MRI is an imaging technique that is best suited for evaluating the response to neoadjuvant chemotherapy for locally advanced breast cancer. We aimed to (a) quantify the response in the molecular subtypes, (b) describe the morphological and dynamic variation, and (c) determine whether the molecular phenotype changes after chemotherapy.
Material and methodsThis is a retrospective study of 75 carcinomas in 69 patients who underwent MRI both before and after neoadjuvant chemotherapy. The response to treatment was classified as (a) complete response, (b) major partial response, (c) minor partial response, or (d) no response. We quantified the response in each molecular subtype (luminal A, luminal B, Herb 2+, and triple negative). The morphological changes were classified as (a) concentric reduction, (b) fragmentation, (c) ductal enhancement, or (d) necrosis. The dynamic changes affected the maximum intensity peak and the post-initial enhancement.
ResultsIn the 4 molecular subtypes, 4 types of response were seen. The response was good in 84.6% of the triple negative subtype, in 76.9% of the luminal B subtype, in 75.6% of the luminal A subtype, and in 69.2% of the Herb 2+ subtype. The predominant morphological change was concentric reduction (75%). The predominant dynamic change was a decrease in the maximum intensity peak (<100% in 64.2%), and type I or II curves were seen in 85.7%.
ConclusionsThe triple negative subtype responded best to neoadjuvant chemotherapy. The most common changes were concentric reduction and a decrease in the maximum intensity peak, with fewer washout curves. We observed a change in the molecular phenotype between the specimen at diagnosis and the final study in 12.2% of cases.
La RM es la técnica de imagen que mejor valora la respuesta a la quimioterapia neoadyuvante en el cáncer de mama localmente avanzado. Los objetivos son: primero, cuantificar la respuesta en los subtipos moleculares; segundo, describir las variaciones morfológicas y dinámicas, y tercero, valorar si el fenotipo molecular se modifica tras la quimioterapia.
Material y métodosEstudio retrospectivo de 75 carcinomas, en 69 pacientes con quimioterapia neoadyuvante. Se realizó RM pre y post-tratamiento. La respuesta se clasificó en: respuesta completa; respuesta parcial mayor; respuesta parcial menor y sin respuesta, cuantificando cada una en cada subtipo molecular (luminal A, luminal B, Herb 2+ y triple negativo). Los cambios morfológicos fueron: reducción concéntrica, fragmentación, realce ductal y necrosis. Los cambios dinámicos afectaron al pico de intensidad máxima y al realce post-inicial.
ResultadosEn los 4 subtipos moleculares se observaron los 4 tipos de respuesta. El triple negativo tuvo 84,6% de buenas respuestas, seguido del luminal B (76,9%), luminal A (75,6%), y Herb 2+ (69,2%). El cambio morfológico que predominó fue la reducción concéntrica en el 75% y el dinámico fue la disminución del pico de intensidad máxima, <100%, en el 64,2%, con predominio de curvas tipo I y II en el 85,7%.
ConclusionesEl subtipo triple negativo es el que mejor respondió a la quimioterapia neoadyuvante. Los cambios más frecuentes fueron la reducción concéntrica y la disminución del pico de intensidad máxima con menos curvas de lavado. El cambio del fenotipo molecular fue del 12,2%, entre la muestra al diagnóstico y el estudio final.
The introduction, at the end of the nineties, of neoadjuvant chemotherapy (NC) as an initial treatment of locally advanced breast cancer (LABC) has provided the following important advantages: (a) assessment of tumor chemosensitivity; (b) reduction in tumor size, improving the surgical outcome, and (c) early treatment of micrometastases.
The major disadvantage is, in case of poor response, the delay in surgery.
Compared with conventional techniques, magnetic resonance (MR) imaging has proven to be the most accurate technique1–3 to evaluate the response to NC.
