Cervical cancer is the third most common gynecological cancer. Its treatment depends on tumor staging at the time of diagnosis, and a combination of chemotherapy and radiotherapy is the treatment of choice in locally advanced cervical cancers. The combined use of external beam radiotherapy and brachytherapy increases survival in these patients. Brachytherapy enables a larger dose of radiation to be delivered to the tumor with less toxicity for neighboring tissues with less toxicity for neighboring tissues compared to the use of external beam radiotherapy alone. For years, brachytherapy was planned exclusively using computed tomography (CT). The recent incorporation of magnetic resonance imaging (MRI) provides essential information about the tumor and neighboring structures making possible to better define the target volumes. Nevertheless, MRI has limitations, some of which can be compensated for by fusing CT and MRI. Fusing the images from the two techniques ensures optimal planning by combining the advantages of each technique.
El cáncer de cérvix es el tercer cáncer ginecológico más frecuente. El tratamiento depende de la estadificación del tumor en el momento del diagnóstico, siendo la combinación de quimioterapia y radioterapia el tratamiento de elección para cánceres localmente avanzados. El uso combinado de radioterapia externa y braquiterapia aumenta la supervivencia en estas pacientes. La braquiterapia permite proporcionar mayor dosis de radiación al tumor con menor toxicidad de los tejidos vecinos en comparación con la radioterapia externa exclusiva. La planificación de la braquiterapia se ha realizado durante años exclusivamente con tomografía computarizada (TC). La reciente incorporación de la resonancia magnética (RM) ha demostrado que aporta información esencial del tumor y de las estructuras vecinas, y permite definir mejor los volúmenes blanco. No obstante, la RM presenta limitaciones, algunas de las cuales se pueden compensar con la fusión de imágenes de TC y RM, con lo que se consigue una planificación óptima al combinar las ventajas de cada técnica.
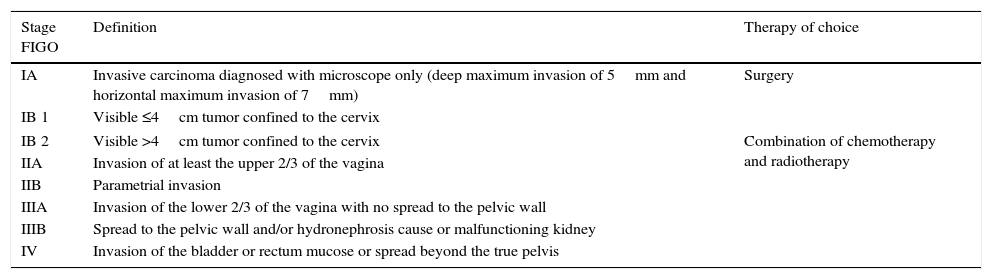
Cervical cancer is the third most frequent gynecological cancer after endometrial and ovarian cancers.1 Staging of cervical cancer is performed clinically following the latest revision by the FIGO (International Federation of Gynecology and Obstetrics) from the year 2009 (Table 1), and magnetic resonance imaging (MRI) is the image modality of choice to assess local extension of the tumor.2 In this article we go through the treatment of locally advanced cervical cancer (Stages IB2 to IVA), we present the usefulness of MRIs in marking out the are of cervical cancer prior to the administration of brachytherapy, we describe the usual MRI modlities for this planning and discuss the technical and safety implications of using MRI for this clinical application. All of this is illustrated with practical examples of the usefulness of MRI for planning brachytherapy for the treatment of patients with advanced cervical cancer.
Cervical cancer staging (International Federation of Gynecology and Obstetrics [FIGO], 2009).
| Stage FIGO | Definition | Therapy of choice |
|---|---|---|
| IA | Invasive carcinoma diagnosed with microscope only (deep maximum invasion of 5mm and horizontal maximum invasion of 7mm) | Surgery |
| IB 1 | Visible ≤4cm tumor confined to the cervix | |
| IB 2 | Visible >4cm tumor confined to the cervix | Combination of chemotherapy and radiotherapy |
| IIA | Invasion of at least the upper 2/3 of the vagina | |
| IIB | Parametrial invasion | |
| IIIA | Invasion of the lower 2/3 of the vagina with no spread to the pelvic wall | |
| IIIB | Spread to the pelvic wall and/or hydronephrosis cause or malfunctioning kidney | |
| IV | Invasion of the bladder or rectum mucose or spread beyond the true pelvis | |
The treatment of cervical cancer depends on the stage of the tumor at the moment of diagnosis, and the combination of chemotherapy with curative intention is considered the treatment of choice for locally advanced cancers (Stages IB2 to IVA).3 The most frequently used type is cisplatin-based chemotherapy, with or without 5-fluorouracil, and it is more effective in the early stages of locally advanced tumors (IB to IIB).3 Although surgery is offered as treatment for patients on stage IIA in some centers, the current trend is to offer chemotherapy and radiotherapy as choice treatments, since surgery is usually not curative and radiotherapy after surgery has higher toxicity and morbidity for the patient.3
The present radiotherapy protocols include the application of external and local radiotherapy (brachytherapy). The combined use of external radiotherapy and brachytherapy increases survival at 4 years in patients with advanced cervical carcinoma in comparison with the exclusive use of external radiotherapy.3,4 Brachytherapy facilitates the administration of higher radiation doses to cervical tumors, with less affectation to the neighboring structures in comparison with external radiation.3
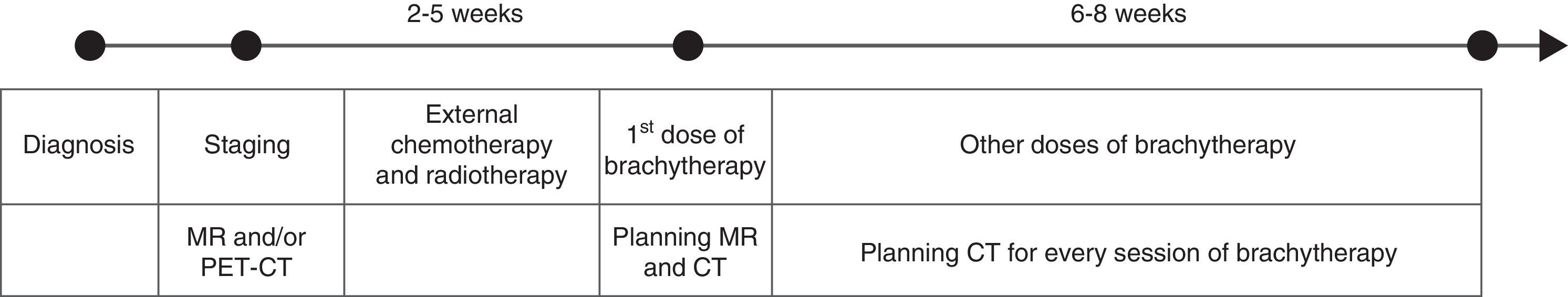
Brachytherapy is usually started once the tumor size has been reduced, between two and five weeks after external radiation treatment has started (Fig. 1). The total radiation applied with brachytherapy is administered in several sessions, one or two sessions in the case of low-dose brachytherapy (0.4–2Gy/h) and between three and six sessions if high doses are used (>12Gy/h). Both radiation application protocols are similarly effective.3 However, high-dose brachytherapy has the advantage that it can be administered in outpatient settings and it allows greater control on the distribution of the dose. Although theoretically there is a higher risk of toxicity in the healthy tissue with this type of brachytherapy due to the higher dose, randomized studies completed to date have not shown a greater index of complications secondary to the radiation of organs adjacent to the tumor.3,5 Low-dose radiotherapy requires hospitalization of the patient with the application of treatment for several days, but it causes less toxicity in healthy tissue, since the radiation dose is smaller.
