To evaluate the diagnostic performance of the length of the tumor contact with the capsule (LTC) and the apparent diffusion coefficient (ADC) map in the prediction of microscopic extracapsular extension in patients with prostate cancer who are candidates for radical prostatectomy.
Material and methodsWe used receiver operating curves to retrospectively study the diagnostic performance of the ADC map and the LTC as predictors of microscopic extracapsular extension in 92 patients with prostate cancer and moderate to high risk who were examined between May 2011 and December 2013.
ResultsThe optimal cutoff for the ADC map was 0.87×10−3mm2/s, which yielded an area under the ROC curve of 72% (95% CI: 57%–86%), corresponding to a sensitivity of 83% and a specificity of 61%. The optimal cutoff for the LTC was 17.5mm, which yielded an area under the ROC curve of 74% (95% CI: 61%–87%), corresponding to a sensitivity of 91% and a specificity of 57%. Combining the two criteria improved the diagnostic performance, yielding an area under the ROC curve of 77% (95% CI: 62%–92%), corresponding to a sensitivity of 77% and a specificity of 61%. We elaborated a logistic regression model, obtaining an area under the ROC curve of 82% (95% CI: 73%–93%).
ConclusionsUsing quantitative measures improves the diagnostic accuracy of multiparametric magnetic resonance imaging in the staging of prostate cancer. The values of the ADC and LTC were predictors of microscopic extracapsular extension, and the best results were obtained when both values were used in combination.
Evaluar el rendimiento diagnóstico de la longitud del contacto tumoral con la cápsula (LCT) y la cuantificación del mapa del coeficiente de difusión aparente (ADC) en la predicción de la extensión extracapsular (EEC) microscópica en pacientes con cáncer de próstata candidatos a prostatectomía radical.
MétodoSe realizó un estudio retrospectivo de prueba diagnóstica con curvas receptor-operador (ROC) evaluando el rendimiento diagnóstico del valor de ADC y LCT como predictores de EEC microscópica en 92 pacientes con cáncer de próstata de moderado y alto riesgo, entre mayo de 2011 y diciembre de 2013.
ResultadosEl punto de corte óptimo para el valor del mapa de ADC fue de 0,87 × 10−3 mm2/s, con un área bajo la curva ROC del 72% (intervalo de confianza del 95% [IC95%]: 57-86%), una sensibilidad del 83% y una especificidad del 61%. Para la LCT, el punto de corte óptimo fue de 17,5mm, con un área bajo la curva ROC del 74% (IC95%: 61-87%), una sensibilidad del 91% y una especificidad del 57%. Empleando ambos criterios, el rendimiento diagnóstico mejoró con un área bajo la curva ROC del 77% (IC95%: 62-92%), una sensibilidad del 77% y una especificidad del 61%. Se calculó un modelo de regresión logística y se obtuvo un área bajo la curva ROC del 82% (IC95%: 73-93%).
ConclusionesEl uso de criterios cuantitativos mejora la exactitud diagnóstica de la resonancia magnética multiparamétrica en la estadificación del cáncer de próstata. Se encontró que los valores de ADC y de LCT son predictores de EEC microscópica, y que se obtienen mejores resultados si se usan de manera conjunta.
