To determine whether there is iron overload by calculating the T2* value in the liver and myocardium in patients with secondary hemochromatosis. To analyse the correlation of the values obtained with the iron levels in blood, with the liver iron concentration (LIC) calculated using magnetic resonance (MR) imaging, and the correlation between them.
Materials and methodsA total of 16 patients (13 males, 3 females), with a mean age of 61 years, were included and evaluated in the years 2008 and 2009. Fifteen of them had received multiple transfusions, and one was diagnosed with hereditary sideroblastic anaemia.
The measurements included, blood ferritin, LIC by MRI, cardiac function using MRI and the T2* value by means of multi-echo sequences in the liver (TR/TE1/ΔTE/No of echos/α: 21/1.18/1.0/20/35°) and myocardium (26/1.04/0.8/30/60°). A correlation-regression analysis was performed by comparing the cardiac and liver T2* values with the ferritin, LIC and between each of them.
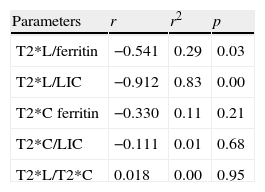
ResultsA total of 13 patients had ferritin values greater than 1000ng/ml (median/minimum/maximum: 1762/294/3785ng/ml). An increased LIC greater than 80μmol/g (median/minimum/maximum: 125.4/41.2/241.5μmol/g) was observed in 13 patients. In all cases cardiac function was conserved, and in 15 cases the liver T2* value was less than 6.3ms. The myocardium T2* value was less than 20ms in only one case. A high correlation was observed between the liver T2* values and the LIC (r: −0.912). The correlation was statistically significant between the liver T2* value and ferritin (r: −0.541). The correlations between myocardium T2* and ferritin, myocardium T2* and LIC, and myocardium T2* and liver T2* were not statistically significant.
ConclusionsThe liver T2* showed a high correlation with LIC and a statistically significant correlation with ferritin. No association was observed between the myocardium T2* values and ferritin in blood, the LIC or the liver T2* value.
Valorar la sobrecarga férrica mediante el cálculo del valor T2* en el hígado y el miocardio en los pacientes con hemocromatosis secundaria. Evaluar la correlación de los valores obtenidos con los niveles de ferritina en sangre y la concentración de hierro hepático (CHH) calculada mediante resonancia magnética (RM), y la correlación de los valores T2* entre sí.
Material y métodosSe incluyeron 16 pacientes (13 varones y 3 mujeres), evaluados entre los años 2008 y 2009, con una edad media de 61 años. Quince eran pacientes politransfundidos y uno estaba diagnosticado de anemia sideroblástica hereditaria.
Se estudió la ferritina en sangre, la CHH por RM, la función cardíaca mediante RM y el valor T2* mediante secuencias multieco en el hígado (TR/TE1/ΔTE/no. ecos/α: 21/1.18/1.0/20/35°) y el miocardio (26/1,04/0,8/30/60°). Se realizó el análisis de correlación-regresión de los valores T2* cardíaco y hepático con los valores de ferritina y CHH, y entre sí.
ResultadosTrece pacientes mostraron valores de ferritina superiores a 1.000ng/ml (mediana/mínimo/máximo: 1.762/294/3.785ng/ml). Trece pacientes presentaron CHH elevada, mayor de 80μmol/g (mediana/mínimo/máximo: 125,4/41,2/241,5μmol/g). En todos los casos la función cardíaca estaba preservada. En 15 pacientes el valor T2* hepático fue menor de 6,3ms. Solo en un caso, el valor T2*miocárdico fue menor de 20ms. Se observó una alta correlación para los valores T2*hepático/CHH (r: −0,912). La correlación fue estadísticamente significativa para T2* hepático/ferritina (r: −0,541). La correlación T2*miocárdico/ferritina, T2*miocárdico/CHH y T2*miocárdico/T2*hepático no fue estadísticamente significativa.
ConclusiónLos valores T2* hepático muestran una alta correlación con la CHH y una correlación estadísticamente significativa con la ferritina. No se observó correlación entre los valores T2* miocárdico y la ferritina en sangre, la CHH, ni con el valor T2* hepático.