In addition to the dose differences, there are different ways of administering the treatment with brachytherapy: intracavitary, with applicators introduced in the vagina and the uterus; or interstitial, directly inserting needles in the tumor with the radiation sources. The latter is generally used when there is extensive affectation of the vagina3 or when the fornices of the vagina are fibrotic.5
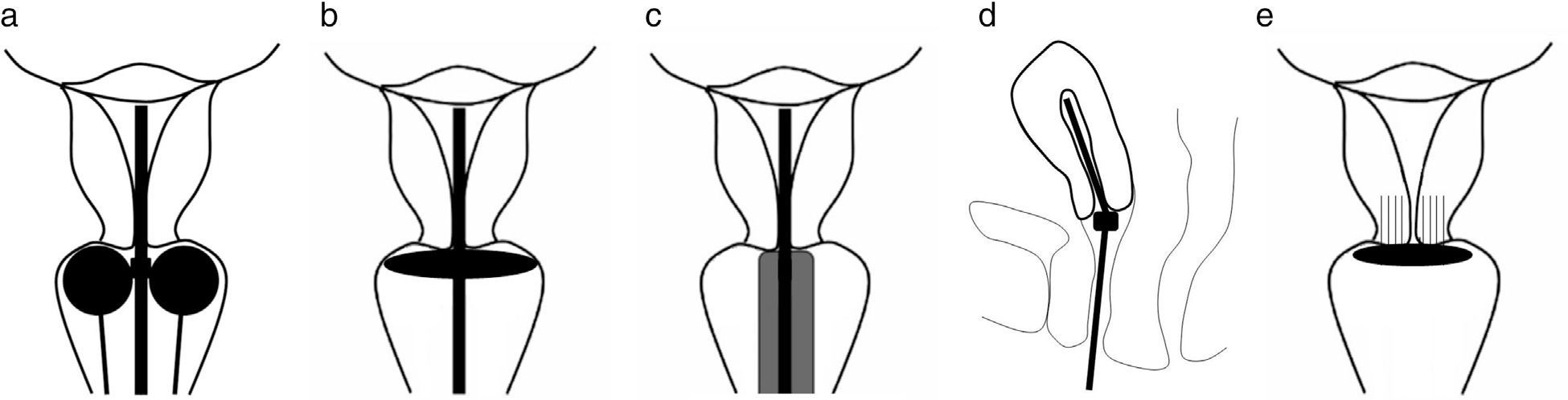
The applicators used in intracavitary brachytherapy can be metallic or non-metallic. Among them we can find, for example, a uterine tandem with a vaginal ring, a uterine tandem with vaginal ovoids or a uterine tandem with a vaginal cylinder3 (Fig. 2). The type of applicator used depends on the preferences of the radiotherapist, the patient's comfort and anatomical considerations (in cases of patients who have been hysterectomized, the uterine tandem cannot be used; therefore, only a vaginal cylinder is used). At the Texas Southwestern University a titanium uterine tandem with vaginal ovoids is generally used. The applicators have canals through which the radioactive source is introduced. The ideal brachytherapy applicator should be simple, comfortable for the patient, tension-resistant, cheap, lasting, safe, non-toxic, sterilizable, compatible with high radiation doses (both pulsed and low), with materials to fasten it and relocate it, easy to identify in the CT and MRI, and it should cause as little artifacts as possible.6
Schematic drawings of correctly deployed applicators. (a) Uterine tandem with vaginal ovoids; the marginal ovoids need to be placed next to the external cervical orifice. (b) Uterine tandem with ring. (c) Uterine tandem with cylinder. (d) The edge of the uterine tandem needs to be placed in the uterine fundus. (e) Applicator of interstitial brachytherapy with needles inserted in the cervical stroma.
The goal of planning brachytherapy is to define the target volume to apply the maximum radiation to the tumor and thus minimize the dose in normal adjacent tissues.7 At the beginning of the use of brachytherapy for treating cervical cancer, planning was made via simple X-rays.3,8 Once the applicator was placed, an anteroposterior X-ray of the pelvis was obtained and the reference point was located, it was called “point A”, which is located 2cm above the ovoids and 2cm laterally to the applicator. This point was used as anatomic reference to standardize the radiation dose.2,5 It did not take into consideration, however, the characteristics of the tumor or the patient's anatomy. This approach was simple, quick and generally safe, but it did not allow obtaining optimal results in all the cases, planning was not individualized specifically for the patient's anatomy or the tumor in question.
