Life-threatening hemoptysis is a severe condition that requires rapid diagnosis and treatment. One of the treatments of choice is embolization. The initial assessment aims to locate the origin and cause of bleeding. The technological advance of the development of multidetector computed tomography (MDCT) has changed the management of patients with life-threatening hemoptysis. MDCT angiography makes it possible to evaluate the cause of bleeding and locate the vessels involved both rapidly and noninvasively; it is particularly useful for detecting ectopic bronchial arteries, nonbronchial systemic arteries, and pulmonary pseudoaneurysms. Performing MDCT angiography systematically before embolization enables better treatment planning. In this article, we review the pathophysiology and causes of life-threatening hemoptysis (including cryptogenic hemoptysis) and the MDCT angiography technique, and we review how to systematically evaluate the images (lung parenchyma, airways, and vascular structures).
La hemoptisis amenazante es una situación grave que precisa de un diagnóstico y tratamiento rápidos. Uno de los tratamientos de elección es la embolización. La evaluación inicial se dirige a localizar el origen y la causa del sangrado. El avance tecnológico de la TC multidetector (TCMD) ha supuesto un cambio en el manejo de estos pacientes. La angio-TCMD permite evaluar la causa rápida e incruentamente, y localizar los vasos implicados; es particularmente útil para detectar arterias bronquiales ectópicas, arterias sistémicas no bronquiales o seudoaneurismas pulmonares. Hacer sistemáticamente una angio-TCMD antes de la embolización permite planificar mejor el tratamiento. En este artículo revisamos la fisiopatología y las causas de la hemoptisis amenazante (incluyendo la hemoptisis criptogenética), la técnica del estudio de la angio-TCMD y describimos cómo evaluar sistemáticamente las imágenes (parénquima pulmonar, vía aérea y estructuras vasculares).
Hemoptysis is expectoration of blood coming from tracheobronchial tree or the pulmonary parenchyma.1 Depending on the amount expectorated, it is classified as massive or non-massive. Although there is not a unanimous definition differentiating them, hemorrhage surpassing 400–600ml in 24–48h or that surpassing 100–200ml in 1h is usually classified as massive.2 Considering that quantifying bleeding is difficult, it is clinically more useful to use the term life-threatening hemoptysis to define a situation in which there is immediate risk for the patient's life.3 Immediate risk is compromise of airway, therefore, clinical significance of an hemoptysis episode should take into account not only the volume of blood expectorated, but also its effect on the respiratory and cardiovascular reserves.3,4 Although it stands for only a small proportion of all the hemoptysis cases, life-threatening hemoptysis treated inadequately has a mortality rate surpassing 50%.5 Urgent surgical intervention also has high morbimortality, and that is why embolization is at present the treatment of choice in most cases.6,7