Iron is an essential element in countless metabolic and biological processes. The iron levels in the body depend exclusively on the regulation of its absorption in the gastrointestinal system, since the human body does not have excretion mechanisms. When it is present in excess, it produces important tissue damage due to the fact that it generates free radicals and induces oxidative stress. If iron overload is not treated in time, the damage caused may get to be irreversible.
Iron overload may be due to an abnormally high gastrointestinal absorption of iron (primary or hereditary hemochromatosis) or else it may be secondary to multiple transfusions. Hematological diseases that occur with diserythropoiesis are found in the latter group, which, in turn, may condition an increase in iron absorption and contribute to or be the cause of iron overload.
The excess iron is accumulated in different organs, the liver and the heart among them, which may lead to hepatic cirrhosis and hepatocarcinoma in the case of liver iron overload, and to cardiomyopathy, arrhythmia, heart failure and even sudden death in the case of heart iron overload.1,2 Transfusion of more than 20–30 units may cause high iron deposits. The appearance of iron chelating drugs opens up the possibility of controlling iron excess in the body, but these drugs are not devoid of adverse reactions and their price is high. Therefore, early determination of the existence and extent of iron overload in these organs is crucial.
The serum markers used in the follow-up have a limited predictive value to determine the appearance of the disease in these organs.3 Ferritin values above 1000ng/ml may indicate an iron overload, but these values may also be elevated due to conditions other than iron overload.
The best parameter to assess actual iron deposit is by quantifying liver iron concentration (LIC) and to that end, a liver biopsy is required, it is an invasive method not lacking morbidity.
Magnetic resonance is a non-invasive technique that has been evaluated in many works and it aims at analyzing heart and liver iron overload, with very good results.4–9 This technique detects iron overload indirectly due to the paramagnetic effect of the deposit of said element in the tissues, which determines a shortening of the T2 which entails a decrease of tissular signal proportional to the concentration of iron. The two methods used in magnetic resonance (MR) to estimate iron are the signal intensity ratio and relaxometry.10 In the signal intensity ratio method, the signal intensity measured in the target organ is divided by the signal intensity measured in an organ not affected by iron overload, such as muscles or fat. Relaxometry measures the time of transversal relaxation directly T2, or T2* if gradient echo sequences are used, and the shorter it is, the greater the iron overload.
Some works have evidenced that quantifying LIC by means of the signal intensity ratio method correlates well with the LIC measurement of liver biopsy.4,5 In 2002 our center validated an LIC quantification model based on the measurement of liver/muscle signal intensity ratio. The correlation with the iron concentration measured by means of biopsy was high, so much so, that in our hospital, MR has replaced biopsy in determining the liver iron levels. When the LIC measured with this model is greater than 85μmol/g, the positive predictive value to determine liver iron overload is 100%. An LIC under 20μmol/g goes against iron overload.4
The relaxometry method makes it possible for us to evaluate liver or heart iron concentration by measuring the shortening of the relaxation time T2 or T2* due to the increase of field heterogeneity caused by the iron deposit in the tissues. In the liver, it is considered that a liver T2*value (T2*L) greater than 6.3ms goes against iron overload, and a T2*L value lower than 1.4ms, suggests a serious iron overload.11 In the heart, the T2* value has proved to be useful in the management of patients with secondary iron overload, since it is highly predictive of the risk of myocardiopathy by iron overload.7,8,12,13 The patients with T2*myocardial values (T2*M) surpassing 20ms (no heart iron detected) do not develop heart failure, whereas the patients with T2*M lower than 10ms have a high risk of cardiac decompensation.3,13 In addition, this technique has proved to be reproducible and transferable.7,8,14,15
Taking into account these data, and given the growing interest in determining liver and heart iron by MR in our hospital's Hematology Service, our objective was to assess by means of MR the T2*L and T2*M values in patients with secondary hemochromatosis and evaluate its correlation with the ferritin and LIC values.
