast oedema | 4 (8.5%) | 1 (1%) | |
| DFS according to associated findings | |||
| No | Mean 97 | Prob. DFS 5 years=74.4 | 0.001 |
| NAC retraction | Mean 17.2 | Prob. DFS 5 years=0 | |
| Skin retraction | |||
| Skin thickening | Mean 63.3 | Prob. DFS 5 years=49.4 | |
| Breast oedema | Mean 27.1 | Prob. DFS 5 years=20 | |
| TTP (time to peak) | |||
| 1st minute | 14 (30%) | 29 (31%) | 0.53 |
| 2nd minute | 19 (40%) | 35 (38%) | |
| 3rd minute | 2 (4%) | 13 (14%) | |
| 4th minute | 7 (15%) | 9 (10%) | |
| 5th minute | 2 (4%) | 2 (2%) | |
| 6th minute | 3 (6%) | 5 (5%) | |
| TTP (time to peak) | |||
| Mean | 2.43±1.48 | 2.3±1.35 | 0.79 |
| Median | 2 (1–3.5) | 2 (1–3) | 0.98 |
| Intensity of tumour enhancement | |||
| Mean | 1582.4±390 | 1701±390 | 0.21 |
| Median | 1562 | 1690 | 0.28 |
| Functional curve | |||
| Type 1 | 2 (4%) | 4 (4%) | 0.97 |
| Type 2 | 28 (60%) | 57 (61%) | |
| Type 3 | 17 (36%) | 32 (34%) | |
| Mean ADC | |||
| Mean | 1.27×10−3±0.24×10−3 | 1.24×10−3±0.25×10−3 | 0.34 |
| Median | 1.25×10−3 | 1.20×10−3 | 0.84 |
| Diffusion restriction (%) | |||
| Mean | 38.05±18.5 | 33.07±16.5 | 0.07 |
| Median | 43 (26–48) | 33.5 (21–41) | 0.14 |
NAC: nipple-areola complex; P: statistical significance; Prob. DFS 5 years: probability of disease-free survival.
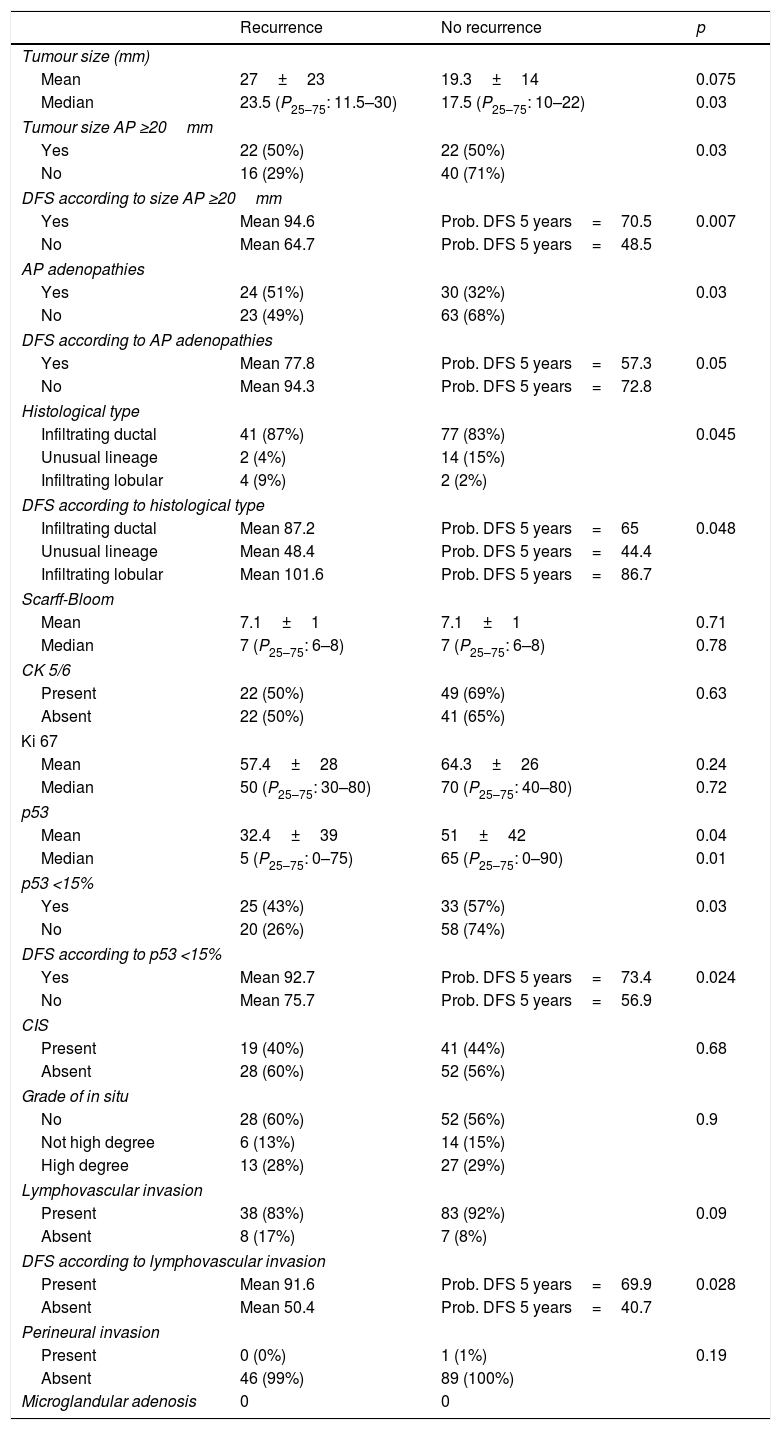
Results of the anatomopathological factors.
| Recurrence | No recurrence | p | |
|---|---|---|---|
| Tumour size (mm) | |||
| Mean | 27±23 | 19.3±14 | 0.075 |
| Median | 23.5 (P25–75: 11.5–30) | 17.5 (P25–75: 10–22) | 0.03 |
| Tumour size AP ≥20mm | |||
| Yes | 22 (50%) | 22 (50%) | 0.03 |
| No | 16 (29%) | 40 (71%) | |
| DFS according to size AP ≥20mm | |||
| Yes | Mean 94.6 | Prob. DFS 5 years=70.5 | 0.007 |
| No | Mean 64.7 | Prob. DFS 5 years=48.5 | |
| AP adenopathies | |||
| Yes | 24 (51%) | 30 (32%) | 0.03 |
| No | 23 (49%) | 63 (68%) | |