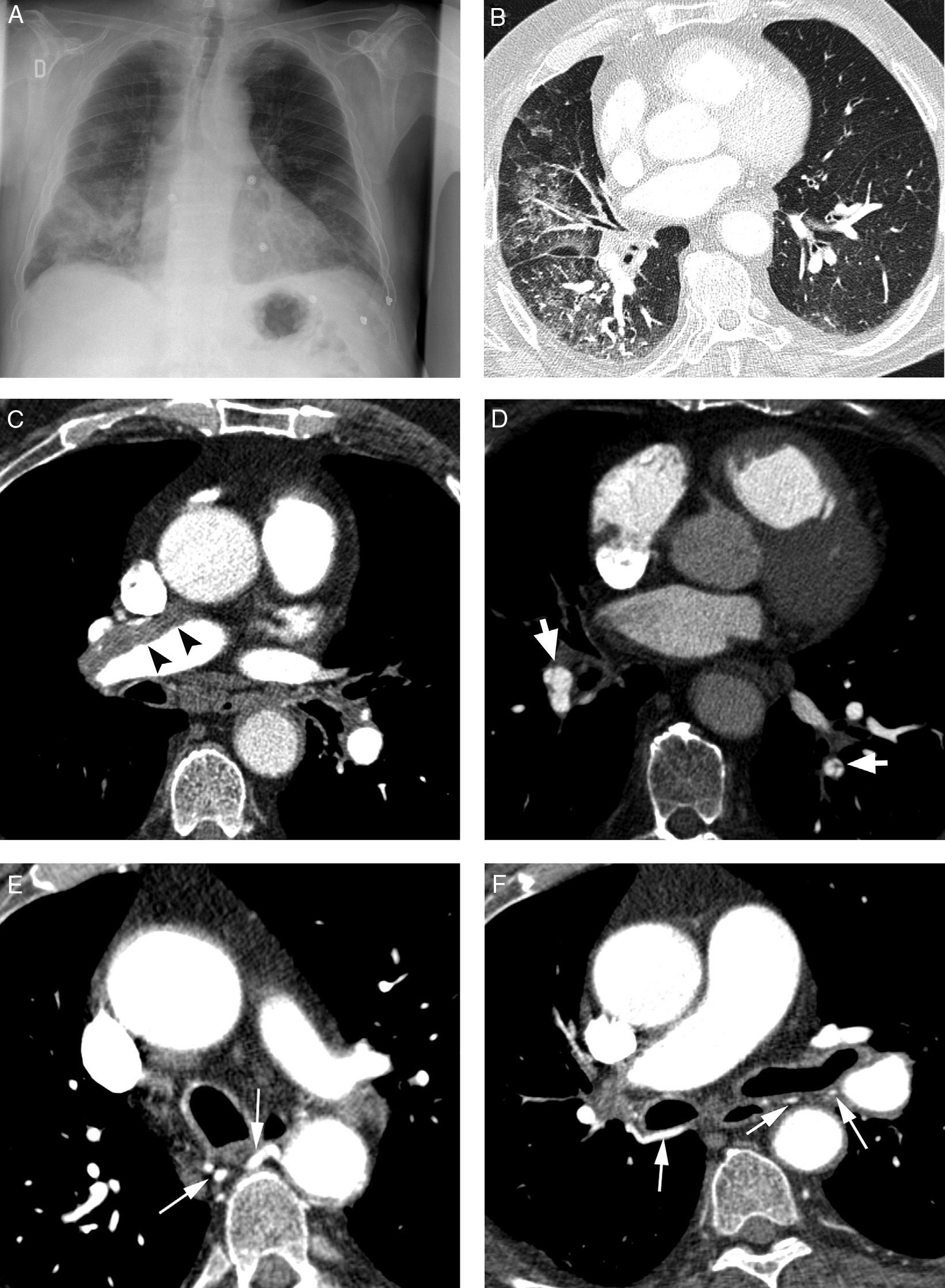
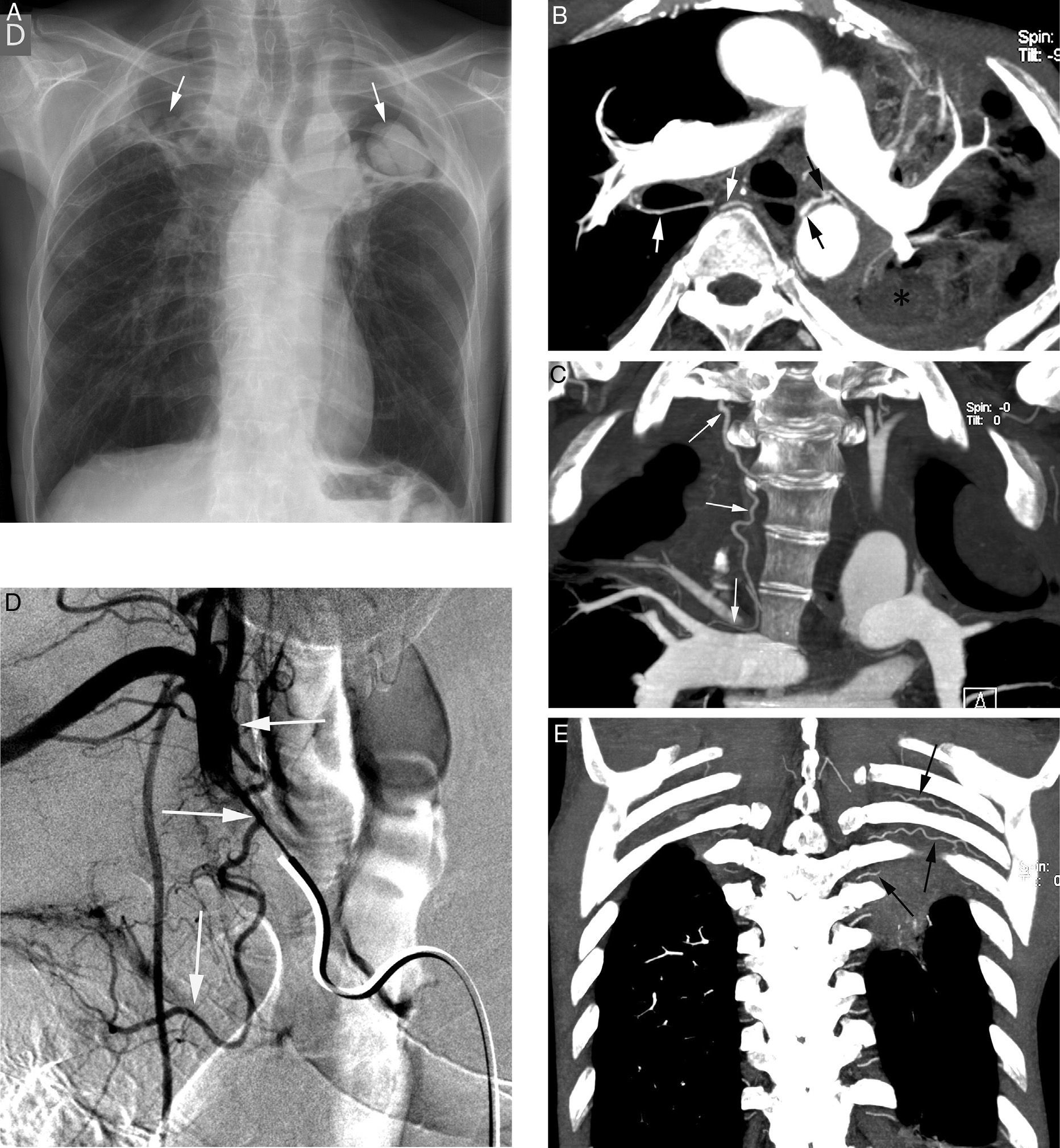
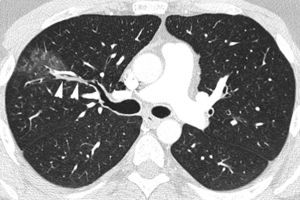
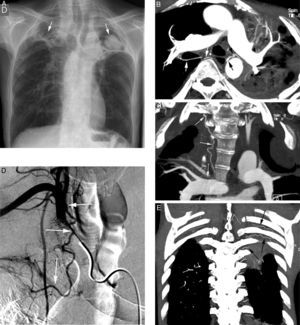
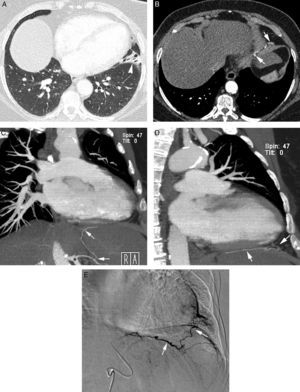
Initial evaluation of life-threatening hemoptysis patients aims at locating the origin and the cause underlying bleeding. The intervention protocols vary depending on the center and they should include urgent availability of CT, fibrobronchoscopy, arterial embolization and, eventually, surgical treatment.8 In our hospital, the first examination performed on a patient with life-threatening hemoptysis is a thorax radiography (which in many cases locates the bleeding area) (Fig. 1A), followed by a thoracic angio-CT (there is no possibility of urgent fibrobronchoscopy), which will serve as a guide for treatment, which in most cases will be embolization; fibrobronchoscopy has been relegated to the few cases in which CT does not identify the cause of bleeding.
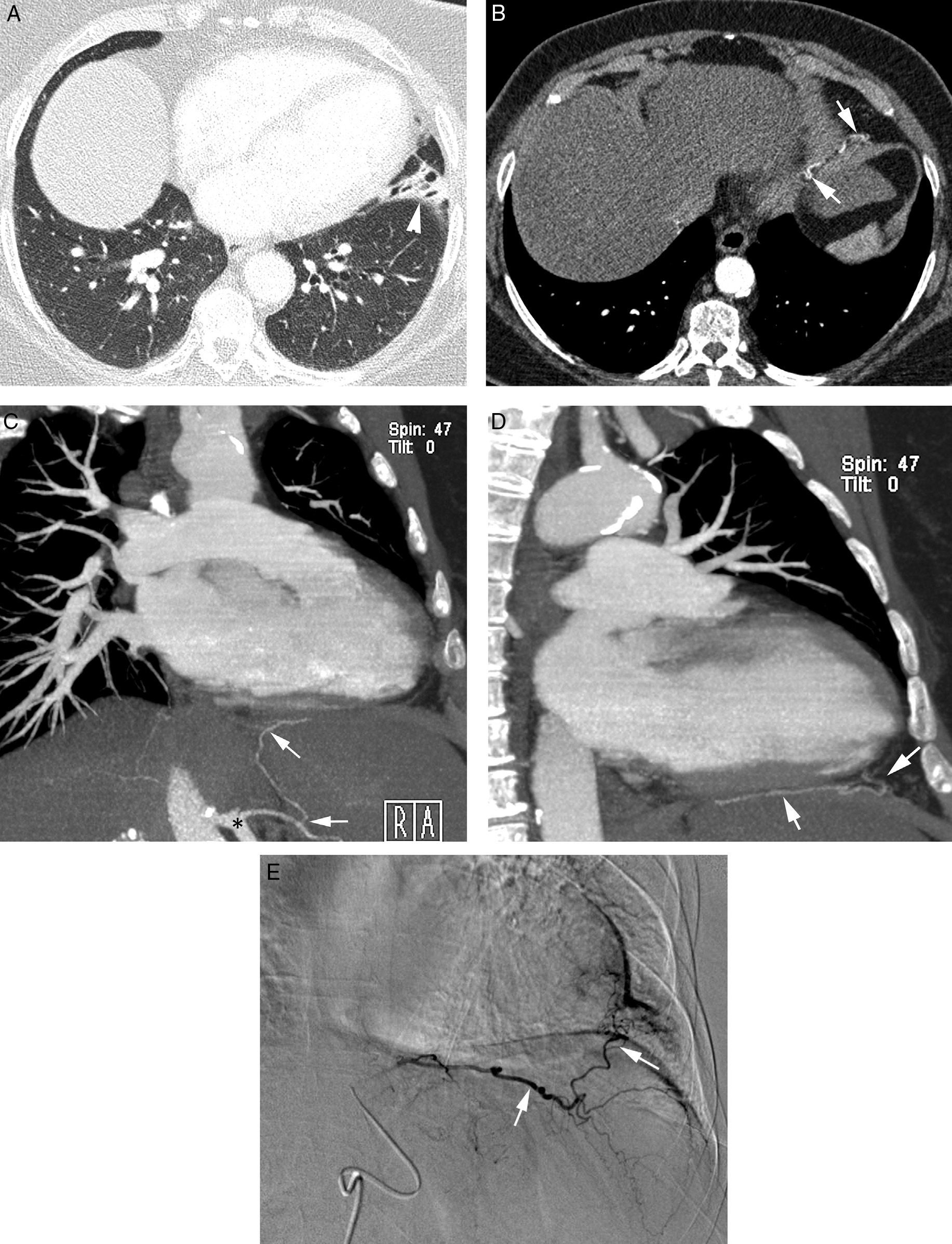
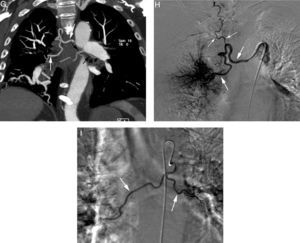
Patient with chronic pulmonary thromboembolism who comes due to life-threatening hemoptysis. (A) Thorax radiography with consolidations in the right pulmonary base corresponding to the area of bleeding. (B) The CT with lung window shows frosted-glass areas in the middle lobe and the right lower lobe. (C–H) Angio-MDCT images. Axial cuts (C and D) showing signs of chronic pulmonary thromboembolism with a large peripheral thrombus in the main right pulmonary artery (arrow heads), as well as linear images inside the lobar and segmentary branches and in the right lower lobe and the left lower lobe respectively, corresponding to residual bands (arrow). Linear and punctiform images (E and F) in the mediastinum, with contrast similar to that of the aorta (arrows), which correspond to hypertrophied bronchial arteries. The coronal MIP reconstruction (G) shows a right intercostobronchial trunk (white arrows) and a hypertrophied common bibronchial trunk (black arrows). The findings were confirmed in the arteriography, which showed the right intercostobronchial trunk's hypertrophy (H: arrows, thin arrows in the intercostal branch) and the bibronchial trunk hypertrophy (I: arrows). Observe the analogy of the arteriography and the angio-MDCT images.
Technological advances of multidetector CT (MDCT) have entailed a change in managing patients with life-threatening hemoptysis. It makes it possible to determine the location and cause of bleeding in a high percentage of the cases,9,10 to analyze in detail the mediastinum and the pulmonary parenchyma and obtain thoracic angiographic (systemic and pulmonary circulation) and upper abdomen studies, which are useful in planning embolization and, occasionally, surgical procedure.4,9,10
This article revises the physiopathology and causes of life-threatening hemoptysis (including cryptogenetic hemoptysis), the technique of angio-MDCT study and the systematic evaluation of images (pulmonary parenchyma, airways and vascular structures).