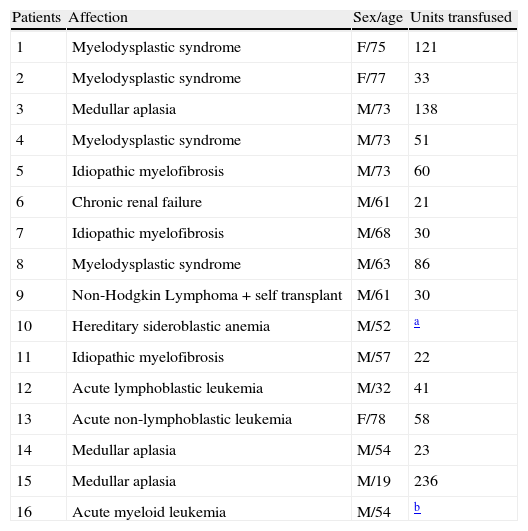
Material and methodPatientsDuring the years 2008 and 2009 16 patients (13 males and three females) were included, their ages ranged from 19 to 78 years (median: 61 years). The 16 studies were performed at the request of their doctors to study heart and liver iron overload by MR. To this end, the usual protocol was followed and the sequences aimed at studying iron overload that are later described were performed. This did not presuppose a significant prolongation of the examination nor did it pose an added risk for the patients. All of them gave their informed consent for the test. For these reasons, permission from the ethics committee was not requested. 15 of them were polytransfused patients (4 due to myelodysplastic syndrome, 3 due to myelofibrosis, due to medullar aplasia, 1 in the context of liver transplant, 1 non Hodgkin lymphoma, 1 acute lymphocytic leukemia, 1 acute myeloid leukemia and 1 acute non-lymphocytic leukemia). In one case, the iron overload was secondary to a hereditary sideroblastic anemia. In 14 of the 15 postransfusional cases more than 20 units were transfused. A patient underwent intense polytransfusion, but we have not been able to determine the number of units (Table 1). In all of them, the blood ferritin values were obtained (normal value: adult male: 12–300ng/ml; adult female: 10–150ng/ml).
Description of the patients.
| Patients | Affection | Sex/age | Units transfused |
| 1 | Myelodysplastic syndrome | F/75 | 121 |
| 2 | Myelodysplastic syndrome | F/77 | 33 |
| 3 | Medullar aplasia | M/73 | 138 |
| 4 | Myelodysplastic syndrome | M/73 | 51 |
| 5 | Idiopathic myelofibrosis | M/73 | 60 |
| 6 | Chronic renal failure | M/61 | 21 |
| 7 | Idiopathic myelofibrosis | M/68 | 30 |
| 8 | Myelodysplastic syndrome | M/63 | 86 |
| 9 | Non-Hodgkin Lymphoma+self transplant | M/61 | 30 |
| 10 | Hereditary sideroblastic anemia | M/52 | a |
| 11 | Idiopathic myelofibrosis | M/57 | 22 |
| 12 | Acute lymphoblastic leukemia | M/32 | 41 |
| 13 | Acute non-lymphoblastic leukemia | F/78 | 58 |
| 14 | Medullar aplasia | M/54 | 23 |
| 15 | Medullar aplasia | M/19 | 236 |
| 16 | Acute myeloid leukemia | M/54 | b |
M: male; F: female.
The MR examinations were performed with MR 1.5T equipment (Philips Health Care, Achieva). In one and the same study Liver Iron Concentration (LIC) was evaluated by MR following the model that had been previously validated in our center,4 heart function and T2* value by means of gradient echo (GE) sequences multi-echo in the liver (T2*L) and the myocardium (T2*M). The median duration of the complete study was 60min.