| DFS according to AP adenopathies | |||
| Yes | Mean 77.8 | Prob. DFS 5 years=57.3 | 0.05 |
| No | Mean 94.3 | Prob. DFS 5 years=72.8 | |
| Histological type | |||
| Infiltrating ductal | 41 (87%) | 77 (83%) | 0.045 |
| Unusual lineage | 2 (4%) | 14 (15%) | |
| Infiltrating lobular | 4 (9%) | 2 (2%) | |
| DFS according to histological type | |||
| Infiltrating ductal | Mean 87.2 | Prob. DFS 5 years=65 | 0.048 |
| Unusual lineage | Mean 48.4 | Prob. DFS 5 years=44.4 | |
| Infiltrating lobular | Mean 101.6 | Prob. DFS 5 years=86.7 | |
| Scarff-Bloom | |||
| Mean | 7.1±1 | 7.1±1 | 0.71 |
| Median | 7 (P25–75: 6–8) | 7 (P25–75: 6–8) | 0.78 |
| CK 5/6 | |||
| Present | 22 (50%) | 49 (69%) | 0.63 |
| Absent | 22 (50%) | 41 (65%) | |
| Ki 67 | |||
| Mean | 57.4±28 | 64.3±26 | 0.24 |
| Median | 50 (P25–75: 30–80) | 70 (P25–75: 40–80) | 0.72 |
| p53 | |||
| Mean | 32.4±39 | 51±42 | 0.04 |
| Median | 5 (P25–75: 0–75) | 65 (P25–75: 0–90) | 0.01 |
| p53 <15% | |||
| Yes | 25 (43%) | 33 (57%) | 0.03 |
| No | 20 (26%) | 58 (74%) | |
| DFS according to p53 <15% | |||
| Yes | Mean 92.7 | Prob. DFS 5 years=73.4 | 0.024 |
| No | Mean 75.7 | Prob. DFS 5 years=56.9 | |
| CIS | |||
| Present | 19 (40%) | 41 (44%) | 0.68 |
| Absent | 28 (60%) | 52 (56%) | |
| Grade of in situ | |||
| No | 28 (60%) | 52 (56%) | 0.9 |
| Not high degree | 6 (13%) | 14 (15%) | |
| High degree | 13 (28%) | 27 (29%) | |
| Lymphovascular invasion | |||
| Present | 38 (83%) | 83 (92%) | 0.09 |
| Absent | 8 (17%) | 7 (8%) | |
| DFS according to lymphovascular invasion | |||
| Present | Mean 91.6 | Prob. DFS 5 years=69.9 | 0.028 |
| Absent | Mean 50.4 | Prob. DFS 5 years=40.7 | |
| Perineural invasion | |||
| Present | 0 (0%) | 1 (1%) | 0.19 |
| Absent | 46 (99%) | 89 (100%) | |
| Microglandular adenosis | 0 | 0 | |
AP: anatomopathological; CIS: carcinoma in situ; Prob. DFS 5 years: probability of DFS at 5 years.