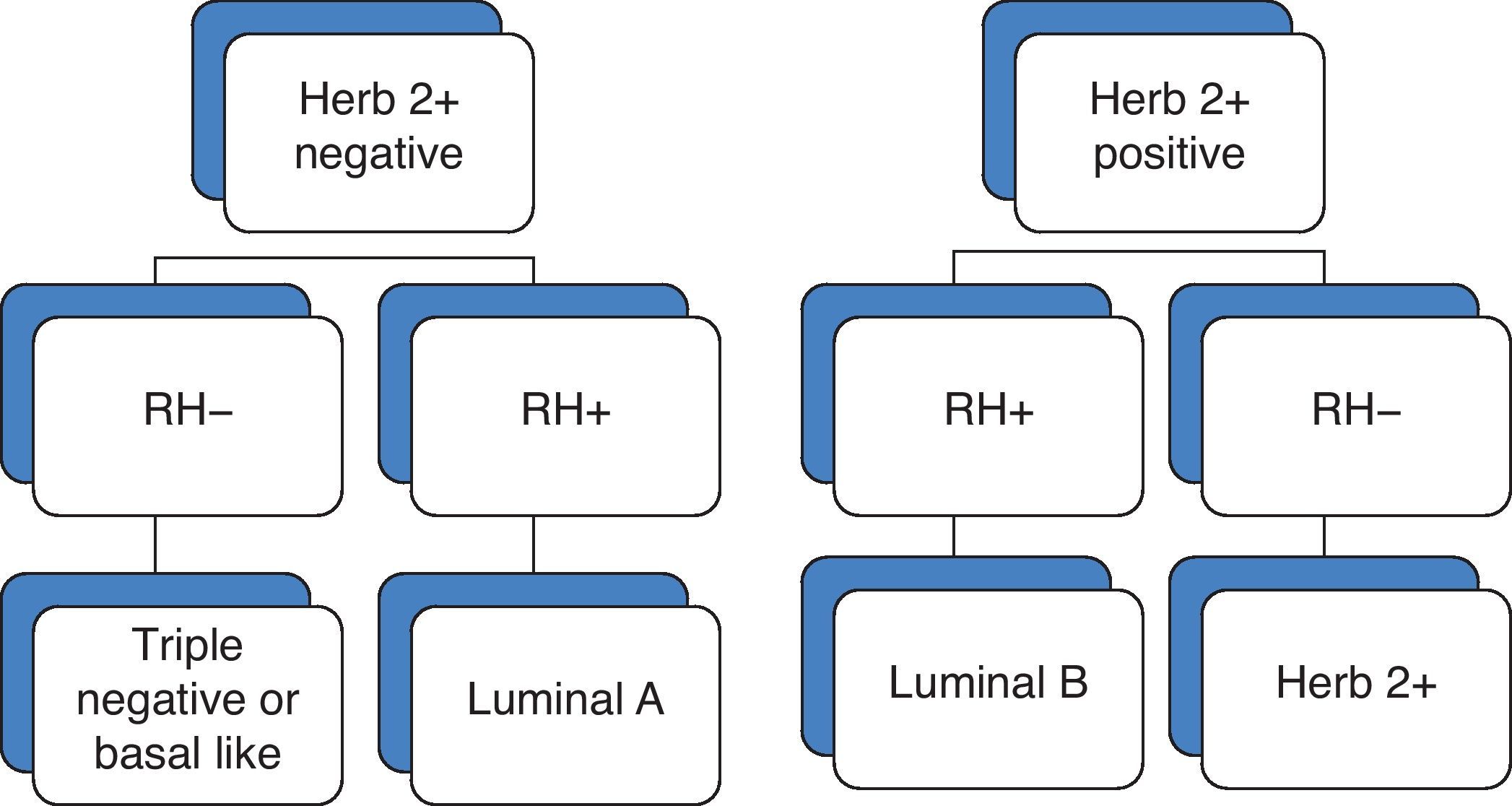
NC is given on the basis of the immunohistochemical analysis of estrogen receptor (ER), progesterone receptor (PR) and epidermal growth factor receptor 2 (Herb 2+). Recent studies suggest that breast cancer is a group of distinct neoplasms4,5 that can be classified according to the molecular phenotype as luminal A, luminal B, basal-like or triple negative, and Herb 2+ subtypes (Fig. 1).
We aim to quantify the response to NC in the molecular subtypes, describe the morphological and dynamic variations undergone by the tumor after NC, and quantify changes in the molecular phenotype in those patients with immunohistochemical studies available before and after NC.
Material and methodsDescriptive retrospective study was conducted between 2003 and 2009, of 75 carcinomas in 69 patients (68 females and 1 male) treated with NC. The age range was 31–74 years (mean 53.4 years), 44% of patients were <50 years and 2 had pregnancy associated breast cancer detected during the first two months of lactation.
All patients underwent an MRI examination before and after the NC.
From 2003 to 2006, the MRI examinations were performed with a GE Signa scanner using FSE T1 (375/14) sequences, T2 (2975/85) sequences with fat saturation, and one axial dynamic 3D sequence, with 6 phases of 90s (5 with contrast and 1 without). From 2006 to 2009, all MRI examinations were performed with a GE Excite scanner using FSE T1 (600/10) sequences, sagittal T2 (4000/85) sequences with fat saturation, and one dynamic 3D VIBRANT sequence with 6 phases of 90s (5 with contrast and 1 without). The dose of gadolinium was 0.15mmol/kg.
The morphological and dynamic pattern of enhancement was classified according to BI-RADS as mass and non-mass enhancement (diffuse, regional, focal area and ductal enhancement). In the dynamic assessment, the initial enhancement during the first 2min was evaluated and considered as moderate when <100% and intense when >100%. The post-initial enhancement was classified as persistent (curve I), plateau (curve II), and washout (curve III). The most suspicious curve was selected in all cases.
Tumor volumes were estimated using the 3D MIP images of the MRI examinations performed before and after the NT, with 4 types of response:
- (1)
Complete response (CR): absence of enhancement or reduction in the volume of enhancement >99%.