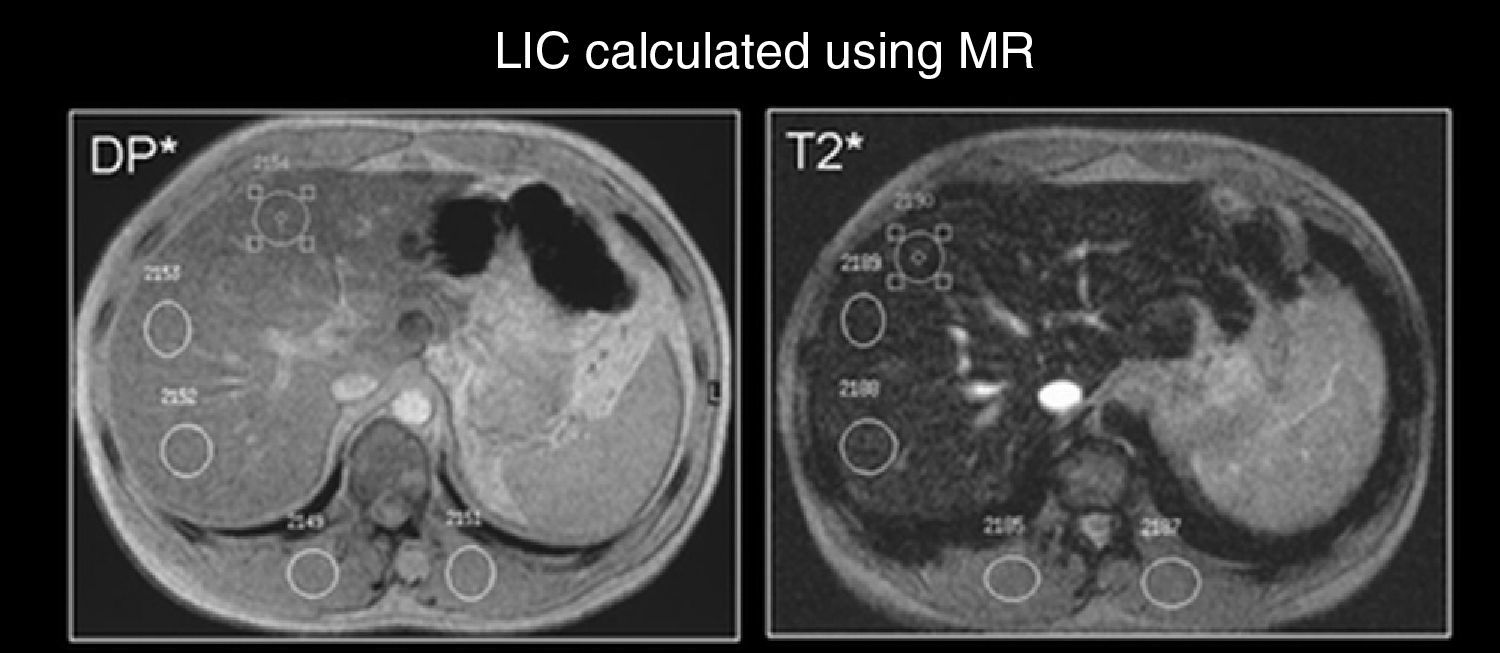
In order to determine LIC, two GE sequences were obtained (protonic density and T2; TR/TE/α: 120/4–4/20°) with a quadrature coil integrated into the equipment, in incidence the axial plane, with a 20-s apnea. The signal intensity (SI) was measured in each sequence with regions of interest (ROI) of more than 1cm2 in the right liver lobe and in the right and left paravertebral musculature (Fig. 1). All the values were introduced in a spreadsheet to quantify the LIC (Fig. 2).
The T2*L calculation was performed by means of a multi-echo axial GE sequence (TR/first echo (TE1)/interval between echoes (ΔTE)/no. echoes/α: 21/1.18/1.0/20/35°), with only one cut through the center of the liver, in a 15-s apnea. The signal of the different echoes was adjusted to a mono-exponential model, with the equation:
where S(TE) is the signal intensity for each of the echoes; S0 is the value of the signal for TE equal zero; T2* is the relaxation time and TE echo time for each of the images. From this adjustment, the value of T2* was estimated for image pixel16 building thus an image parametric map T2*. For the measurement, a ROI was placed on the parametric map T2* excluding vascular structures (Fig. 3).For the calculation of T2*M three parallel cuts were performed in the short axis projection on the left ventricle, one on the base, another in the mid third and the other in the apical segment, using the multi-echo GE sequence T2* (TR/TE1/ΔTE/no. echoes/α: 26/1.04/0.8/30/60°), with heart synchronicity and a 17-s apnea. Each cut was acquired at the end of the diastole. Just like in the liver study, a parametric map T2* was estimated with the signal from the different echoes. A ROI was drawn on said map in the interventricular septum, excluding the endocavitary lumen and areas near the coronary veins (Fig. 4).