Irrigation of pulmonary parenchyma and causes of hemoptysisThe lungs have 2 independent vascular systems: pulmonary arteries and the bronchial systemic arteries. Pulmonary arteries are the main component; they provide 99% of arterial blood to the lungs and participate in the gaseous exchange. Bronchial arteries represent 1% of the cardiac output, they perform the function of nourishing multiple structures (trachea, bronchi, nerves, lymphatic ganglia, vasa vasorum of vascular structures, pleura, esophagus), and they do not participate, in normal conditions, in the gaseous exchange.4 The 2 systems create a right-left physiologic short-circuit by means of the anastomosis among pulmonary and bronchial capillaries, which represent 5% of cardiac output.4,11,12
In circumstances that steadily diminish pulmonary circulation and produce ischemia (for example, in chronic thromboembolism), bronchial circulation responds with hypertrophy and focal vascular proliferation through the anastomosis to replace pulmonary circulation; on the other hand, neoplasias and chronic inflammation (for example, bronchiectasis and chronic infections), by means of angiogenic growth factors, produce neovascularization and increase of systemic circulation. These hypertrophied neoformed systemic vessels are generally very fragile and they are exposed to systemic pressure; therefore, they tend to rupture in their most distal portion (capillary) toward the bronchial lumen or the alveoli, causing hemoptysis.13,14 In most hemoptysis cases (90%) bronchial arteries are implicated, but their origin may also be in non-bronchial systemic arteries or, on rare occasions, in pulmonary arteries.15
The underlying causes of hemoptysis vary depending on the geographic location of the studies, of tuberculosis prevalence and the use of CT.16 In our milieu, the most frequent causes of life-threatening hemoptysis are bronchiectasis, tuberculosis and its sequels, and lung cancer.17–19Table 1 contains the main causes.
Causes of life-threatening hemoptysis.
| Acquired |
| Chronic inflammation of pulmonary parenchyma |
| Bronchiectasis |
| Cystic fibrosis |
| Chronic bronchitis |
| Aspergilloma |
| Necrosis of pulmonary parenchyma |
| Tuberculosis |
| Bacterial necrotizing pneumonia |
| Septic embolisms |
| Metastatic or primary pulmonary neoplasia |
| Vascular alterations |
| Chronic occlusion of pulmonary artery: chronic pulmonary thromboembolism, vasculitis |
| Aneurysm (Behçet's disease and Hughes-Stovin's syndrome) and pulmonary artery pseudoaneurysm |
| Bronchial artery aneurysm |
| Iatrogenic or traumatic penetrating lesions |
| Misplacement of Swan-Ganz's catheter (pulmonary artery pseudoaneurysm) |
| Penetrating wounds |
| Cryptogenetic hemoptysis |
| Congenital |
| Sequestration |
| Systemic irrigation to normal lung |
| Arteriovenous malformations (Rendu-Osler's disease) |
We have at our disposal n MDCT of 16 rows of detectors (MDCT Sensation 16; Siemens, Erlagen, Germany). The parameters used are: 120kV, 70–120mAs (variable values according to caredose®), rotation time 0.42s, collimation 0.75mm and «pitch» 0.85. The thickness of image reconstruction is 1mm with a 0.7mm interval.
Acquisition is performed with the patient lying supine and in maximum inspiration, in craniocaudal direction, covering from the base of the neck to the medium third of the kidneys (at the level of the renal arteries) to include the supraaortic trunks and the infradiaphragmatic arteries.4
100ml of non-ionic iodized contrast is administered (Iopromide, Ultravist 300; Schering, Berlin, Germany), at a rate of 4ml/s, and then 20ml of saline are instilled. The study begins automatically using the bolus tracking technique. To this end, the region of interest is located in the ascending aorta, we establish a threshold of 120 UH, and the study is started 10s after the threshold is reached.
Axial cuts will be useful to detect the origin of systemic arteries. The Maximum Intensity Projection (MIP) reconstructions will be indispensable to study the tortuous trajectory of these arteries. Coronal plane reconstructions are the most adequate to study intercostal and inner mammary arteries, and the axial ones to study lower phrenic arteries and branches of the celiac trunk. Oblique sagittal plane reconstructions are also useful to study the trajectory of phrenic and mammary arteries. The degree of obliquity and the thickness of reconstructions are adapted to each case.4 MIP reconstructions of pulmonary arteries on different planes will also be necessary when it is suspected that they are affected. Multiplane and minimal intensity projection reconstructions are useful to assess permeability of airways.
Image assessmentThoracic angio-MDCT offers us a guiding map for embolization. We must assess the pulmonary parenchyma, the ariway and the mediastinum systematically (looking for the place and the cause of bleeding) (Fig. 1B, C and D), and analyze the vessels that are possibly implicated: the bronchial arteries, the non-bronchial systemic arteries and the pulmonary arteries.
Pulmonary parenchyma and airwayAssessing the pulmonary parenchyma may identify, among others, bronchiectasis, lung neoplasia and acute or chronic pulmonary infections (particularly those causing parenchyma necrosis) as the cause of bleeding.4
The most frequent signs of bleeding in the pulmonary parenchyma are centrilobular nodules, frosted glass opacities and/or condensations (Figs. 1B and 2). These findings help us identify the places of bleeding if they are focal or unilateral, and they are less helpful if affectation is extensive and bilateral20; in these cases multiplane reconstructions are useful to assess the zonal prevalence of bleeding.21 When there are cavities they may be filled with blood (Fig. 3A and B) which may conceal intracavity lesions such as mycetomas; occasionally hyperdense areas are identified due to clots.21 At times the clots may simulate nodules or masses; that is why it is advisable to conduct a control CT weeks after the hemoptysis episode to see the evolution of suspicious images (Fig. 4).4
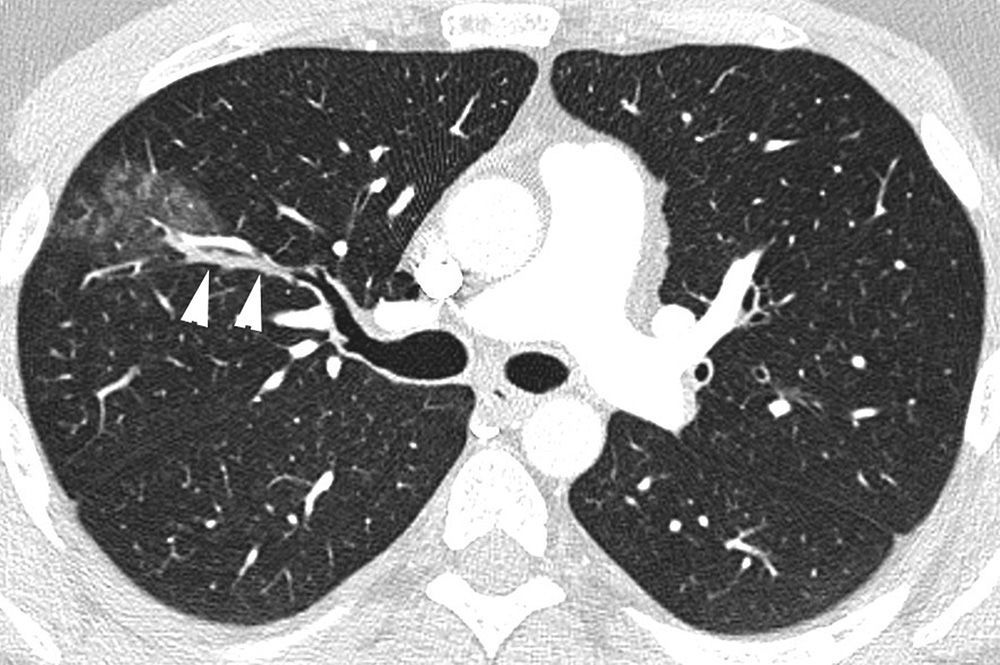
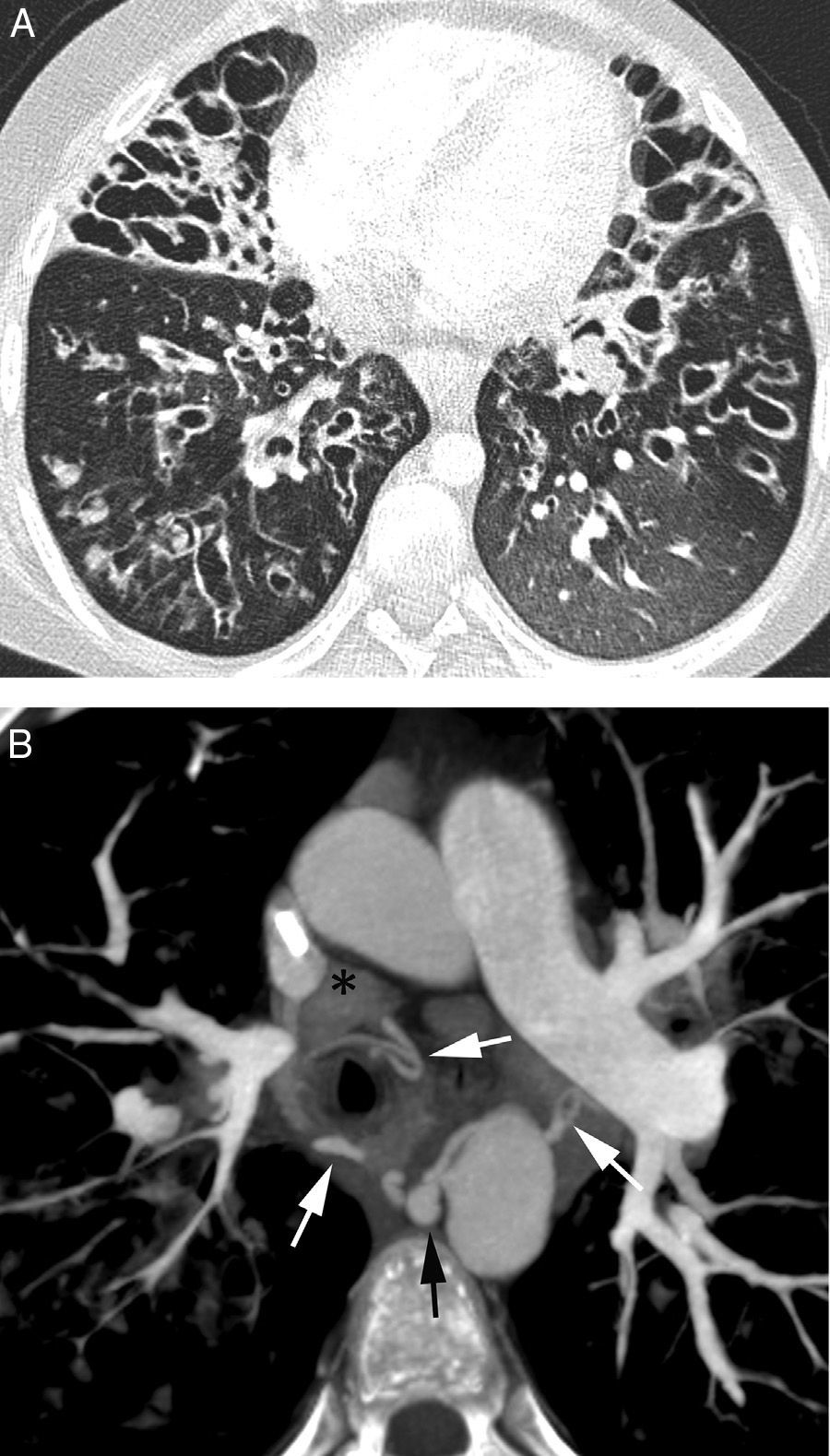
Patient who comes due to right pleuritic pain and life-threatening hemoptysis. CT with lung window showing signs of bleeding in the right upper lobe in the form of frosted glass and centrilobular nodules, with occupation of a subsegmentary bronchus (arrow heads) which runs parallel to the pulmonary artery.