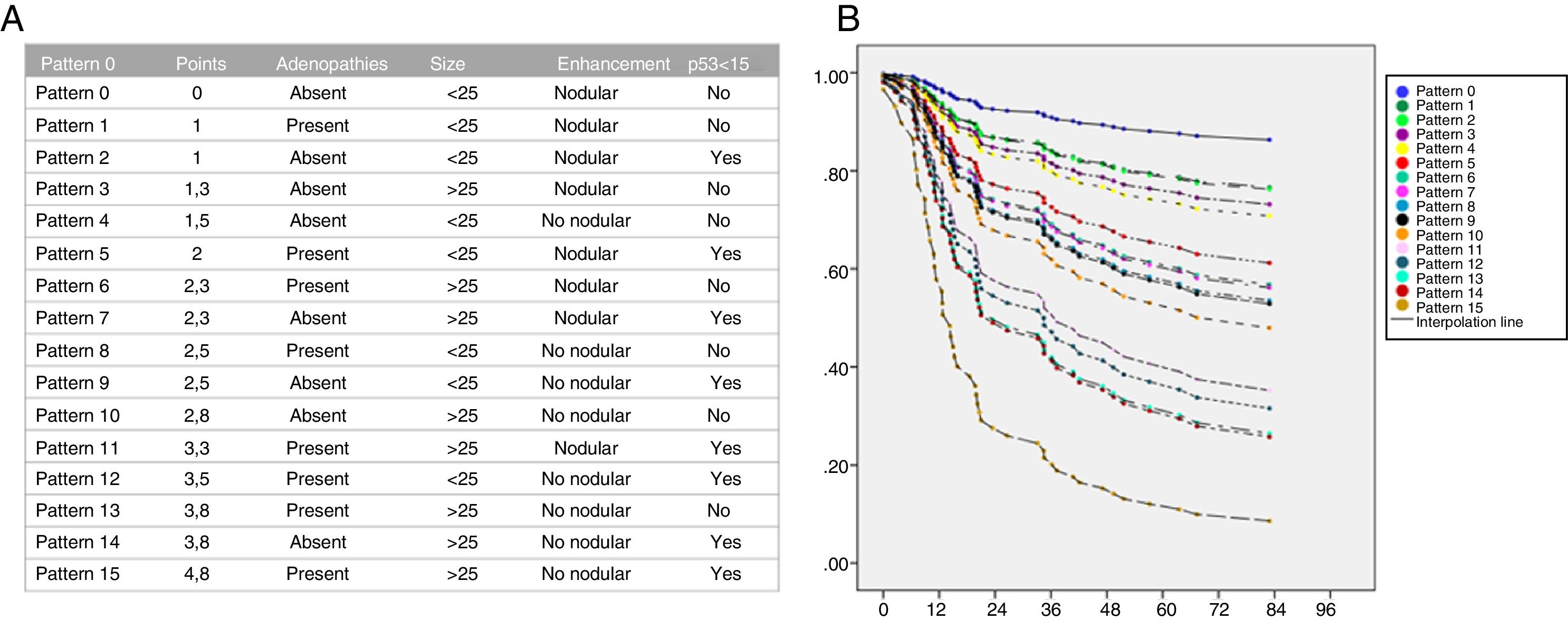
Regarding the Cox regression analysis, we obtained the following odds ratio: adenopathies on MRI=1.8; tumour size ≥25mm=2.1; non-nodular enhancement=2.3 and p53<15%=1.8.
The regression formula was:
And the following scores were defined:
- •
Adenopathies on MRI=1.