High-risk clinical target volume (HR CTV) includes high-risk recurrence areas due to persistence of macroscopic residual tumor after external radiotherapy and it also includes the entire cervix. The total dose applied to this volume must be the highest possible to be able to eradicate the entire macroscopic residual tumor. Intermediate-risk clinical target (IR CTV) includes areas where there was macroscopic tumor at the moment of diagnosis (before the external radiotherapy) but not at the beginning of the brachytherapy, therefore, they are areas with potential microscopic residual disease. The planning target volume (PTV) includes the clinical target volume, both the high-risk and the intermediate-risk ones, with a safety margin that considers possible movements of the patient and inaccuracies in the administration of brachytherapy.2,7
Image modalities: pros and limitationsComputed tomographyCT locates accurately the intracavitary applicator, defines the thickness of the uterine wall and shows the anatomic relations of the applicator with the neighboring structures.11 The main limitation of CT is that it does not visualize tumor volume very well due to a low contrast level in soft tissue consequently making it difficult to distinguish the cervical tumor from the uterus and define the limit between the cervix and the vagina, as well as to mark out accurately the bladder and the rectum.8 The latter distinction is particularly difficult in patients with perivaginal plexus atrophy and scarce pelvic fat in whom the anatomic separation planes between the vagina, the cervix, the uterus and/or the tumor with the bladder and the rectum can be very thin or non-existent. In addition, the standard metal applicators can cause artifacts in the CT images, which can make it difficult to determine tumor volume, the anterior rectum wall and the posterior bladder wall.
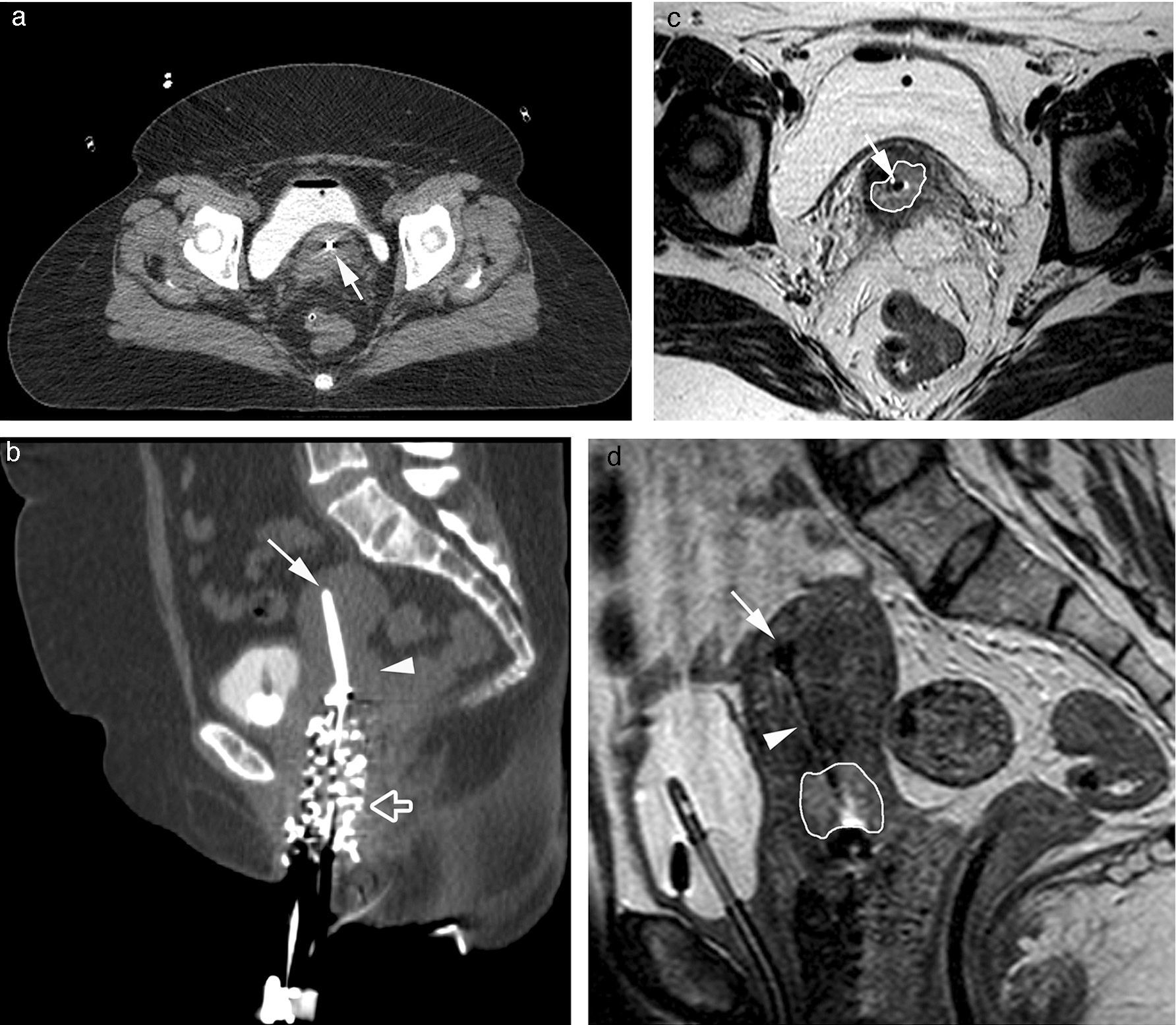
Magnetic resonanceMRI presents advantages and drawbacks as opposed to CT when planning brachytherapy.8,12–14 MRI presents better soft tissue contrast, which provides better definition of the size and location of the cervical cancer, which is moderately hyperintense in T2-weighted sequences with respect to the normal cervical stroma, and it allows us to distinguish it from the normal uterus (Fig. 3). Also the MRI facilitates the visualization of adjacent tissues and other conditions that make it difficult to mark out the tumor in the CT scan (Fig. 4). Optimization of planning via MRI improves significantly the dosimetry applied to the rectum, the sigma and the bladder compared to treatment after conventional planning with X-rays, especially in patients with small tumors at the time of diagnosis or those whose size decreases significantly after external radiotherapy.15
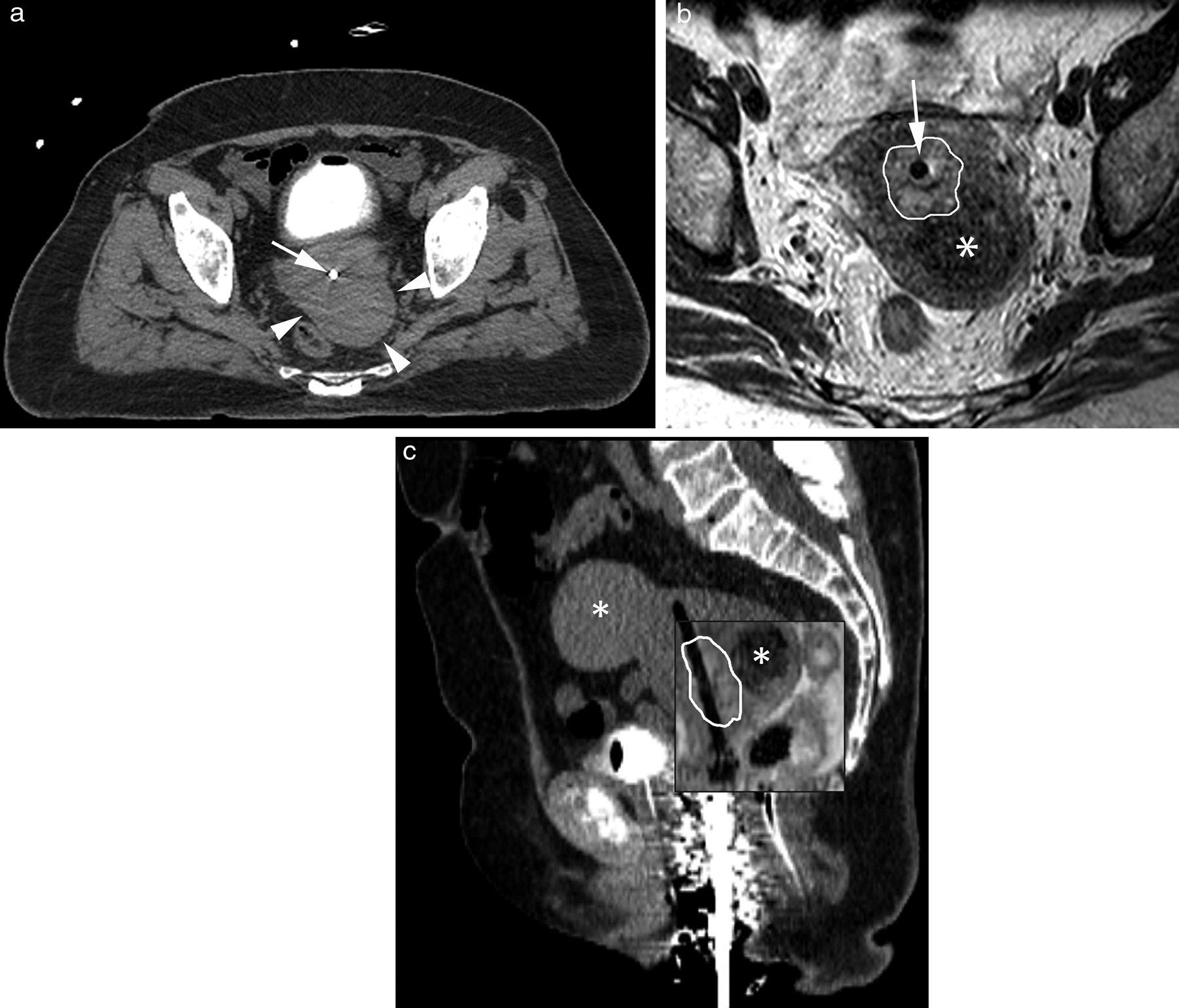