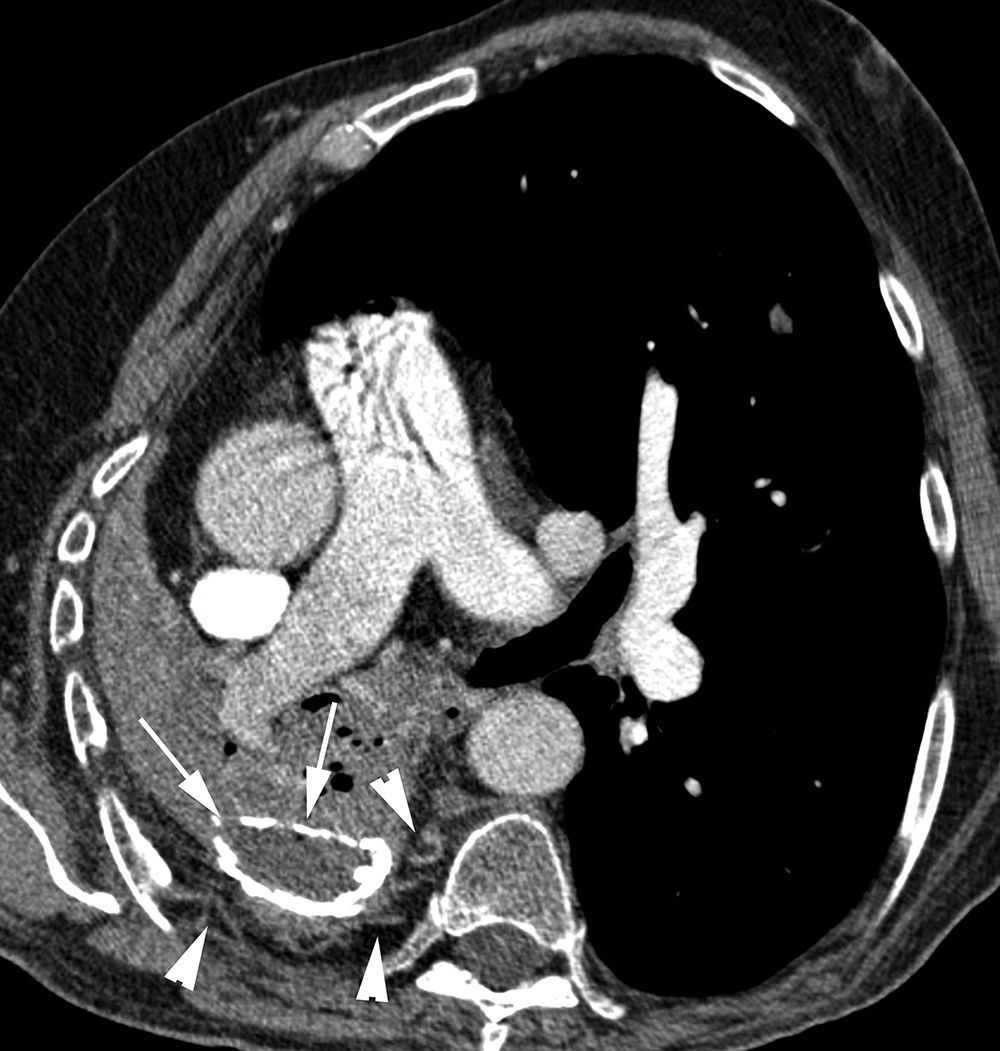
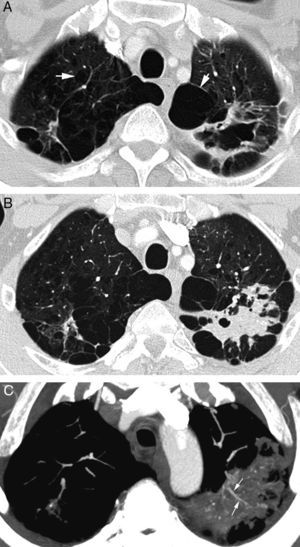
Patient in treatment for atypical mycobacterial infection presenting life-threatening hemoptysis. (A) CT (lung window) performed 5 months before the hemoptysis episode showing an irregular-wall cavitated lesion in the upper left lobe. In addition, it is possible to observe an important affectation by centrilobular and paraseptal emphysema (arrows). (B) CT (lung window) where it is possible to observe cavity occupation by the bleeding. (C) Axial MIP reconstruction, showing branches of the pulmonary arteries (arrows) in the lower peripheral portion of the cavity. Moreover, many small focal images are observed that correspond with calcified granulomas.
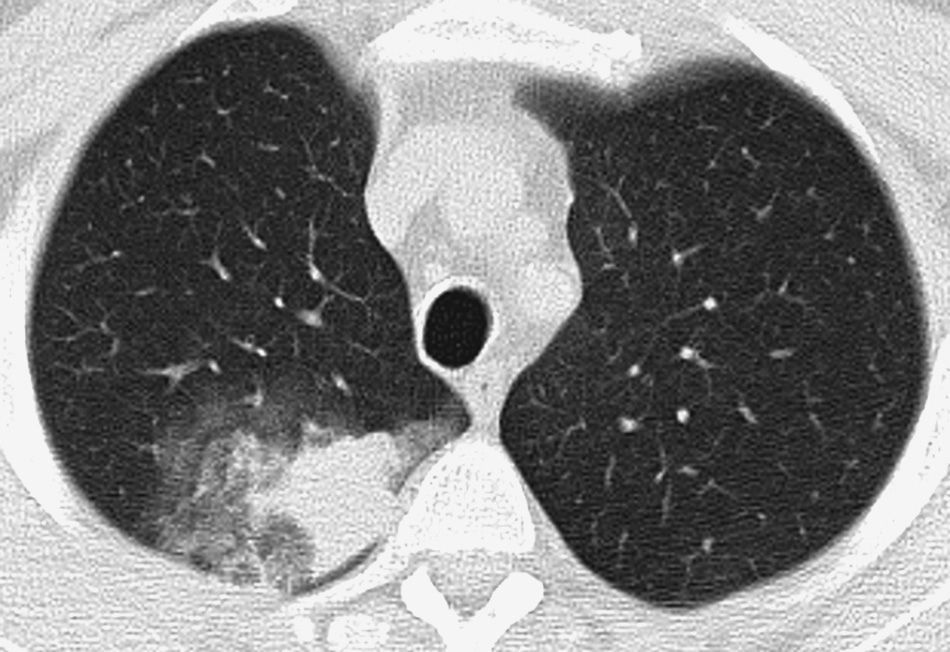
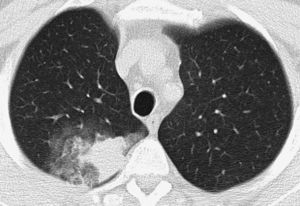
Patient with life-threatening hemoptysis. CT with lung window, where it is possible to observe a consolidation in the posterior segment of the right upper lobe, secondary to the bleeding, with an adjacent opaque glass area, findings resolved completely in the control CT performed a month later (not shown).