- (2)
Major partial response (MPR): reduction in the volume of enhancement >50%.
- (3)
Minor partial response (mPR): reduction in the volume of enhancement <50%.
- (4)
No response (NR): absence of changes or tumor progression.
Sixty-five patients underwent surgery, while 4 patients did not because they had stage IV disease. In 33 of the 65 patients who underwent surgery, the immunohistochemical analysis of the surgical specimen was repeated after chemotherapy. In 32, this analysis was not performed, in 9 of them because of complete pathological response.
Core needle biopsy (CNB) of the residual tumor after chemotherapy was repeated in 1 of the 4 patients who did not undergo surgery because of stage IV disease.
At diagnosis, the histological analysis included histological type and grade and immunohistochemical determination of ER, PR, c-erbB2, p 53 and ki 67. Herb 2+ was considered positive with 3+, and in case of 2+ or a weak positive a FISH test was used to determine gene amplification and confirm or rule out its positivity. After NC, the study included the histological type and grade of the infiltrative and non-infiltrative component, size, location, margins, prognostic factors, and associated lesions. The immunohistochemical analysis was repeated only in 33 cases.
ResultsThe histological type was non-specific (NS) ductal carcinoma in 55 patients (73.3%), lobular in 10 (13.3%), mixed ductal-lobular in 2 (2.6%), papillary in 1 (1.3%), and 7 (9.33%) inflammatory carcinomas of the ductal type in 5 patients (2 were bilateral).
There were 6 cases (8%) of bilateral carcinoma, 2 inflammatory and 4 were incidentally discovered on the MRI examination.
The histological diagnosis was obtained after CNB in 58 patients, surgical biopsy in 8, surgical lymph node biopsy in 4 (without a previous mammogram), and CNB of lymph nodes in 1. Three of the 4 bilateral carcinomas incidentally discovered were diagnosed with fine needle aspiration biopsy (FNA), and 1 with FNA of the suspicious lymph node.
The stage distribution was IIA (10.6%); IIB (17.3%); IIIA (28%); IIIB (30.6%); IIIC (8%), and IV (5.3%).
The presentation was unifocal in 38.6% of patients and multicentric in 61.3%.
The luminal A subtype was 2.5 times more common than the rest, accounting for 48% of carcinomas; HERB 2+ accounted for 20%; and luminal B for 18.6%. The least common subtype was the triple negative, accounting for 13.3%.
Magnetic resonance before chemotherapySixty cases (80%) exhibited mass enhancement, and 15 (20%) exhibited non-mass enhancement (5 diffuse, 4 regional, 2 segmental, 3 focal areas, and 1 ductal enhancement with branching pattern).
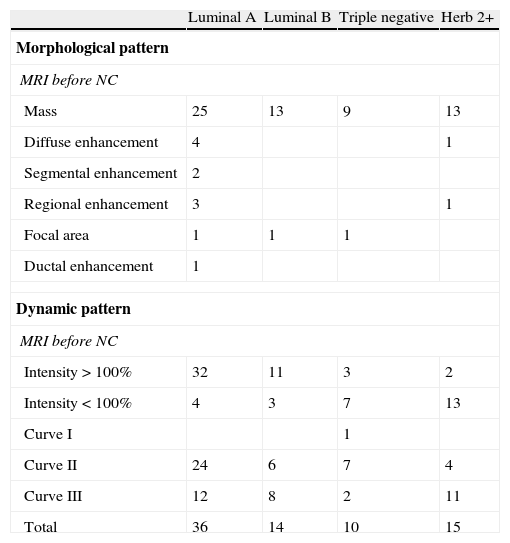
The enhancement was rapid and intense >100% in 48 cases (64%), and <100% in 27 cases (36%). After the initial enhancement, plateau enhancement (curve II) was found in 41cases (54.6%) and washout (curve III) in 33 (44%). Persistent enhancement (curve I) was found in only one case (1.33%), with intensity <100%. Table 1 shows these findings according to the distribution of the four molecular subtypes.
Morphological and dynamic pattern of the different molecular subtypes at the MRI examination performed before NC.
| Luminal A | Luminal B | Triple negative | Herb 2+ | |
| Morphological pattern | ||||
| MRI before NC | ||||
| Mass | 25 | 13 | 9 | 13 |
| Diffuse enhancement | 4 | 1 | ||
| Segmental enhancement | 2 | |||
| Regional enhancement | 3 | 1 | ||
| Focal area | 1 | 1 | 1 | |
| Ductal enhancement | 1 | |||
| Dynamic pattern | ||||
| MRI before NC | ||||
| Intensity>100% | 32 | 11 | 3 | 2 |
| Intensity<100% | 4 | 3 | 7 | 13 |
| Curve I | 1 | |||
| Curve II | 24 | 6 | 7 | 4 |
| Curve III | 12 | 8 | 2 | 11 |
| Total | 36 | 14 | 10 | 15 |
NC, neoadjuvant therapy.