The calculation of the left ventricular ejection fraction (LVEF) was performed by means of Simpson's method17 using cine multi-echo multi-cut sequences SSFP, short axis projection (TR/TE/phases: 3.1/1.5/30).
Both for the T2*L calculation and for the complete heart study we used the 5-element phase-array coil T2*.
The data were collected by several experienced people. All these data were supervised by one and the same person who is also very experienced.
Statistical analysisA study was performed on the correlation-regression of the T2*L values with the ferritin and LIC, of the T2*M values with the ferritin and LIC values and between the T2*L and T2*M values. α=0.05 was considered in the analysis. The statistical study was performed by means of the statistic software SYSTAT V9.0. (Chicago, IL). The Shapiro–Wilk test was used to determine the distribution variable.
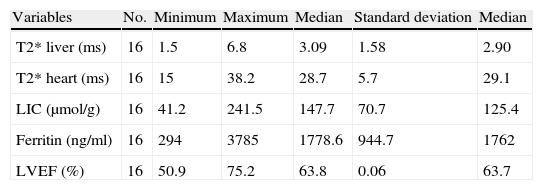
ResultsThe blood ferritin values ranged from 294 to 3785ng/ml (median: 1762ng/ml). Thirteen patients showed blood values greater than 1000ng/ml. Thirteen patients presented a high LIC, greater than 80μmol/g (median: 125.4μmol/g). In 15 patients the T2*L values were less than 6.3ms (median: 2.90ms). Only in one case the T2*M value was less than 20ms (median: 29.1ms).
The LVEF values ranged from 50.9 and 75.2% (median: 63.7%). They did not indicate left ventricular dysfunction in any case.
According to the Shapiro–Wilk test all the variables followed a normal distribution, except for LIC which strayed slightly from normalcy (p=0.045).
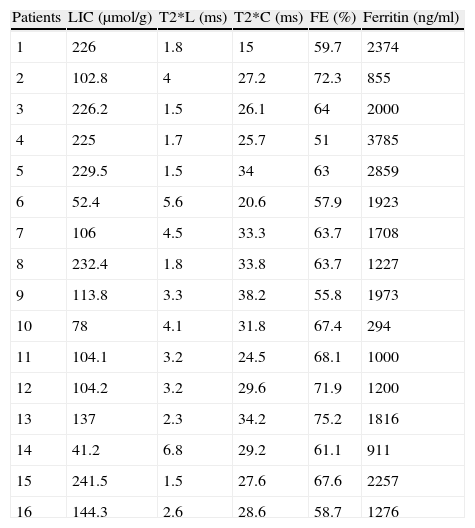
Table 2 presents the LVEF, T2*M, T2*L, LIC and ferritin values obtained for each patient. Table 3 describes the variables.
Values obtained for each patient.
| Patients | LIC (μmol/g) | T2*L (ms) | T2*C (ms) | FE (%) | Ferritin (ng/ml) |
| 1 | 226 | 1.8 | 15 | 59.7 | 2374 |
| 2 | 102.8 | 4 | 27.2 | 72.3 | 855 |
| 3 | 226.2 | 1.5 | 26.1 | 64 | 2000 |
| 4 | 225 | 1.7 | 25.7 | 51 | 3785 |
| 5 | 229.5 | 1.5 | 34 | 63 | 2859 |
| 6 | 52.4 | 5.6 | 20.6 | 57.9 | 1923 |
| 7 | 106 | 4.5 | 33.3 | 63.7 | 1708 |
| 8 | 232.4 | 1.8 | 33.8 | 63.7 | 1227 |
| 9 | 113.8 | 3.3 | 38.2 | 55.8 | 1973 |
| 10 | 78 | 4.1 | 31.8 | 67.4 | 294 |
| 11 | 104.1 | 3.2 | 24.5 | 68.1 | 1000 |
| 12 | 104.2 | 3.2 | 29.6 | 71.9 | 1200 |
| 13 | 137 | 2.3 | 34.2 | 75.2 | 1816 |
| 14 | 41.2 | 6.8 | 29.2 | 61.1 | 911 |
| 15 | 241.5 | 1.5 | 27.6 | 67.6 | 2257 |
| 16 | 144.3 | 2.6 | 28.6 | 58.7 | 1276 |
LIC: liver iron concentration; T2*L: T2* liver; T2* c: T2* heart.