Images of high dose-intracavitary brachytherapy planning in a patient with cervical cancer. Axial (a) and sagittal (b) CT images where the uterine tandem (white arrow) showing beam hardening artifacts in the cervix. The tumor in the cervix cannot be identified (white arrowhead). With the help of a Foley catheter the bladder partially filled with contrast and vaginal packing (hollow white arrow) can be seen here. In the T2-weighted 2D FSE axial (c) and sagittal (d) MRIs the uterine tandem (white arrow) can be seen and the tumor is precisely marked out showing one intermediate signal in the T2-weighted sequences (white line) surrounded by the hypointense cervical stroma yet the visualization of the uterine tandem (white arrowhead) and the uterine tandem edge inside the uterine fundus (white arrow) is worse.
Planning images of high-dose intracavitary brachytherapy in one patient with cervical cancer. Axial (a) CT image where the uterine tandem of the applicator (white arrow) and one mass located at the left posterior-lateral side of the cervix can be seen (white arrowheads). T2-weighted axial 2D FST image (b) showing the uterine tandem of the applicator (white arrow) and cervical cancer spread (white arrow) while confirming that the left posterior-lateral cervical mass observed in the TC scan is to a fibroma (asterisk). In the fused sagittal images of CT and MRI (c) the tumor spread can be accurately identified in the MRI (white line). Two (2) different fibromas (asterisks) can be identified too, the bladder using the Foley catheter and the vaginal packing material.
Among the drawbacks of MRI as opposed to CT, the following stand out: greater image acquisition time of MRI, worse definition of bone anatomy than CT, worse delimitation of the applicator, especially its tip and the need to use MRI-compatible applicators – more expensive than conventional applicators6.
Positron emission tomographySome authors propose using PET to plan brachytherapy.6,10 This modality has the advantage of not requiring specific applicators and the images can be fused with those of CT to improve spatial resolution. Nevertheless, its drawback is that non-tumoral inflammatory tissue can show uptake of 18F-fluorodeoxyglucose6 and lead to the resulting error in the planning of brachytherapy. PET-CT has, in addition, the same drawback as the CT scan in its difficulty to mark out adjacent anatomic structures. In our own experience, the low resolution of PET can make it difficult to identify subtle tumor spread, for example, in the parametrium.
Technical considerations in magnetic resonance for the planning of brachytherapyMRIs provide information about tumor spread and it helps locate adjacent structures in the three dimensions of space, all of it with the intracavitary applicator placed in its therapeutic position. It is recommended to perform an MRI prior to the beginning of external radiotherapy treatment and another MRI before brachytherapy with the applicator being placed in its usual therapeutic position.