CR was obtained in 11 cases (14.6%): 4 luminal A (3 ductal and 1 lobular), 2 luminal B (1 ductal and 1 lobular), 3 triple negative (ductal type), and 1 Herb 2+ (ductal type).
MPR was obtained in 45 patients (58.6%), with an average reduction in the volume of enhancement of 81.52%.
mPR occurred in 11 cases (14.6%): 6 luminal A (1 papillary, 2 lobular and 3 ductal), 2 luminal B (ductal), 1 triple negative (ductal), and 2 Herb 2+ (ductal).
NR occurred in 8 cases, 2 of them were difficult to evaluate, one because of a predominant cystic component, which did not change, and the other was a pregnancy associated breast cancer.
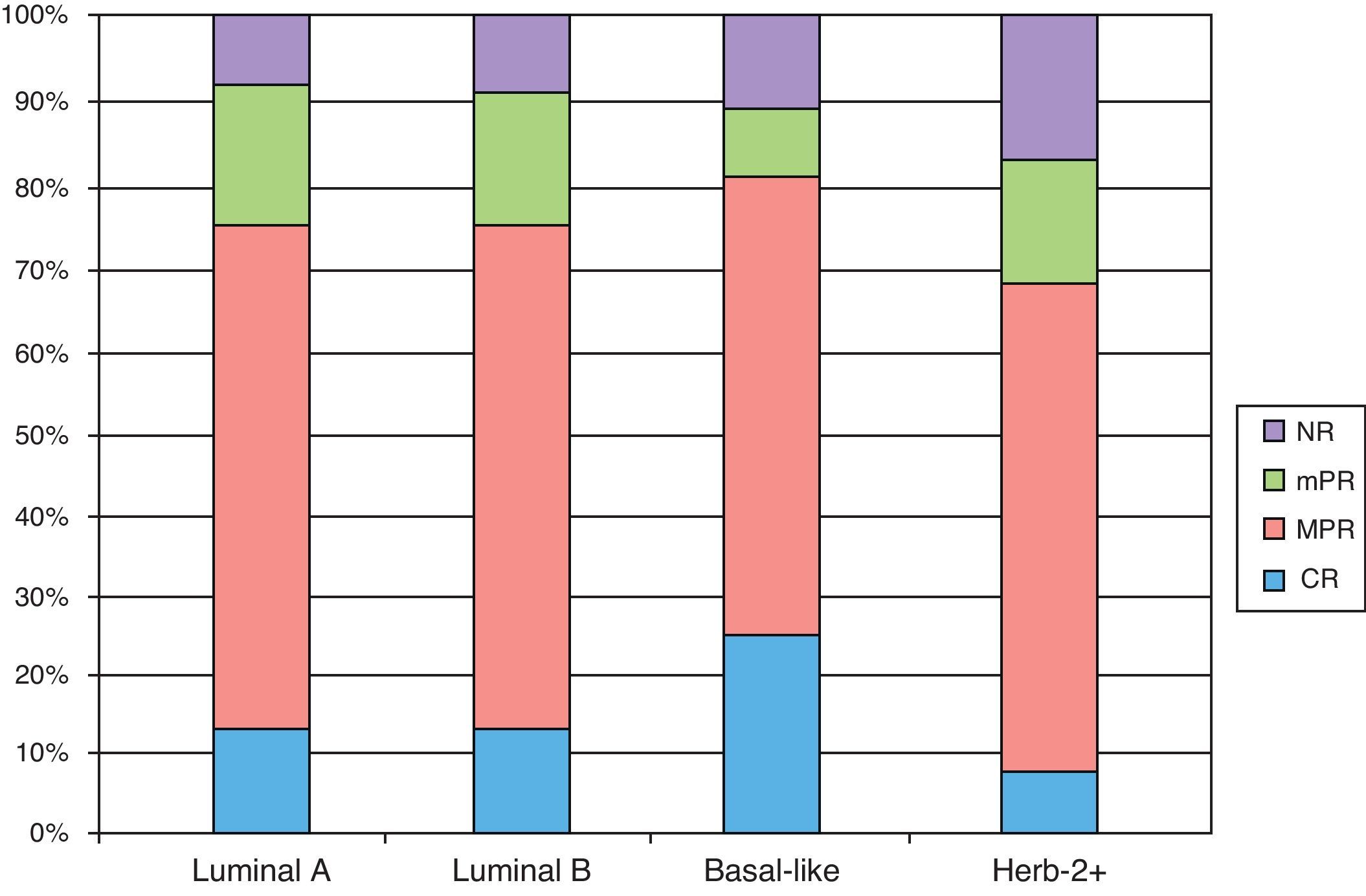
The triple negative subtype had the highest rate of good response (CR and MPR), with 84.6%. Luminal A and B subtypes showed similar rates (75.6 and 76.9%, respectively) and Herb 2+ showed the lowest rate (69.2%) (Fig. 2).