It is indispensable to analyze the airway's permeability minutely. If the lumen is occupied, it may be due to clots that may secondarily produce atelectasis (Fig. 2); the blood may hide small endobronchial tumors.21 On rare occasions, it is possible to observe extravasation of contrast to bronchial lumen.20
In sum, bleeding in the parenchyma and the airway may conceal the origin of hemoptysis; that is why, in case the cause is not found, it is advisable to perform a control CT a few weeks after the episode.4,21
VesselBronchial systemic arteriesIn 90% of the cases of hemoptysis, bronchial systemic arteries are the origin of hemorrhage.4,9,20,22 Bronchial arteries are those that go to the lung through the pulmonary hilius following the bronchial Tree.12 Orthotopic bronchial arteries are those that originate in the descending thoracic aorta, at the level of vertebral bodies T5–T6 (approximately the in the area of the carina). The bronchial arteries that do not originate at this level are called ectopic bronchial arteries.4,23 The arteries that do not reach the pulmonary parenchyma through the pulmonary hilius are called non-bronchial systemic arteries.
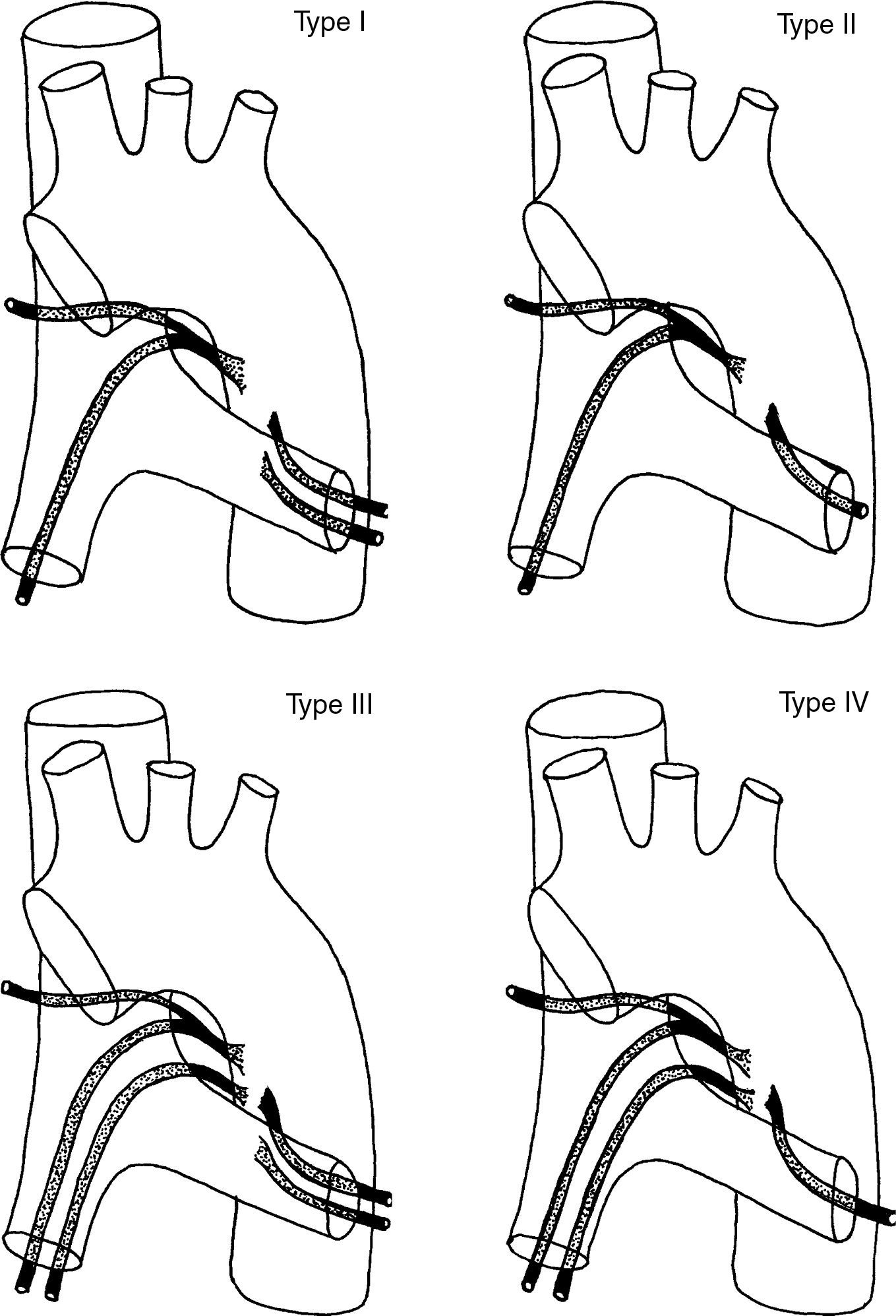
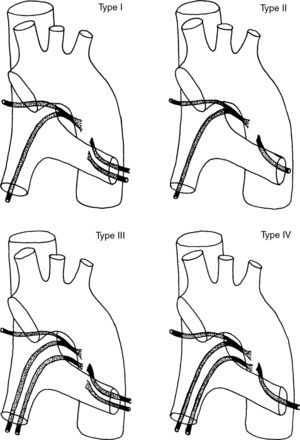
Orthotopic bronchial arteries have a very variable origin, ramification and trajectory (Fig. 5).24 The right intercostobronchial artery is the one that presents a more constant location. It is present in almost 90% of the cases and it is the easiest to identify in angio-MDCT. It originates from the right posterolateral side of the descending aorta to follow first a cranial trajectory, before it ramifies into the first right intercostal artery and the right bronchial artery, and after a caudal trajectory to reach the main right bronchus (Fig. 1G and H). Left bronchial arteries generally emerge from the anteromedial side of the aorta. There are usually 2, an upper and a lower one; its mediastinum trajectory is very short and its course is harder to see in the angio-MDCT; it is not frequent either for there to be a common right and left trunk (Fig. 1G and I).4,25,26
Schematic representation of the 4 classic ramification patterns of the bronchial arteries: Type I: 2 bronchial arteries on the left and one on the right, originated from an intercostal trunk, known as right intercostobronchial trunk (40.6%); Type II: a bronchial artery on the left and an intercostobronchial trunk on the right (21%); Type III: 2 bronchial arteries on the left and 2 on the right (an intercostobronchial trunk and a bronchial artery) (20%); Type IV: a bronchial artery on the left and 2 on the right (an intercostobronchial trunk and a bronchial artery) (18%).
In adults, the normal diameter of bronchial arteries is less than 1.5mm in their origin and 0.5mm at the point in which they enter the bronchiopulmonary segment.4,22,25 A diameter greater than 2mm is considered pathological and it indicates the artery that must be embolized, although, unfortunately, there is not a good correlation between artery size and risk of bleeding.4,23,26 Yoon et al.27, in a retrospective study comparing angio-MDCT to angiography in 22 patients with hemoptysis, showed statistically significant differences between the arteries that caused hemoptysis and those that did not; the trajectory of the ones causing it was easier to see from their origin to the pulmonary hilius than that of the arteries not responsible for hemoptysis.
In angio-MDCT we will see bronchial arteries as small nodular or linear images in the mediastinum (they are almost imperceptible if they are not hypertrophied), around the main bronchi, the esophagus and the aortopulmonary window (Fig. 1E and F). It is indispensable to perform MIP reconstructions in different planes so as to be able to see their origin and trajectory (Fig. 1G).4
Ectopic bronchial arteries may be observed in 8.3 to 35% of the cases.4,25 The most frequent ectopic origins are concavity of the aortic arc (74%), ipsi- or contralateral subclavian artery (10.5%) (Fig. 6C), abdominal aorta (8.5%), the ipsilateral brachiocephalic trunk (2%), the ipsilateral inner mammary artery (2.5%) and the ipsilateral thyrocervical trunk (2.5%).28
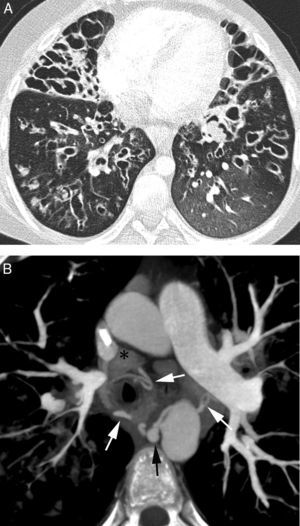
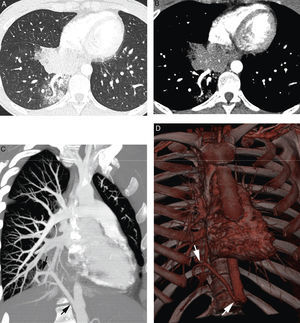
Patient with bilateral aspergillomas and life threatening hemoptysis. (A) Thorax radiography showing important volume loss of the upper lobes with large cavities in both. Nodular images are observed within the cavities (arrows) corresponding to the aspergillomas. (B) Axial MIP reconstruction where we observe hypertrophy of the right (white arrows) and left (black arrows) bronchial arteries, as well as the mycetoma (*). (C) Coronal MIP reconstruction showing an ectopic bronchial artery originating in right subclavian artery and going into the lung along the hilius (arrows). (D) Arteriography showing the ectopic bronchial artery originated from the right subclavian (arrows). (E) Posterior coronal MIP reconstruction showing in addition hypertrophy of the first left intercostal arteries (arrows) adjacent to the pleural thickening accompanying the left upper lobe aspergilloma.