ApplicatorsThe applicators that are considered “conditional”, and therefore usable in the MRI setting are non-metallic and made out of titanium.9 Plastic applicators, for example, do not interfere with the magnetic field and appear as a signal void in the MRIs. To facilitate the detection of the applicator, the canals can be filled with a copper sulfate (CuSO4) solution, which reduces the relaxation time of water though the small volume that can be used due to the small size of the canals can limit the signal in the MRI. T1-weighted gradient echo images allow a better the visualization of the applicator with CuSo4 compared to T2-weighed spin echo sequences.9 These applicators with CuSo4 can be reused though the signal degrades approximately 3 months after its initial use.9
Titanium applicators are safe in magnetic fields of up to 1.5T, but cases of applicator heating and displacement have been described in studies with phantoms in 3T magnetic fields.16 Reconstructing the MRIs of titanium applicators is more complicated than the MRIs os plastic ones because they cause susceptibility artifacts, particularly in the areas where the applicator is thicker (end of the tandem, ovoids and ring), in addition, it renders the use of the CuSO4 solution in the canals useless. The susceptibility artifact can be minimized when the applicator is placed parallel to the main magnetic field.9,17,18 Fusing MRI and CT images facilitates the visualization of the applicator tip.
Getting the patient readySedation or general anesthesia is used to deploy the applicator.5 It is not necessary to carry out intestinal preparation before the procedure. The authors place the applicator under mild sedation (midazolam fentanyl) in the Department of Radiotherapeutic Oncology which is next to the MRI area in the Department of Radiology. The tandem must be placed in the uterine cavity with the distal end in the fundus of the uterus and the ovoids in the vagina next to the external cervical orifice (Fig. 2). After deploying the applicator, the vagina is packed with cotton gauzes to keep the tandem in the desired position and thus prevent displacement when the patient is moved. Once the position of the applicator has been secured, the patient is transferred on a stretcher to the MRI ward and placed in supine position on the MRI table. It is essential for the patient to be in a similar position during the MRI and CT acquisitions, so that the images are correctly be fused as well as for the treatment session. Bladder refilling must be similar during the acquisition of the planning MRI and CT images. In most cases, a vesical catheter (Foley) allows us to keep the bladder collapsed during the MRI and the CT and the treatment. Bladder distension is useful, however, when the image scans prior to the treatment show small intestine loops near the uterine cervix. In these cases, the authors distend the bladder using 200ml of diluted iodized contrast (Isovue, 10% in saline solution). The distended bladder, with liquid content inside with high signal intensity in T2-weighted images and high attenuation in the planning CT, displaces the small intestine upwardly thus diminishing the possibility of radiation toxicity. Once the MRI examination is over, the patient is once again transferred to the Department of Radiotherapeutic Oncology for a pelvis CT examination and the subsequent treatment with special care so that the applicator does not move during the transfer.
Magnetic resonance image protocol for the planning of brachytherapyA surface antenna is used in the pelvis to increase the signal-noise relation of the region of interest. The basic sequences in the image protocol are T2-weighted bi-dimensional (2D) fast spin echo (FSE) sequences because soft tissue contrast inherent to these acquisitions offers optimal visualization of the tumor, the neighboring structures and the applicator.6,9 The tumor is, in general, slightly hyperintense when it comes to the unaffected cervical stroma. Signal hyperintensity of the unaffected cervical stroma in these sequences provides an anatomic model to determine tumor spread outside the cervix.6 It is better to use FSE sequences as opposed to conventional spin echo sequences because they require less acquisition time.6 T2-weighted FSE sequences without fat saturation, on both the axial and sagittal planes, enable us to identify tumor spread in the parametrium, as well as visualize the posterior bladder wall and anterior rectum wall and their position with respect to the tumor thanks to the signal hyperintensity of fat planes.6 The authors usually use 2D acquisitions on the axial plane or axial reconstructions of the 3D sagittal acquisition (see below) to trace the target volume since it is very easy to fuse these images with CT axial images. Sagittal images or multiplanar reconstructions on other spatial planes are useful to clarify tumor spread in the cranial–caudal direction and to avoid partial volume problems with the fornices of the vagina oriented horizontally due to the position of the ovoids.
In general, T1-weighted echo gradient sequences are not necessary but they are useful in some cases to evaluate the presence of pelvic adenopathies or characterize soft tissues. A better uterus-tandem contrast has been described with weighted sequences in proton density compared to the T-2 weighted acquisitions.19 However, if the T2-weighted FSE images are fused with CT images routinely, the benefit in the visualization of the tandem is probably minimal.
Recently the use of diffusion images (DWI) has been proposed with echo-plane spin echo sequences to mark out tumor volume. It has been suggested that the area showing restriction on the map of the apparent diffusion coefficient (ADC) corresponds with the metabolically active tumor volume in the PET-CT.20 Even though the DWI sequences are part of the image protocol, in our experience the distortion caused by the presence of the tandem can restrict the acquisition of images with enough quality to be able to mark out the tumor correctly. It is difficult to assess the impact that this distortion can have on the ADC quantification of the tumor. Moreover the correlation between tumor volume on the ADC map and volume of the metabolically active tumor in the PET-CT can be due to the limited spatial resolution of both modalities. However it is possible that new breakthroughs in DWI sequences, such as FSE acquisitions and acquisitions with limited fields of view can solve both problems partially. Similarly we can anticipate that the PET spatial resolution will improve with the recent advances seen in detector technology.
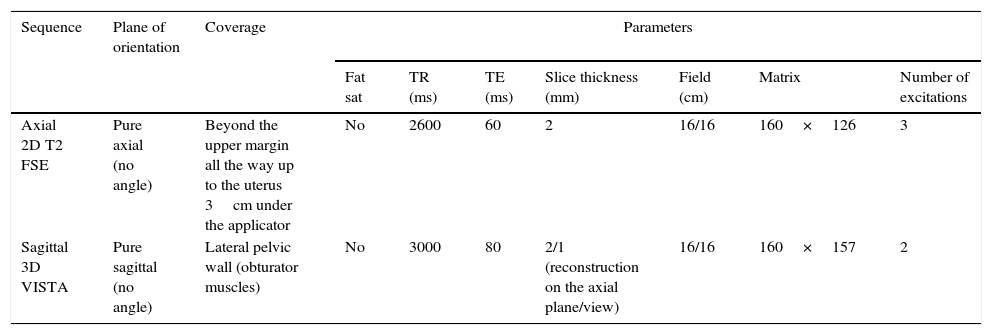
The Texas Southwestern University image protocol includes 2D FSE axial sequences and 3D FSE sagittal sequences, both T2-weighted (Table 2). The main advantage of 3D sagittal sequences as compared with 2D acquisition is the possibility to make multiplanar reconstructions with good spatial resolution on all planes, facilitated by the interpolation in the direction of slice thickness (3D acquisition with a slice thickness of 2mm and interpolated to generate 1mm-thick slices). However, in our experience, axial reconstructions of the 3D acquisition do not replace 2D acquisitions on the axial plane due to a greater vulnerability to movement and the less spatial resolution on the axial plane of the former ones. We consider therefore that 2D and 3D acquisitions are complementary.