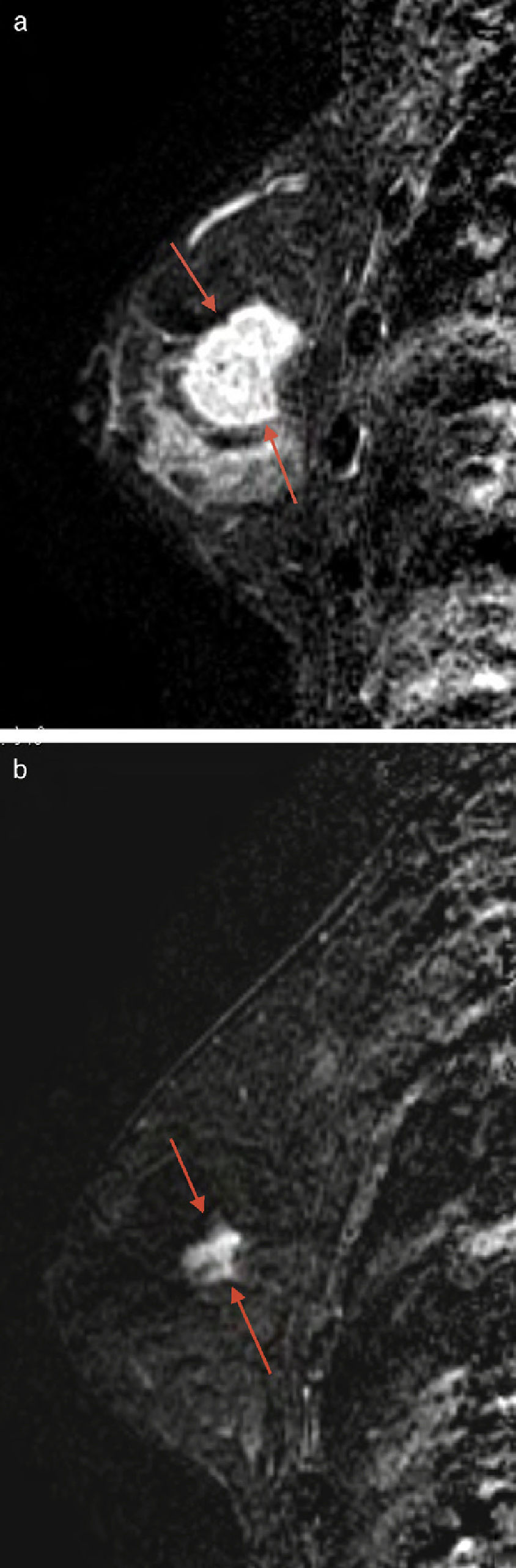
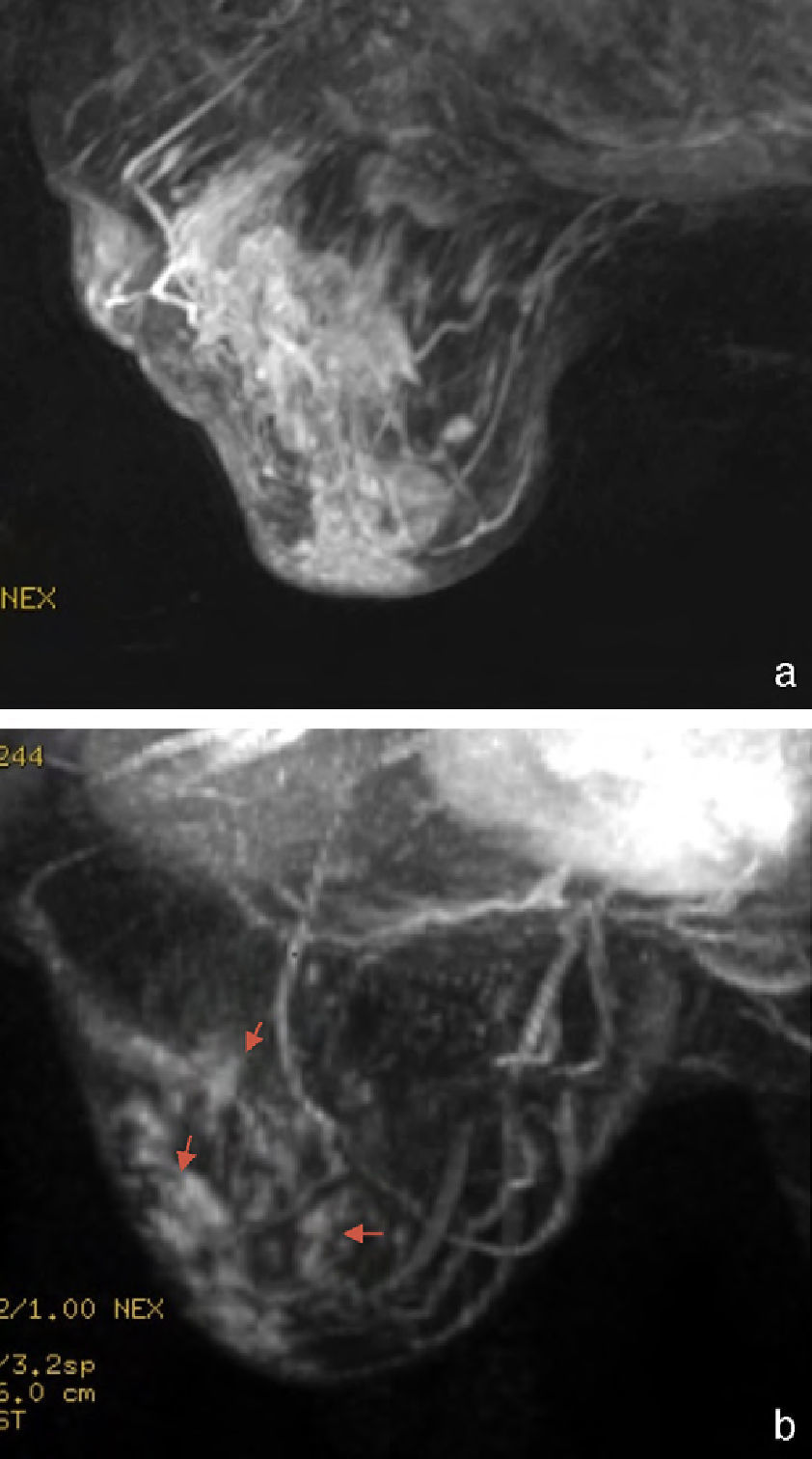
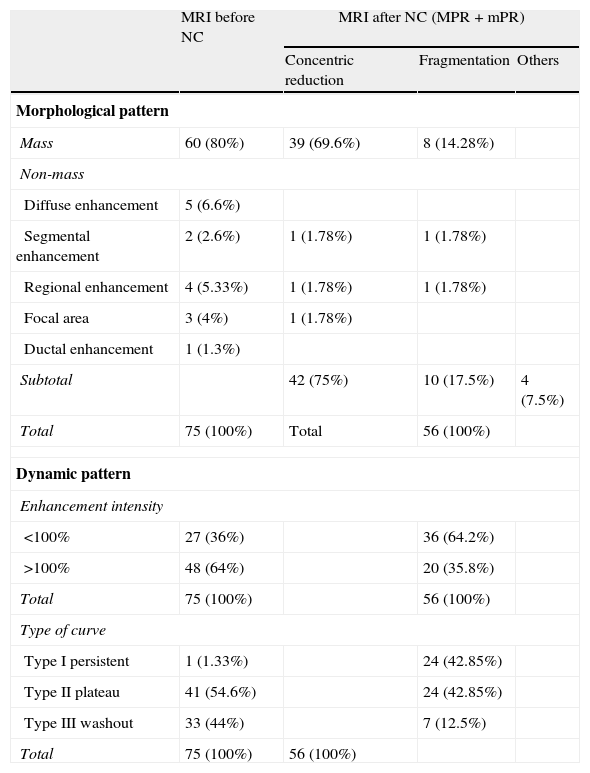
Morphological changesThe morphological changes in the MPR and mPR subgroups were classified as concentric reduction in 42 cases (75%) (Fig. 3); fragmentation in 10 (17.85%) (Fig. 4), and others, such as the appearance of new cystic areas in 2 cases (3.57%) and ductal enhancement in 2 (3.57%) (Table 2).
Morphological and dynamic variations of breast carcinomas treated with NC.
| MRI before NC | MRI after NC (MPR+mPR) | |||
| Concentric reduction | Fragmentation | Others | ||
| Morphological pattern | ||||
| Mass | 60 (80%) | 39 (69.6%) | 8 (14.28%) | |
| Non-mass | ||||
| Diffuse enhancement | 5 (6.6%) | |||
| Segmental enhancement | 2 (2.6%) | 1 (1.78%) | 1 (1.78%) | |
| Regional enhancement | 4 (5.33%) | 1 (1.78%) | 1 (1.78%) | |
| Focal area | 3 (4%) | 1 (1.78%) | ||
| Ductal enhancement | 1 (1.3%) | |||
| Subtotal | 42 (75%) | 10 (17.5%) | 4 (7.5%) | |
| Total | 75 (100%) | Total | 56 (100%) | |
| Dynamic pattern | ||||
| Enhancement intensity | ||||
| <100% | 27 (36%) | 36 (64.2%) | ||
| >100% | 48 (64%) | 20 (35.8%) | ||
| Total | 75 (100%) | 56 (100%) | ||
| Type of curve | ||||
| Type I persistent | 1 (1.33%) | 24 (42.85%) | ||
| Type II plateau | 41 (54.6%) | 24 (42.85%) | ||
| Type III washout | 33 (44%) | 7 (12.5%) | ||
| Total | 75 (100%) | 56 (100%) | ||
NC, neoadjuvant chemotherapy.