In order to study bronchial arteries in MDCT the following is necessary9,26: (a) to locate the bronchial arteries ostia and see whether they are orthotopic or ectopic; (b) to describe the exit of the aortic wall (right or left anterior, posterior, lateral); it is also useful to describe atheroma plaques and the angle of the vessel with the aorta, which, if very acute, it may be difficult to catheterize; (c) to measure the diameter of the bronchial artery; and (d) to determine the total number of pathological bronchial arteries on each side.
Bronchial artery aneurysms are infrequent; they may be located in the intramediastinal portion of the bronchial artery or in the intrapulmonary portion. Angio-MDCT may show them (Fig. 7).29 Mediastinal bronchial aneurysms may cause symptoms by compression of structures. Rupture of more proximal mediastinal aneurysms may occur with acute thoracic pain that simulates an aortic dissection. Rupture of intrapulmonary aneurysms may give rise to a massive, often catastrophic hemoptysis.29,30 Embolizing the mediastinal aneurysms may be difficult if they are too close to the aorta's exit orifice.29
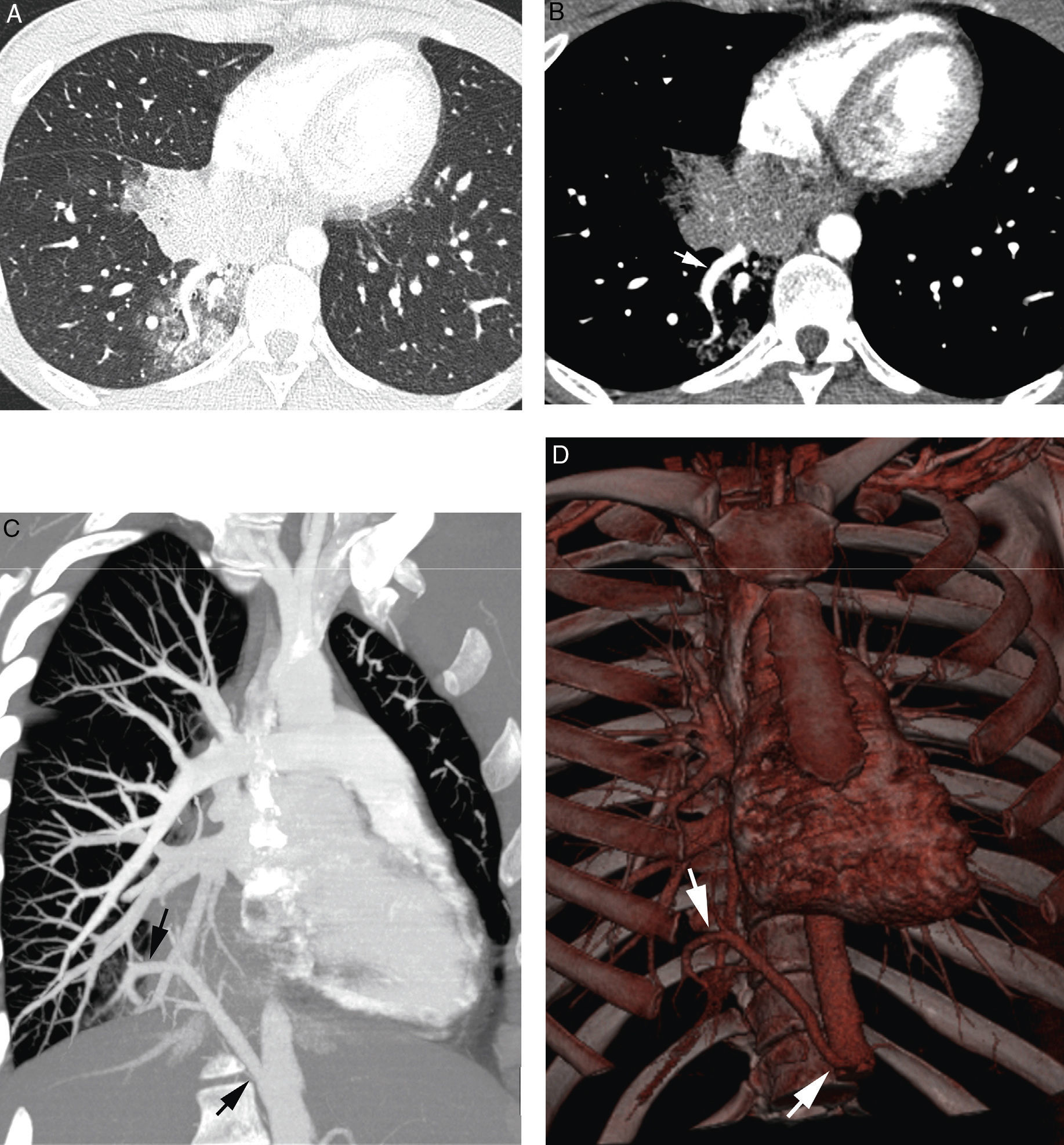
Patient with cystic fibrosis and life-threatening hemoptysis. (A) CT with lung window in which it is possible to observe extensive bilateral affectation by bronchiectasis (predominant in the middle lobe and the lingula), mucous impacts and air trapping areas in both lower lobes. (B) Axial MIP reconstruction showing multiple pathological bronchial arteries (white arrows). The right intercostobronchial trunk presents aneurysm (black arrow). Multiple mediastinal adenopathies reactive to repetition infections may be observed (*).
One of the most feared complications of the procedure is embolizing inadvertently the anterior medullar artery, which results in paraplegia. The anterior portion of the spinal cord irrigates the anterior spinal artery. In the thoracic region, the anterior spinal artery comes from a single long anterior medullar artery (Adamkiewicz's artery), which originates between T5 and L4.6 Although it is infrequent (5%), Adamkiewicz's artery may originate from the costal portion of the right intercostobronchial trunk, with a characteristic hairpin shape in the arteriography. Embolizing this artery produces a medullar ischemia and it must be avoided.31 If Adamkiewicz's artery is seen during the aortography, embolization must be performed beyond its exit, so as not to occlude it.32 Unfortunately, Adamkiewicz's artery is very thin and it is rarely seen in the angio-MDCT of hemoptysis patients.4
In sum, given the great anatomical variability and prevalence of ectopic bronchial arteries (which may be difficult to identify during aortography), proving those variables with a non-invasive method before procedure may reduce relapses, often related with unoccluded ectopic bronchial arteries.4,33–35
Non-bronchial systemic arteriesNon-bronchial systemic arteries are implicated in 41–88% of the cases of hemoptysis according to several articles.4,27 They may be the primary cause of bleeding or be a cause additional to bronchial artery bleeding. Unlike bronchial arteries, they do not enter the lung through the hilius and their course is not parallel to the bronchi. These arteries enter the pulmonary parenchyma through the pleura or the lower pulmonary ligament.36
Non-bronchial systemic arteries may originate in the intercostal arteries (the ones most frequently implicated), the branches of the supraaortic trunks (the innominate trunk, subclavian arteries, thyrocervical and costocervical trunks), axillary arteries, inner mammary arteries and infradiaphragmatic aortic branches (lower phrenic arteries, gastric arteries, celiac trunk).26,36
Seeing abnormally dilated and tortuous arteries in the extrapleural fat in the MDCT, with pleural thickening (greater than 3mm) and affectation of adjacent parenchyma (bronchiectasis, tuberculous sequels) must make us suspect its implication in hemoptysis (Figs. 6 and 8).27,35 Once the bleeding has been located in the MDCT, non- bronchial arteries that may potentially vascularize that area must be sought systematically: the lower phrenic artery (Fig. 9) (lower lobes and lower lingula segment), intercostal arteries (posterior pleura), inner mammary artery (anterior segment of superior lobes, the medial lobe and the lingula) and branches of the subclavian and axillaries arteries (the pulmonary apex).20,27 When the inner mammary artery has a diameter greater than 3mm or the phrenic artery one greater than 2mm, their involvement in the bleeding must be suspected.35 Not to recognize these systemic arteries may be motive of an early recurrence of hemoptysis after successfully embolizing the bronchial arteries.4,9,34,35
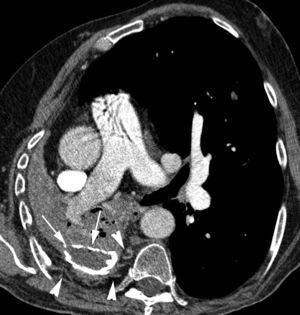
Patient with tuberculous sequels and necrotizing pneumonia affecting the right lower lobe. CT with contrast showing an important volume loss of the right hemithorax, with calcified chronic pleural collection on the right hemithorax base (arrows). An increase of subpleural fat is observed, along which the hypertrophic, tortuous intercostal artery runs (arrow heads).