Description of the variables.
| Variables | No. | Minimum | Maximum | Median | Standard deviation | Median |
| T2* liver (ms) | 16 | 1.5 | 6.8 | 3.09 | 1.58 | 2.90 |
| T2* heart (ms) | 16 | 15 | 38.2 | 28.7 | 5.7 | 29.1 |
| LIC (μmol/g) | 16 | 41.2 | 241.5 | 147.7 | 70.7 | 125.4 |
| Ferritin (ng/ml) | 16 | 294 | 3785 | 1778.6 | 944.7 | 1762 |
| LVEF (%) | 16 | 50.9 | 75.2 | 63.8 | 0.06 | 63.7 |
LIC: liver iron concentration; LVEF: left ventricular ejection fraction.
Of the 15 patients for whom the T2*L value was less than 6.3ms, 13 showed ferritin values greater than 1000ng/ml, in one of them the value obtained was 855ng/ml and in another it was normal (294ng/ml). The correlation estimated between the T2*L and the ferritin values was significant (r: −0.541; r2: 0.29).
In the 15 patients with T2*L values under 6.3ms, LIC revealed the existence of iron overload. In 13 cases it was greater than 80μmol/g. Of the other two 2, in 1, the value obtained was near the value considered as serious iron overload (78μmol/g). In the other case, LIC indicated a moderate iron overload (52μmol/g). The correlation obtained between both parameters was high (r: −0.912; r2: 0.83; p<0.05).
No significant correlation was found between the T2*M and the ferritin value (r: −0.330; r2: 0.11; p>0.05) or between T2*M and the LIC value (r: −0.111; r2: 0.01; p>0.05).
Likewise, the correlation T2*L/T2*M (r: 0.018; r2: 0.00; p>0.05) was not significant.
Table 4 details the relation among the variables.
DiscussionIn our study, the great majority of the patients with secondary hemochromatosis presented a high iron overload in the liver and high levels of ferritin.
After the analysis of the association of T2*M and T2*L with ferritin and LIC, and the relation of T2*M-T2*L by means of the correlation-regression analysis, it was possible to observe that the association was mainly significant for the T2*L and LIC values. The higher the LIC level, the lesser the T2*L. Therefore, the MR calculation of liver iron concentration and the liver T2* values turned out to be useful tools and with similar results for determining liver iron overload. The high correlation was to be expected, given the good results that both techniques have proved to have in the bibliography for measuring hepatic iron by RM.5,18–20
The T2*L values were also associated with the ferritin level. Ferritin determination is a good screening technique to know the body iron level and it may reflect a liver iron overload, but it must be remembered that it may be elevated due to other conditions different from iron overload, such as active inflammatory processes, certain hepatopathies or neoplasias.21,22 Contrariwise, it may happen that ferritin is normal and the T2* value indicates that there is a liver iron overload, as it occurred in one of our cases. It is a patient with hereditary sideroblastic anemia, an affection that is classed within the group of the “iron overload anemias”, since it is a congenital anemia that with age, it is accompanied by overload diserythropoiesis itself before the transfusions need to be started. The patient had been diagnosed 20 years earlier before the MR study had been performed. In this period of time, he has been transfused sporadically. He always presented normal or not very high ferritin values, for the level of iron detected in the MR. Discordance between the ferritin levels and iron concentration in the liver may be due to the fact that in the patients with diserythropoiesis, the excess of iron absorbed remains stored in the liver during a long period of time and for this reason, the blood ferritin levels are lower. Said discordance has also been described in intermediate thalassemias which, as in our case, are hardly transfused, and they may occur with low ferritin and liver overload.23
Unlike what happened in the liver, the association of T2*M values with those of ferritin, LIC and T2*L was not statistically significant. Since the advent of MR as a method for the study of heart iron accumulation, it has been observed that there is not a significant association between heart iron concentration and liver iron concentration and the ferritin levels. This is due to the fact that the heart's iron reception and elimination mechanism is different from that of the liver. Iron is deposited and cleared more rapidly in the liver than in the heart.24 In fact, liver overload presupposes a risk factor of heart overload. Nevertheless, there is not a liver overload value that determines whether there is heart overload or not. On the other hand, these differences between the iron's kinetics for both tissues will determine different responses from these organs to different chelating treatments.3,7,25 Thus, after an intense chelating treatment, an important reduction in the levels of liver iron may occur while iron overload still persists in the heart.