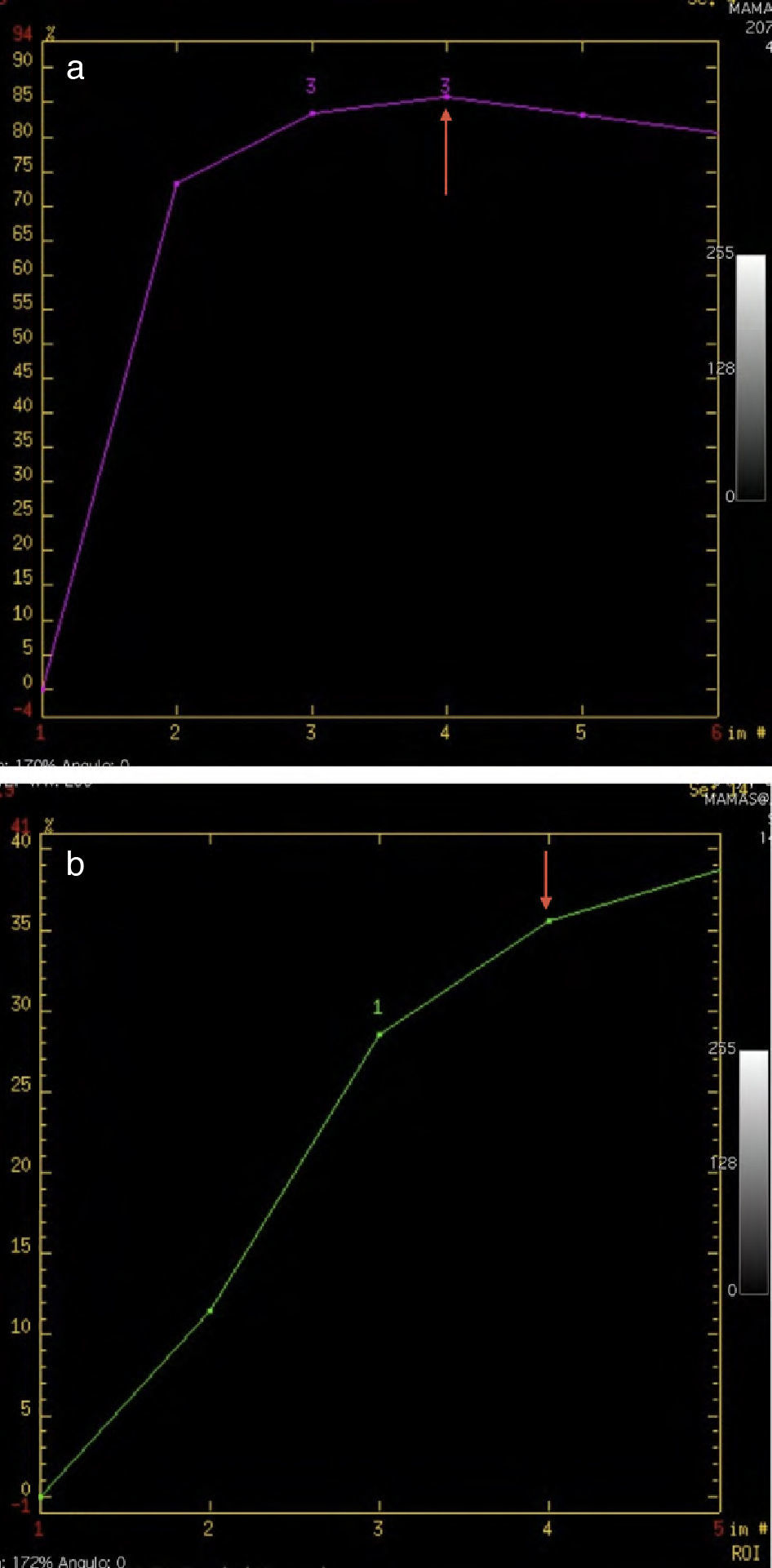
The dynamic changes in MPR and mPR were a decrease in the intensity peak (<100% in 64.2%), and change in the post-initial enhancement in the curve type (Fig. 5), with a predominance of persistent (I) and plateau (II) curves (42.85% in both cases), and a decrease in washout curves (III) to 12.5% (Table 2).
No enhancement was observed in cases of CR.
In one NR case, there was an increase in the intensity peak and a change in the curve type to a washout profile.
Immunohistochemical changesThe immunohistochemical analysis was repeated in 33 residual tumors and changes in marker expression were found in 4 cases (12.2%).
In one patient with stage IV disease, the repeat biopsy revealed a change in the molecular profile from the initial triple negative to luminal B, which led to a change in the chemotherapy regimen.