Patient with bronchiectasis in the lingula who presented life-threatening hemoptysis a year ago and due to that the bronchial arteries were embolized. Now he is presenting a new episode of life-threatening hemoptysis. (A) CT with lung window showing bronchiectasis in the lingula (arrow head). (B–D) Angio-MDCT images; (B) axial cut in the subdiaphragmatic region showing a tortuous left phrenic artery (arrows). Oblique coronal MIP reconstructions (C) and (D) allow us to see the non-bronchial artery trajectory from the celiac trunk (*) to the lingula (arrows). (E) The arteriography confirms the CT findings; the phrenic artery goes into the lingula.
Although it is very infrequent, there may be communication between the coronary arteries and the bronchial arteries. The communication is probably congenital, and in situations with decreased pulmonary flow or chronic pulmonary disease (bronchiectasis) anastomosis between pulmonary and bronchial arteries may be reinforced by collateral circulation from the coronary arteries.4 Most of the patients are asymptomatic but they may cause angina, by the phenomenon of ‘coronary steal’, heart failure, endocarditis and hemoptysis.36 These fistulas are usually retrocardiac, in areas of wide pericardial recesses. There may be inverse communications, with flow from the bronchial to the coronary arteries in cases of arteriosclerotic coronary stenosis.36
In this section we also consider congenital pulmonary malformations presenting systemic irrigation, such as bronchiopulmonary sequestration (non-functioning pulmonary tissue mass, in general without connection to the normal bronchial tree) (Fig. 10) and systemic irrigation of the normal lung, which though infrequent, may be the cause of life-threatening hemoptysis.37 Normal lung systemic irrigation, unlike bronchiopulmonary sequestration, is a purely vascular anomaly, in which a systemic artery irrigates a portion of the normal lung.38 In both cases the anomaly usually affects the lower lobes, the systemic artery usually originates from the abdominal aorta (entering the lung through the lower portion of the pulmonary ligament) and venous drainage is performed through the pulmonary veins. In case it becomes complicated with a life-threatening hemoptysis, it may be efficaciously controlled by embolizing the aberrant systemic artery.4,39 We may suspect the possibility of congenital malformation as the cause of life-threatening hemoptysis especially in young patients, without known previous pulmonary disease (Fig. 10).
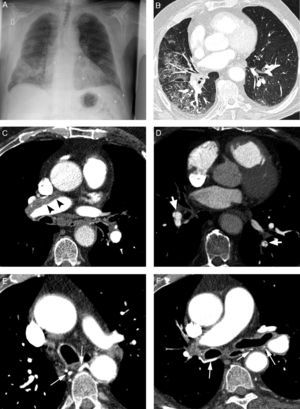
26-year-old patient without known antecedents presenting life-threatening hemoptysis. (A) CT with lung window showing a consolidation in the lower right lobe, with frosted-glass images around it. (B–D) angio-MDCT images. Axial cut (B) showing an anomalous vascular image (arrow) in the right lower lobe adjacent to the consolidation. The oblique coronal MIP reconstruction (C) shows an anomalous vessel originating in the abdominal aorta (arrows) and going into the right lower lobe, confirming that the parenchymatous affectation is a pulmonary sequestration. Volume reconstruction (D) showing the anomalous vessel (arrows). The reconstructions allow us to observe the angle of the anomalous vessel as it exits the aorta.
Thoracic vascular evaluation must always include pulmonary circulation. Bleeding originating in the pulmonary arteries represents, according to recent publications, approximately 10% of the causes for life-threatening hemoptysis.7,40,41
The study of pulmonary arteries mainly aims at identifying aneurysms or, more frequently, pseudoaneurysms (dilatation of the artery that does not include all the wall layers) of pulmonary arteries.7 Both lesions are observed in the CT with contrast as saccular or fusiform dilatations of the pulmonary arteries that are filled with contrast, simultaneously with the rest of the pulmonary arteries (Fig. 11A–D).42 Seeing the branches of the pulmonary arteries in the inner portion of the walls of the necrotic cavities is also a sign suggesting its implication in bleeding (Fig. 3C).20
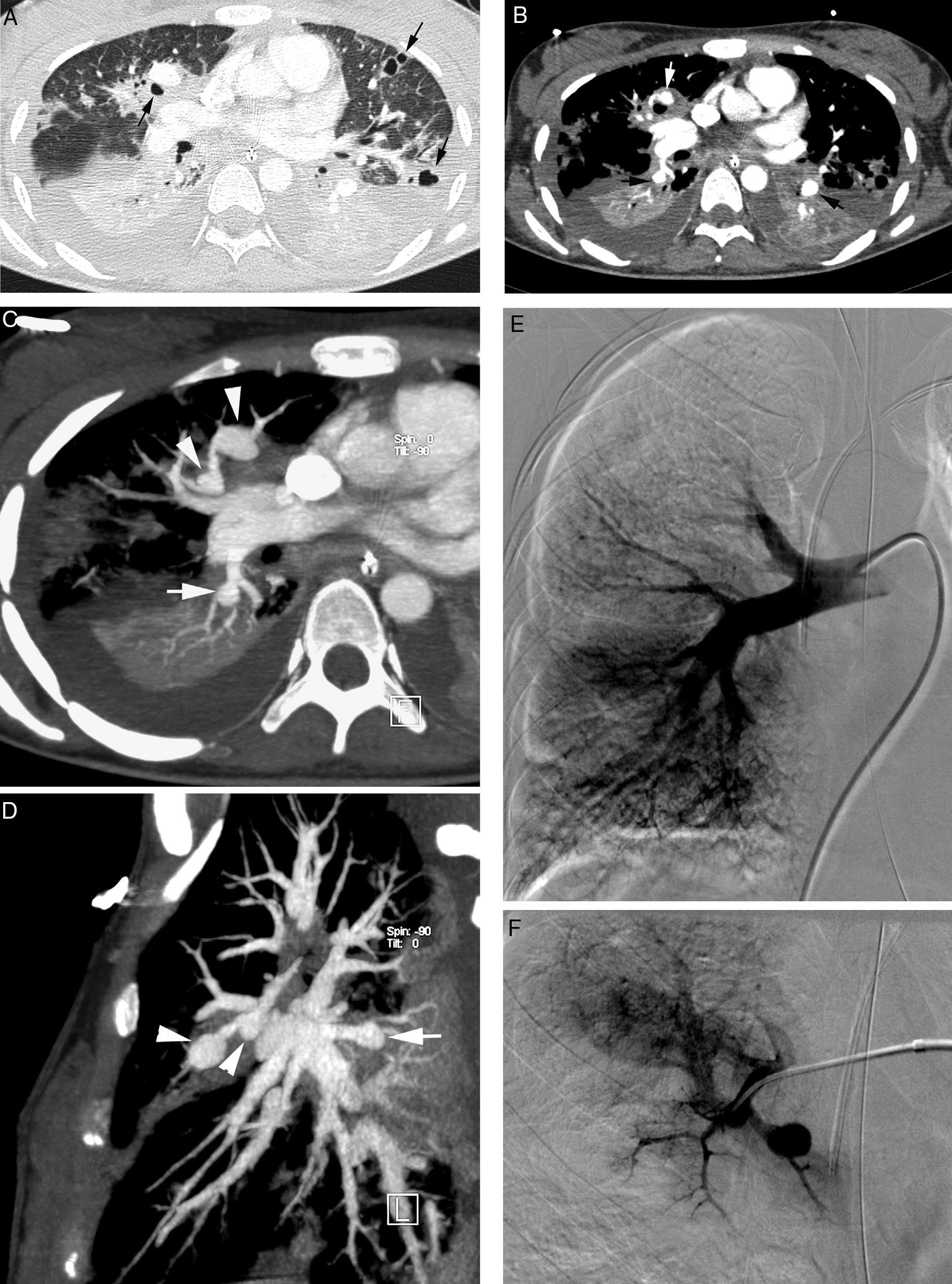
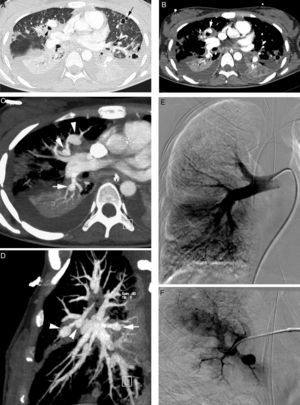
Patient with septic embolisms and life-threatening hemoptysis. (A) CT with lung window in which it is possible to observe multiple bilateral cavitated lesions, some with thin walls, others with thick walls, which correspond with septic embolisms (arrows). (B–E) Angio-MDCT images. It is possible to observe (B) multiple dilatations of the pulmonary arteries (arrows), some of them adjacent to a cavity (white arrow), which corresponds to multiple mycotic pseudoaneurysms. Axial MIP reconstruction (C) showing 2 aneurysms in anterior segmentary branches of the right upper lobe (arrow heads) and another aneurysm in a right lower lobe segmentary branch (arrow). Sagittal MIP reconstruction (D) showing the 3 right aneurysms, 2 in the right upper lobe (arrow heads) and one in the right lower lobe (arrow); global pulmonary arteriography (E) in which the pseudoaneurysms cannot be seen. (F) Supraselective arteriography where one of the right upper lobe aneurysms can be observed.