Prostate cancer is the second most common type of cancer in males worldwide (15 per cent) and the fifth cause of cancer mortality among this population (6.6 per cent).1 In Colombia, the panorama is not any different and it is the second most common cause of cancer in men, with an incidence of 46.5 per 100,000 inhabitants and a mortality rate of 12.6 per 100,000 inhabitants.2
Both choosing treatment and the prognosis of patients with prostate cancer depend on the presence of extracapsular spread (ECS). For the cases of localized prostate cancer, the radical prostatectomy is the treatment of choice, with high rates of survival.3
Patients with localized prostate cancer undergo clinical staging of the risks based upon what decisions are made when it comes to management (radical prostatectomy with or without adjuvant therapy), ECS risk prediction and possible chemical relapse after the prostatectomy–reported in up to 15–40 per cent of the cases.4–7 This staging is performed based on the prostate-specific antigen (PSA), rectal examination, and Gleason grading group.8 Gleason's grading system is based on the sum of the two (2) most prevalent patterns of growth of biopsied tissue, and this score is used to make a five (5) group-grade classification (1: ≤6; 2: 3+4=7; 3: 4+3=7; 4: 8; and 5: 9 and 10), where group-grade 1 is low risk, while groups-grades 4 through 5 are high risk.9,10
Unfortunately, with the use of these clinical parameters we find a 59 per cent substaging,11 with presence of microscopic ECS in 20–50 per cent of the surgical pieces initially classified as localized prostate cancer.12,13 This is why it is recommended to perform one wide surgical resection including the neurovascular bundle that may lead to erectile dysfunction as a possible complication.14 This is the reason why determining the ECS is essential to be able to achieve better surgical outcomes with a lower comorbidity rate and better oncological outomes.15
The multiparametric magnetic resonance imaging (mp-MRI) is the best imaging modality for the tumor staging of this neoplasm. It uses anatomical sequences (T1 and T2-weighted images) and functional sequences (diffusion and dynamics after the administration of contrast). For the diagnosis of ECS we normally use T2-weighted imaging conventional criteria16,17; this is how, in the year 2011, the European Society of Urogenital Radiology established the PI-RADS criteria and determined the score for the prediction of extracapsular involvement (1–5 points) using T2-weighted sequences. These criteria assess the following parameters: capsular contact (1 point); capsular irregularity (3 points); neurovascular bundle thickening (4 points); bulging or capsular loss (4 points); and overt extracapsular spread (5 points). Scores ≥4 regard the presence of ECS as likely.8 In 2015, the PI-RADS criteria (versión 2) were updated, no changes to these ECS criteria were made, but the DWI sequences-appararent diffusion coefficient (ADC) map and tumor contact length (TCL) with the capsule were mentioned as important factors in the prediction of ECS.18
In the medical literature we found great variability in the values of sensitivity and specificity when using these conventional criteria (T2-weighted sequences). One meta-analysis from 2015 showed low sensitivity–53 per cent (95 per cent confidence interval [95 per cent CI]: 44–63 per cent), and high specificity–91 per cent (95 per cent CI: 88–93 per cent).19
In view of the clinical substaging and wide variability of sensitivity and specificity with the use of T2-weighted imaging conventional criteria for the preoperative diagnosis of ESC, during the last years new quantitative markers for mp-MRI have been studied like the TCL and the quantitative values of the ADC map. There are few studies in the medical literature and these imaging markers have only been assessed independently.
Therefore, the goal of this study is to assess the diagnostic performance of the quantification of TCL and ADC map, both separately and together, for the prediction of microscopic ESC in patients with prostate cancer using the radial prostatectomy specimen as the standard of reference.
MethodWe conducted one diagnostic test study with a retrospective review of the clinical histories of patients with confirmed diagnoses of moderate and high-risk prostate cancer treated with radical prostatectomy in a reference center on prostatic diseases from May, 2011 to December, 2013. The study was approved by the institutional research ethics committee which did not deem it necessary to obtain prior written informed consent given the observational and retrospective nature of the study.
PatientsAll patients with prostate cancer treated at the institution with radical prostatectomy who also underwent mp-MRIs during their preoperative assessment were included in the study.
The following patients were excluded from the study: 1) patients with prior prostate cancers who had already received radiotherapy or hormone replacement therapy; 2) patients with overt signs of ECS in the T2-weighted sequences; 3) patients whose mp-MRIs did not show affectation of the prostatic capsule tumor; and 4) patients whose images showed technical artifacts that would not allow a precise estimation of the values of the ADC map.
Imaging modalityAll patients underwent one prostate mp-MRI using one 1.5T, Signa Excite HDxT superonductor machine (GE Medical Systems®, Milwaukee, Wisconsin, USA) with pelvic antenna (16 channel phase array). We included high resolution T1-weighted images (axial cut, TR/TE, 500/11.48, cut thickness/gap 3mm/0mm, FOV 240mm and matrix 256×256), T1-weighted images (axial, sagittal and coronal cuts, TR/TE 4300/61, cut thickness/gap: 3mm/0mm, FOV: 180mm and matrix 256×256), dynamic sequences after the administration of contrast (axial cut, TR/TE 3.3/1.5, cut thickness: 3mm, FOV:180mm and matrix 256×192) and diffusion weighted imaging (DWI)-ADC map (axial cut, values b200, b500, b800s/mm2, TR/TE, 4725/91, cut thickness: 3mm, FOV: 280mm and matrix 256×256).