Magnetic resonance protocol for the planning of intracavitary brachytherapy in the management of cervical cancer used by the Texas Southwestern University.
| Sequence | Plane of orientation | Coverage | Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fat sat | TR (ms) | TE (ms) | Slice thickness (mm) | Field (cm) | Matrix | Number of excitations | |||
| Axial 2D T2 FSE | Pure axial (no angle) | Beyond the upper margin all the way up to the uterus 3cm under the applicator | No | 2600 | 60 | 2 | 16/16 | 160×126 | 3 |
| Sagittal 3D VISTA | Pure sagittal (no angle) | Lateral pelvic wall (obturator muscles) | No | 3000 | 80 | 2/1 (reconstruction on the axial plane/view) | 16/16 | 160×157 | 2 |
Lastly, it is important to keep the total time of MRI examination as short as possible, since the patients with the applicator deployed in therapeutic position tend to feel uncomfortable with the passing of time. Applicator displacement caused by the patient's movement renders the information provided by the MRI study useless; hence it is important to use a short MRI image protocol, one that provides the information necessary to plan treatment.
Picking up the magnetic field: 1.5T vs. 3T1.5T MRIs offer optimal quality for the acquisition of images to plan cervical cancer brachytherapy. The use of 3T MRI is limited due to image distortion and the presence of artifacts caused by the applicator. Also the potential for applicator heating during the execution of the study is also problematic in 3T MRIs.16 It is likely that, in the future, enhancements in MRI sequences that use lower energy levels and/or image parameters that correct the geometric distortion caused by the applicator will allow us to perform MRI examinations in 3T machines with a similar or even greater image quality than 1.5T machines. Also it is possible that the use of non-metallic applicators will facilitate the incorporation of 3T MRI in this clinical application.
Fusion of computed tomography and magnetic resonance imagesEven though the MRIs can be used as a single modality to plan brachytherapy,2,8,9 this proposition has limitations due to its inability to delimit accurately the edge of the applicator via MRIs.9 This setback can be overcome to a large extent by using MRIs and CTs by fusing the images generated by both modalities.
Two planning studies are being conducted at the Texas Southwestern University: that of MRI followed by CT. After completing the MRI examination with the applicator in therapeutic position, the axial CT images for planning are obtained without IV contrast, with a slice thickness of 2mm (in some occasions with the bladder filled up with contrast, as described before). Then the MRI and CT images are fused, treatment is planned and the first fraction of brachytherapy is administered.
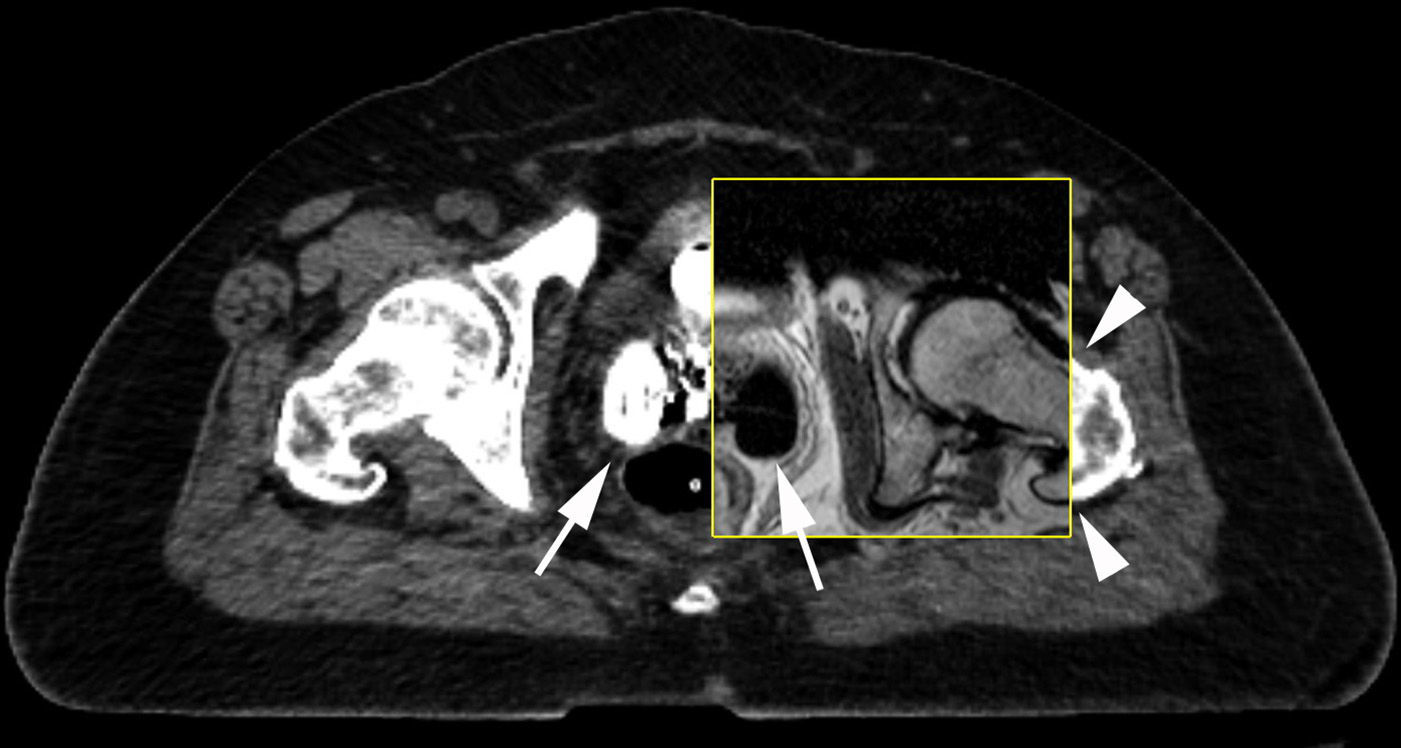
To fuse CT and MRI images, they are imported onto a workstation using a specific software for this application. In general, the sagittal MRIs are used plus a sagittal reconstruction of the planning CT for the anatomic register of the two examinations, since the latter offers a better identification of the intracavitary applicator (Fig. 5). The guide point for the fusion is the area where the tandem and the ovoid of the applicator join that needs to be adjacent to the external cervical orifice. This anatomic model is used because it is easy to identify, has a fixed shape and it is in the vicinity of the high-risk clinical target volume. After aligning the images on the sagittal plane, the correct overlapping of the images on the MRI and CT axial plane is confirmed (Fig. 6), which is used as described before to draw up the target volume (Fig. 7).