LVEF was not indicative of heart systolic dysfunction in any patient, including the one whose T2* value was less than 20ms. Deterioration of heart systolic function is a late sign of toxicity by heart iron overload. Detection of abnormal heart T2* values occur before the abnormal iron deposit affects systolic function. This is due to the fact that MR detects mainly the iron stored in the form of hemosiderin, not ferritin or free iron. Heart dysfunction is due to free iron. As iron accumulates in the storage systems, T2* drops, but it hardly causes any effects on heart function. When there is no more storage capacity, plasmatic free iron increases and myocardial damage occurs as well as the subsequent myocardial alterations.7,25 In our study, the diastolic function was not assessed. Nevertheless, it must be pointed out that that said assessment results of great interest since iron accumulation on ventricular walls causes alterations in ventricular filling by affecting myocardial elasticity and it alters diastolic function before it does the systolic function.26,27 This assessment may be performed in a non-invasive manner both by means of ultrasonography and RM.28
Our study is limited by the small number of patients. Nevertheless, the results are similar to those of other publications.7,29 Although another limitation is the fact that the study of intra-interobserver variability was not conducted, it must be pointed out that at present, it is widely accepted that there is good intra-interobserver correlation.7,30
At present, relaxometry is not a technique available in all the equipment. It is to be expected that, given the progressive importance that MR is acquiring in managing patients with iron overload, the use of this technique will spread to other centers. It is desirable that in the future standardization of the T2* value is attained with acquisition and post-process parameters that allow for the results to be compared among different centers and for the T2*values to be calibrated in μmol/g.
As a conclusion, T2* value shows a high correlation with LIC obtained by MR. The calculation of T2* allows for the heart overload to be evaluated even before systolic dysfunction occurs. Ferritin may alert about the existence of liver iron overload, but it would not be useful for heart iron overload. LIC measured by MR and the T2* liver value do not reflect the heart's iron content either, therefore each organ would need to be evaluated in an independent and specific manner.
MR is a non-invasive technique that may contribute to the diagnosis of patients with heart/liver iron overload. The estimation of myocardial and liver T2*may not only have diagnostic value, but also its use may be extensible to the follow-up and thus contribute to the management of patients with secondary hemochromatosis in what refers to treatment guidelines.
Author contributionsMCB is responsible for the study's integrity. MCB, JMA, and JS contributed in conception of the study. MCBM, JMA, and JS contributed in design of the study. MCB, MU contributed in data acquisition. MCB, CG. and AG contributed in data analysis and interpretation. MCB contributed in statistic treatment. MCB and JMA contributed in bibliographic search. MCB, CG, and JMA contributed in writing of the paper. JMA, MU, CG, and AG contributed in critical revision of the manuscript with intellectually relevant contributions. CG, AG, MCB, JMA, MU, and JS contributed in approval of the final version.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Barrera Portillo MC, et al. Medición del T2* hepático y cardíaco en la hemocromatosis secundaria. Radiología. 2013;55:331–339.