- •
p53 <15%=1.
- •
Size ≥25mm=1.3.
- •
Non-nodular enhancement=1.4.
Annual risk patterns and curves were calculated for each combination of predictive factors according to their score.
Patients without risk factors for recurrence (score 0) would have a five-year probability of DFS of 88%, while those who had a 4 (score 4), of 12% (Fig. 7).
DiscussionTumour size has been considered an independent prognostic factor in TN tumours.13–15 In our work, we established a cut-off point for tumour size on MRI (>25mm) and AP (≥20mm), from which a higher recurrence rate and a lower DFS were observed, a finding not previously defined in the scientific literature.
Non-nodular enhancement was associated with a worse prognosis, but no relationship was established with internal distribution or enhancement. There are no authors who conclude that non-nodular enhancement conditions a worse prognosis; however, it has been described that it could be related to the presence of CIS, which on MRI usually shows a non-nodular enhancement.16–18
Breast oedema and NAC retraction associated a higher recurrence rate and a lower DFS, findings related to lymphovascular invasion14,19 and with large tumours, near the nipple and multifocal.20
Tumour adenopathies have been considered the most important independent prognostic factor of BC. There are authors who do not conclude prognostic correlation and others have demonstrated a lower DFS in tumours with N2 involvement compared to N1 or N0.21 We agree that the presence of adenopathies associates a worse prognosis.
The histological subtype could also be a prognostic factor.22–26 In our study, all patients with infiltrating lobular carcinoma were treated with NCT with a higher percentage of recurrences and a lower DFS. This result agrees with other authors who claim that lobular carcinomas associate a lower response to chemotherapy, which would condition lower rates of complete pathological response and a worse prognosis.
Overexpression of p53 protein is more prevalent in TN tumours; in contrast, its prognostic association is much debated.27–30 Overexpression of p53 has been considered to result from mutations in the gene TP53. However, the correlation between mutations of the gene and its protein expression is inconclusive.31–33 We have established a cut-off point of p53 (15%) and the expression below 15% associated a worse prognosis.
Lymphovascular invasion has been considered an independent risk factor for tumour recurrence.34 We agree with other authors that lymphovascular invasion is associated with a lower DFS.
Microglandular adenosis is a rare proliferative lesion that could be a precursor to TN tumours.35 However, we did not identify it in any of the surgical specimens; therefore, if it were a precursor, its presence would not imply a worse prognosis.
LimitationsOur prognostic score has been obtained retrospectively in a heterogeneous sample, without categorisation according to the treatment received, and must be validated by a prospective study with patient homogenisation.
Radiopathological response, considered one of the most relevant prognostic factors, has not been assessed36,37 as there was not a sufficient number of patients treated with NCT.
ConclusionsWe have defined the independent radiopathological factors of lower DFS and created a recurrence risk profile that could condition an individualised follow-up of patients with a high risk score. However, these results must be validated by a prospective analysis with a more homogeneous sample.
As radiologists, we must consider the inclusion of MRI in the follow-up of patients with TN BC who have been treated with conservative surgery, who present a high-risk score and associate a dense breast pattern. The use of MRI is justified by the greater sensitivity of this imaging technique for the detection of tumour recurrence.
Authorship- 1.
Responsible for the integrity of the study: CSS.
- 2.
Drafting of the article: CSS, CGM and SCC.
- 3.
Design of the article: CSS, CGM and ISA.
- 4.
Data collection: CSS, CGM, SCC and DRC.
- 5.
Data analysis and interpretation: CSS, CGM, SCC, BGB and ISA.
- 6.
Statistical processing: BGB.
- 7.
Literature search: CSS, CGM, SCC, DRC and ISA.
- 8.
Drafting of the article: CSS, CGM, SCC and BGB.
- 9.
Critical review of the manuscript with intellectually relevant contributions: CSS, CGM, SCC, DRC, ISA and BGB.
- 10.
Approval of the final version: CSS, CGM, SCC, DRC, ISA and BGB.
The authors declare that they have no conflicts of interest.
Please cite this article as: Sebastián Sebastián C, García Mur C, Gros Bañeres B, Cruz Ciria S, Rosero Cuesta DS, Suñén Amador I. Análisis de los factores radiopatológicos del cáncer de mama triple negativo y determinación de perfiles de riesgo. Radiología. 2020;62:365–375.