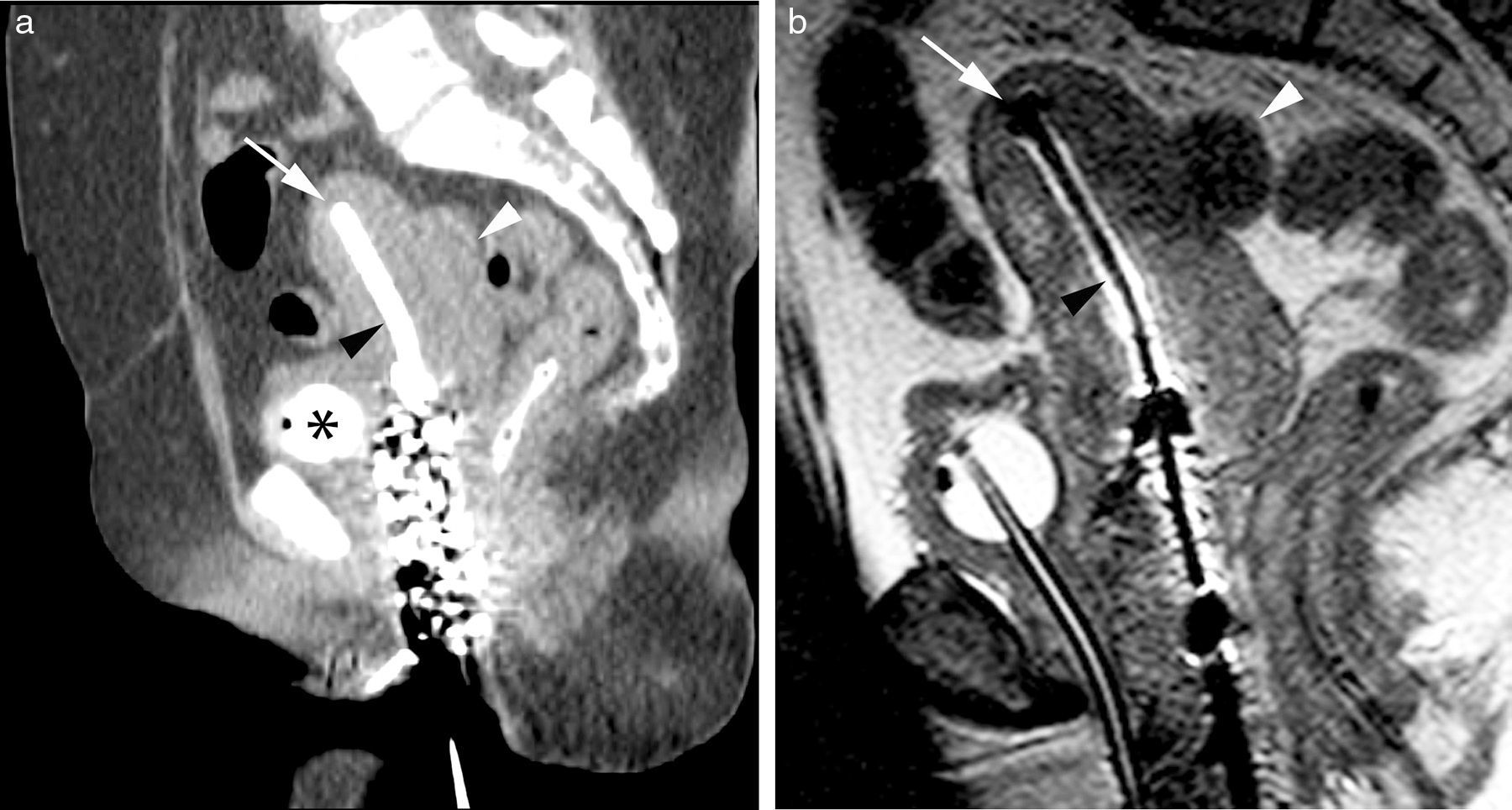
Planning images of high-dose intracavitary brachytherapy in one patient with cervical cancer. Sagittal CT reconstruction (a) showing the uterine tandem (black arrowhead) with the edge (white arrow) in the uterine fundus. The posterior uterine wall shows one lobulated edge (white arrowhead) that could be compatible with tumor spread. The bladder has one Foley catheter with the inflatable balloon filled with iodinated contrast (asterisk). The vaginal packing material can be seen. The T2-weighted sagittal 2D FSE sequence (b) of the same patient shows the uterine tandem (black arrowhead) with a poor definition of its edge due to susceptibility artifact (white arrow). However the uterine wall is better defined with a clear distinction between such wall and the adjacent sigmoid colon (white arrowhead).