Angio-MDCT, with MIP and multiplane reconstructions, accurately locates the pseudoaneurysm and its nourishing artery. On occasion, distal pseudoaneurysms are not visible in the global of lobar pulmonary arteriography and they can only be seen in a supraselective angiography of pulmonary arteries. The information provided by the angio-MDCT is vital, since it indicates accurately what the affected vessel is so as to be able to perform a supraselective embolization7 (Fig. 11E and F).
The potential causes of bleeding originating in the pulmonary arteries are many and they include: diseases with necrosis of the pulmonary parenchyma (Fig. 11A and B) (active or chronic tuberculosis, pulmonary abscess, necrotizing pneumonia, aspergillosis, neoplasias), vasculitis (Behçet's disease, Hughes-Stovin's syndrome), iatrogenesis/traumatisms (catheters, penetrating wounds) and arteriovenous malformations (AVM).7 In patients with necrosis of pulmonary parenchyma (secondary to infection or neoplasia), hemoptysis originating in the pulmonary arteries is produced by erosion of the arteries which form a pseudoaneurysm.43 Rasmussen's aneurysms are the pulmonary artery pseudoaneurysms originated in areas of tuberculous inflammation; in the CT they appear as round images on the walls of the tuberculous cavities that take up much contrast. In the cases in which a Swan-Ganz catheter is misplaced, the catheter's distal end erodes the artery wall, producing a pseudoaneurysm that is contained by the adventitia and on occasion by the thrombosis.43 Vasculitis produces an inflammation of the middle layer vasa vasorum, destruction of the elastic fibers and dilatation of vascular lumen.
Life-threatening hemoptysis may occur, although it is rare, by rupture of pulmonary AVM. These are anomalous communications between pulmonary arterial and venous circulation that result into a right-left short-circuit. Most of them are congenital and associated to Rendu-Osler's disease. Embolization of AVM whose afferent vessel is greater than 3mm4 is usually recommended electively.
Before the introduction of MDCT, hemoptysis originating in the pulmonary arteries was suspected when embolization of systemic arteries did not control bleeding.44 Khalil et al.15 compared 2 consecutive groups of patients with hemoptysis; one of them received angio-MDCT before the endovascular treatment and the other did not. Angio-MDCT increased diagnosis of hemoptysis of pulmonary origin and decreased urgent surgical resections and pulmonary arteriographies without embolization. In the group without angio-MDCT the pulmonary embolizations were performed before the recurrence of hemoptysis.
Cryptogenetic hemoptysisIt is the hemoptysis whose underlying cause cannot be found, despite conducting a complete study, including thoracic CT and fibrobronchoscopy; it is an exclusion diagnosis. It represents approximately 15% of the hemoptysis45 and, in one third of the cases; it may be life-threatening.4 It occurs more often in smoking patients.4,45,46 Menchini et al.46 studying the angiographic findings of cryptogenetic hemoptysis in smoking patient, pointed out that there is a marked hypertrophy of bronchial arteries in 80% of the patients. In these cases, embolizing the bronchial arteries solves the hemoptysis.
In Herth et al.’s study47 6% of the patients with cryptogenetic hemoptysis developed cancer during the 3-year follow-up period. This fact highlights the importance of studying the pulmonary parenchyma and the bronchi in detail to rule out a small lung carcinoma, and the importance of performing a follow-up CT months later, so as to be able to detect an interval cancer. However, in a subsequent study that followed up the evolution of 81 patients with cryptogenetic hemoptysis, Savale et al.45 did not show a greater incidence of lung neoplasia. In their sample, 13 of the patients with cryptogenetic hemoptysis were operated on due to the presence of bleeding and more than half presented findings in the submucosa compatible with Dieulafoy's disease. This disease is characterized by the anomalous dilatation of vessels in the submucosa with a tendency to bleeding. It was originally described in the gastrointestinal tract (its typical location is the stomach followed by the duodenum)48 and, more recently, in the bronchi. It generally coexists with chronic inflammatory processes, such as chronic bronchitis,48 and most patients who are diagnosed with it are heavy smokers. In none of these patients were findings found neither in the fibrobronchoscopy nor in the CT that would raise suspicion of the disease, therefore it is believed that Dieulafoy's disease may be implicated in some cases of life-threatening cryptogenetic hemoptysis in smoking patients.45,49
Causes of rebleedingAfter the embolization, immediate control of hemoptysis is attained in 73–99% of the patients.50–53 However, it is not infrequent for hemoptysis reoccur, which happens in 10–53% of the cases.53–56 Early recurrence, in the first few weeks, is associated with incomplete occlusion of the vessels involved, whether because there is an underlying cause with very extensive affectation or because not all the participating vessels were examined thoroughly.53–55 Tardive recurrence is due to recannulation of the previously embolized vessels, not having embolized other implicated vessels, or due to revascularization by collateral circulation caused by persistence or progression of the underlying pathology. Of the causes of life-threatening hemoptysis, aspergilloma and pulmonary neoplasias are the ones that present the worst immediate and tardive control of hemoptysis53,54; in an article on our subject, the most frequent tardive relapse occurred in the patients with bronchiectasias.18
Due to what is explained above, it is important to identify and embolize all the vessels that may contribute to anomalous irrigation, including any systemic non-bronchial or pulmonary artery. On the other hand, the information about atherosclerosis and stenosis of the bronchial artery ostia that is contributed by the angio-MDCT to the interventionist radiologist may prevent failure in catheterization.15
Practical recommendations to assess multidetector computed angiotomography in life-threatening hemoptysis
The study must include the supraaortic trunks and the upper part of the abdomen, because there may be systemic vessels implicated in the bleeding originating in supraaortic and/or infradiaphragmatic branches.
It is advisable to start the image evaluation with the lung window, which most of the times, gives clues about the causes of bleeding and its location.
The airway must be checked systematically in the axial cuts or by means of multiplane reconstructions or in minimum intensity projection.
Axial images with mediastinum windows allow us to study the implicated vessels initially and they are useful to see the systemic aorta artery ostium or that of its branches, but the MIP and multiplane reconstructions (with adjusted thickness and planes in each case) are indispensable to show the tortuous trajectory of the systemic vessels or the alterations of pulmonary arteries.
If signs of bleeding are observed in the upper lobes, the subclavian arteries and the inner mammary arteries must be checked.
If the signs of bleeding are located in the lower lobes, the lingula and the middle lobe, the phrenic arteries must be checked.
In case of acute or chronic infections, or if there are cavitated pulmonary lesions, the pulmonary arteries must be checked to detect pseudoaneurysms.
When there is pleural disease, the possible implication of intercostal arteries must be considered.
In elderly patients with atherosclerosis we must mention the atheroma plaques in the bronchial artery ostia, which may make their catheterization difficult in the angiography.
In order to facilitate catheterism, angio-MDCT may indicate angulation of the vessels in the aorta origin.
In a young patient without known previous pulmonary disease, the congenital lesions must be considered and it must be assessed whether there are systemic vessels coming from the abdominal aorta, going to the lower lobes.
ConclusionLife-threatening hemoptysis is a serious clinical situation that must be diagnosed and treated urgently. The treatment of choice is embolization. Bronchial circulation is the most frequent cause of life-threatening hemoptysis, but systemic non-bronchial arteries or pulmonary arteries may also be the cause of bleeding depending on the underlying disease.
With angio-MDCT the cause, location and possible vessels implicated in hemoptysis are studied in a non-invasive, quick and accurate manner, and it is particularly useful to detect ectopic bronchial arteries, systemic non-bronchial arteries or pulmonary pseudoaneurysms. Performing an angio-MDCT systematically before embolizing makes it possible to plan the treatment better.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments have been conducted with humans or animals for this research.
Data confidentialityThe authors declare that there are no patient data in this article.
Right to privacy and informed consentThe authors have obtained informed consent from the patients and/or subjects referred to in this article. This document is in the possession of the author
Authors contribution- 1
Person responsible for the study's integrity: CS, EC.
- 2
Conception of the study: CS, EC.
- 3
Design of the study: CS, EC.
- 4
Data acquisition: CS, EC, XG, MA, AA.
- 5
Data analysis and interpretation: CS, EC, XG, MA, AA.
- 6
Statistic treatment: not applicable.
- 7
Bibliographic search: CS, EC.
- 8
Writing of the paper: CS, EC.
- 9
Critical revision of the manuscript with intellectually relevant contributions: XG, MA, AA.
- 10
Approval of final version: CS, EC, XG, MA, AA.
The authors declare that they do not have any conflict of interests.
Please cite this article as: Spinu C, Castañer E, Gallardo X, Andreu M, Alguersuari A. La tomografía computarizada multidetector en la hemoptisis amenazante. Radiología. 2013;55:483–498.