DiscussionBreast cancer is now considered as a heterogeneous group of diseases that is categorized into 4 molecular subtypes according to immunohistological findings: luminal A, luminal B, triple negative, and Herb 2+. In our series, the luminal A subtype was the most common (48%) followed by Herb 2+, luminal B, and triple negative, with 20%, 18.6% and 13.3%, respectively. The rate of good responses (CR and MPR) was higher in the triple negative subtype (84.65%), followed by the luminal B (76.5%), luminal A (75.6%), and Herb 2+ (69.2%). However, the four subtypes may show any of the four types of response and, therefore, we cannot predict the response on the basis of the molecular subtype alone. Nonetheless, it is important to monitor the tumor behavior to determine if a change in therapy is required.
The most common morphological change in the MPR and mPR groups was concentric reduction, followed by fragmentation. In this latter case, the assessment of the residual tumor was more difficult. In the series of Thibault, which included 30 patients, 13 of the 15 responders showed concentric reduction and 2 showed fragmentation, which is consistent with the results in our series.6 Unlike what might be expected, necrosis is rare and it is not mentioned in other articles.
NC causes a reduction in microvessel density due to a direct effect on angiogenesis7,8 and, as Hayes explains, indirectly due to a reduction in the concentration of growth factors caused by tumor cell death.8 In our MPR and mPR cases, there was first a decrease in the maximum intensity peak and fewer washout curves, which is consistent with results from other authors.3–6,9,10 Before NC, the MRI showed that 64% had an intensity peak >100%, and 64.2% had a maximum intensity peak <100% after NC, probably related to a reduction in the vascular supply to the tumor. Similarly, there was a significant decrease in type III curves and an increase in type I curves.
Since it seems that it is not possible to predict the response, some authors like Rieber et al.9 advocate the use of MRI between the cycles of chemotherapy and the signal intensity to identify those patients who do not respond adequately to NC. Others, like Padhani et al.11, consider that changes in both size and enhancement are equally sensitive in the identification of patients who would gain no clinical or pathologic benefit from NC. We did not perform MRI examinations between cycles, and in NR cases, the second examination was performed based on the clinical judgment of the oncologist, either before or on completion of the NC. In 2 cases, the MRI was performed after treatment, showing no tumor changes. In 4, the MRI was performed after discontinuing therapy due to lack of response, showing no changes in signal intensity or in the type of curve, except in one case where the enhancement increased and the curve changed to a washout profile. We have therefore found only one case of increased enhancement in a case of NR. The immunohistochemical study of the surgical specimen was repeated in 2 cases of NR revealing a change from the luminal A phenotype to the triple negative phenotype. In our opinion, the immunohistochemical analysis of the surgical specimen is important, especially in cases of NR, to evaluate the correlation between the analyses and the response to the systemic therapy.
We observed a change in the immunohistochemical profile between the specimens obtained before and after chemotherapy in 12.2% of cases. This is probably an overestimation in response to different factors, such as the own tumor heterogeneity and the different volumes of the samples analyzed (cores/residual tumor in the surgical specimen). Nonetheless, we cannot rule out that some of these changes have actually occurred and that they have been caused by the drugs. For this reason, it is important to repeat the immunohistochemical analysis of the residual tumor and to repeat the biopsy in patients with stage IV disease if they worsen, or even in metastases.
One of the most important limitations is some degree of interpathologist, and even intrapathologist, variability since the molecular subtypes were determined by immunohistochemical analysis and not by more accurate techniques like microarray assay.
In conclusion, the triple negative subtype showed the highest rate of good response to NC. However, the 4 molecular subtypes may show any of the 4 types of response, and the type of response cannot be predicted on the basis of the molecular subtype alone, which makes it essential to monitor tumor behavior.
The most common changes after NC were concentric reduction and a decrease in the maximum intensity peak, with fewer washout curves.
Since we observed a change in the molecular phenotype between the specimen at diagnosis and the final study in 12.2% of cases, we believe that it is essential to repeat the immunohistochemical analysis in the residual tumor.
AuthorshipResponsible for the integrity of the study: LMDP.
Conception of the study: LMDP.
Design of the study: LMDP.
Acquisition of data: ATB, MLAM, LMDP, VCDJ.
Analysis and interpretation of data: LMDP, ATB, MLAM, VCDJ.
Statistical analysis: N/A.
Bibliographic search: LMDP, ATB.
Drafting of the paper: LMDP, MLAM.
Critical review of the manuscript: LMDP, MLAM.
Approval of the final version: LMDP, ATB, MLAM, VCDJ.
The authors have no conflict of interest to declare.
Please cite this article as: Marcos de Paz LM, et al. Resonancia magnética de mama: cambios en la imagen del cáncer tratado con neoadyuvancia. Correlación con subtipos moleculares. Radiología. 2012;54:442-8.