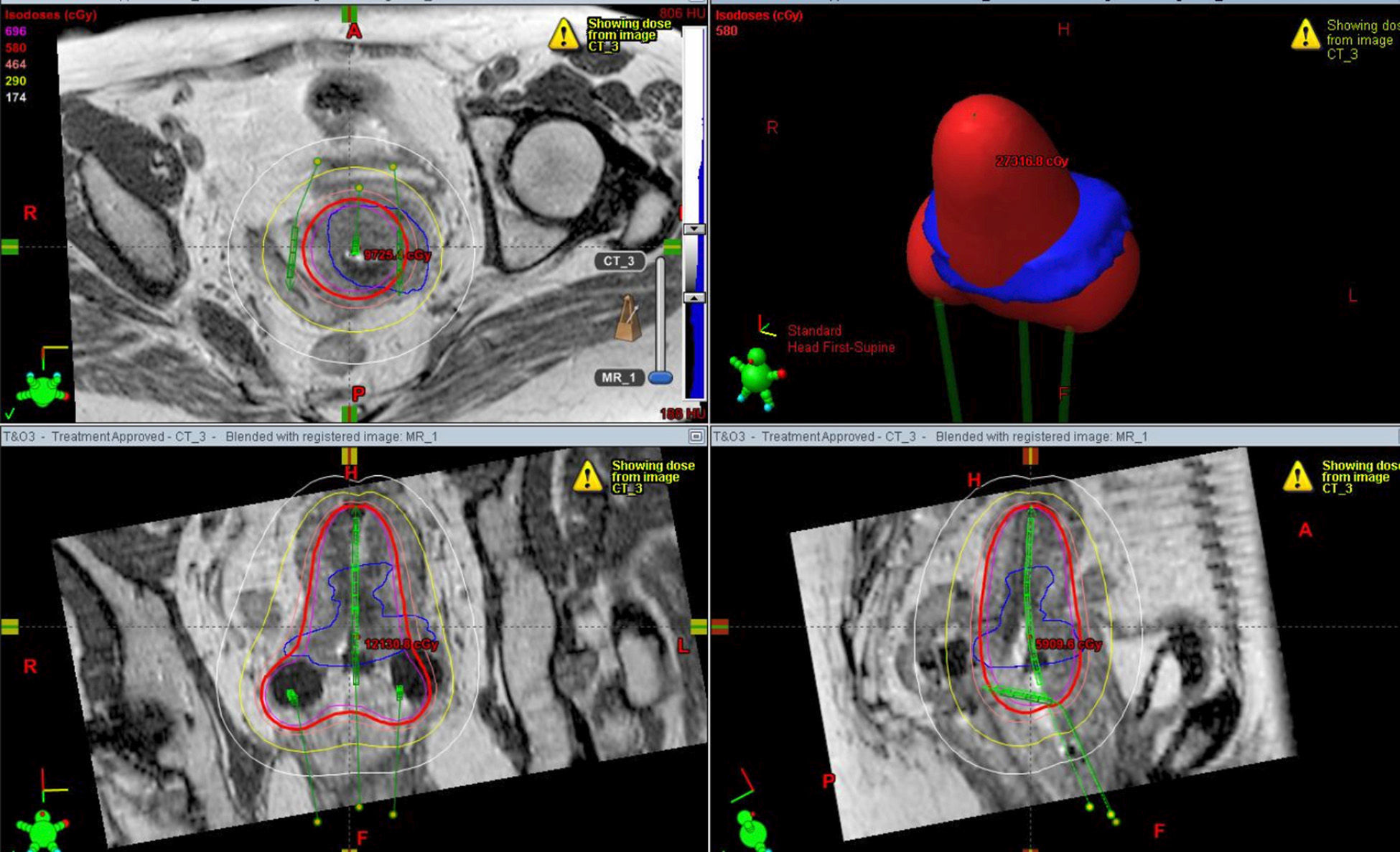
Example of MRI multiplanar planning of intracavitary brachytherapy of cervical cancer. The blue line shows the outside edge of the tumor while the red line shows the isodose of the prescribed radiation. The applicator of the intracavitary brachytherapy is marked out in green. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
For the following brachytherapy sessions a new planning CT is performed in each visit, and each of these CT examinations is fused with the initial MRIs using as point of reference for the fusion the same junction area between the applicator tandem and ovoid. Overall the MRI examination is not repeated. After the fusion, the brachytherapy is planned by identifying the applicator in the CT images and determining the target volume in the MRIs.
ConclusionThe MRIs for the planning cervical cancer brachytherapy provide essential information about the tumor and the location of other important adjacent anatomic structures. The systematic analysis of the finding with the applicator deployed in therapeutic position offers a better definition of the target volumes compared to conventional planning, which allows the optimization of the dose administered to the tumor and reduces the dose administered to the rectum, sigma and bladder.
Visualization of the applicator through MRIs is limited but it can be improved fusing the MRI with CT images using specific software. It is possible that other MRI sequences, such as those weighted in proton density allow us to improve the visualization of the applicator. It is desirable for the sake of technological development that these MRI limitations are overcome in the future so that use of this modality can be generalized as a single modality in the planning of brachytherapy in patients with cervical cancer.
The success of implementing a planning protocol with MRI for cervical cancer brachytherapy requires adjustments in MRI protocols and considerations about the safety measures and logistic needs in the MRI ward during the management of patients with the intracavitary applicator deployed in therapeutic position. It is essential to have a multidisciplinary team with the collaboration of the Department of Images and Radiotherapeutic Oncology.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Data confidentialityThe authors declare that they have followed their center protocols on the publishing of data from patients.
Right to privacy and informed consentThe authors have obtained the written informed consent from patients and/or subjects referred to in this paper. This document belongs to the corresponding author.
Conflict of interestsThe authors declare no conflict of interests.
Authors/contributors- 1.
Manager of the integrity of the study: MOM, IP.
- 2.
Study idea: IP.
- 3.
Study design: IP.
- 4.
Data mining: MOM, DFP, ZW, KA.
- 5.
Data analysis and interpretation: MOM, DFP, IP.
- 6.
Statistical analysis: not applicable to this paper.
- 7.
Reference search: MOM, DFP, IP.
- 8.
Writing: MOM, IP.
- 9.
Critical review of the manuscript with intellectually relevant remarks: DFP, ZW, KA, IP.
- 10.
Approval of final version: MOM, DFP, ZW, KA, IP.
Please cite this article as: Oñate Miranda M, Pinho DF, Wardak Z, Albuquerque K, Pedrosa I. Resonancia magnética en la planificación de la braquiterapia intracavitaria para el tratamiento del cáncer de cérvix localmente avanzado. Radiología. 2016;58:16–25.