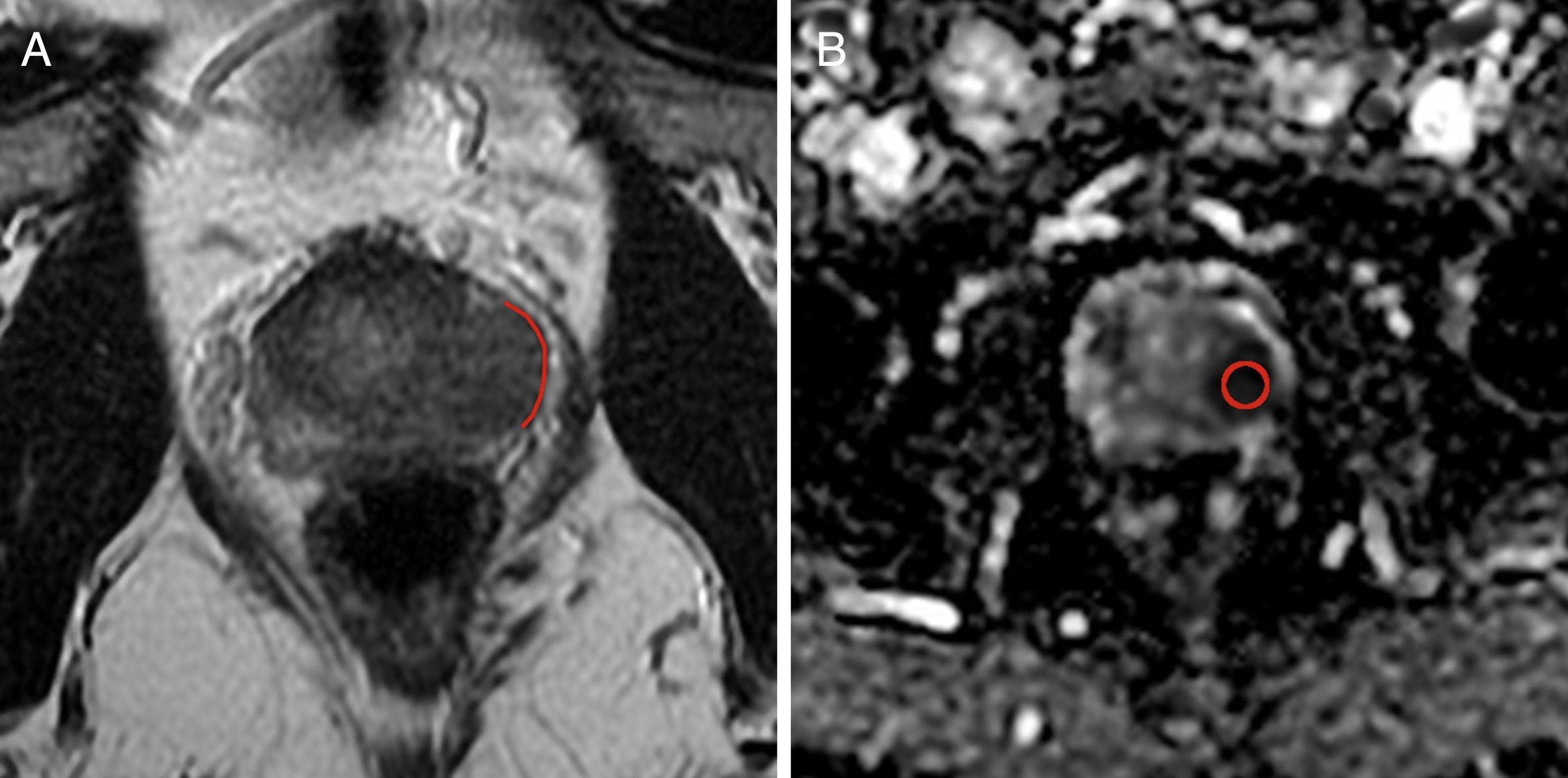
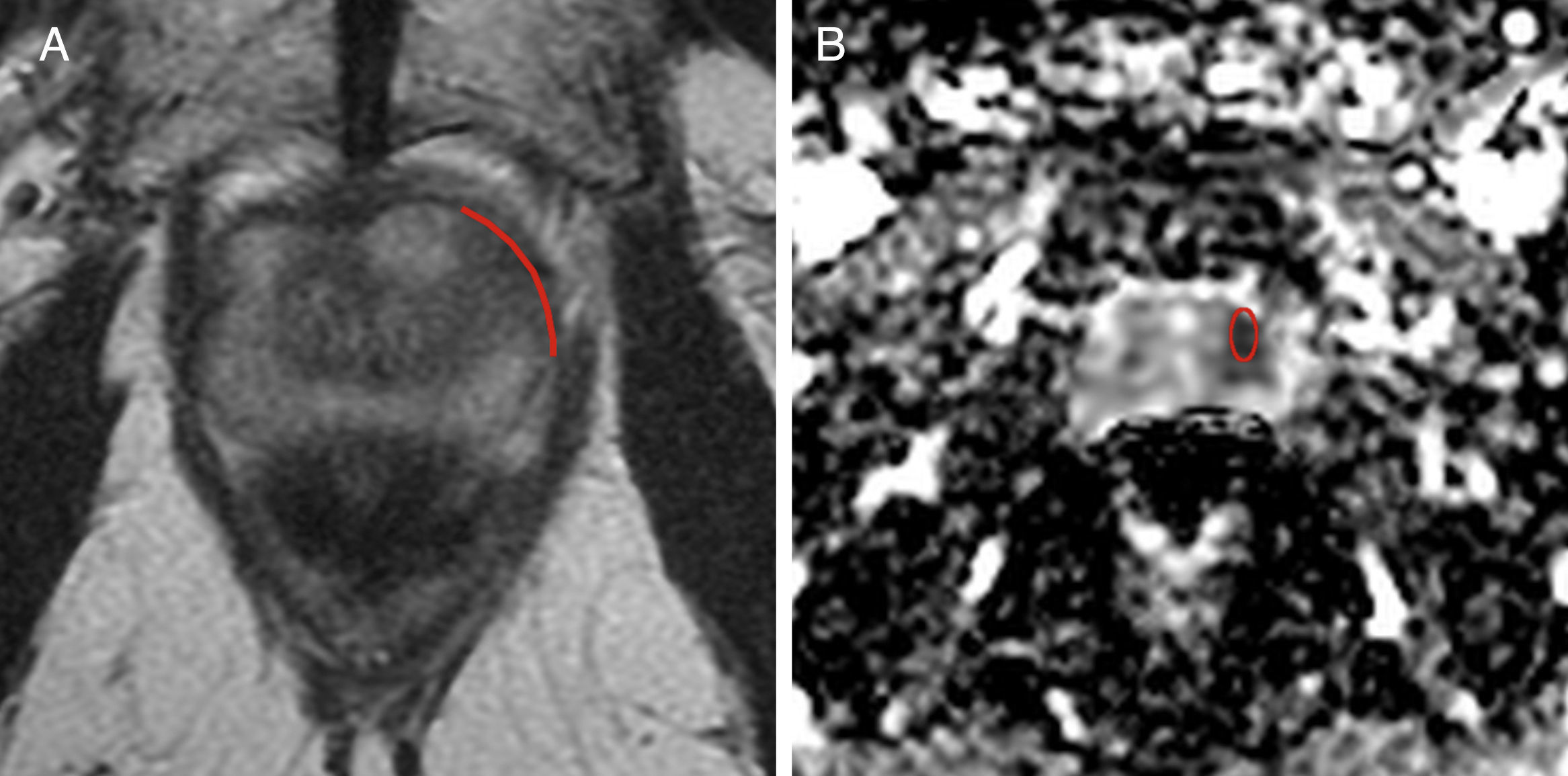
One radiologist identified the disease-induced target lesion and then proceeded to locate it in the T2-weighted images and ADC b800. Using the Advantage WorkStation (GE Medical Systems®, Milwaukee, Wisconsin, USA) the ADC values were assessed using one region of interest (ROI) from the injury reported, while the ADC b800 value and standard deviation were obtained too. Also the TCL of the injury with larger surface of contact to the capsule seen in the axial T2-weighted images was measured in millimeters using one digitized metric function of the working station (Figs. 1 and 2).
Fifty-one year old-patient with Gleason 8 prostate cancer (4+4), located in the peripheral area of the middle third of the left side. (A) Axial T2-weighted image showing one red line as the measurement of tumor contact length with a 19.7mm capsule. (B) Axial image of the apparent diffusion coefficient map (b 800) with a value of 0.645×10−3mm2/s. This injury showed microscopic extracapsular spread in the pathology report.
Sixty-five year old-patient with Gleason 8 prostate cancer (4+4) located in the peripheral area of the middle third of the left side. (A) Axial T2-weighted image showing one red line as the measurement of tumor contact length with a 26.5mm capsule. (B) Axial image of the apparent diffusion coefficient map (b 800) with a value of 0.798×10−3mm2/s. This injury showed microscopic extracapsular spread in the pathology report.
After the radical prostatectomy, one pathologist with twelve years of experience in genitourinary diseases conducted one detailed study of the surgical piece using the following protocol: 3mm cut sections were obtained, first in the axial plane, then in the sagittal plane and eventually in apex-to-base quadrants. Three (3)μm cuts were performed and hematoxylin–eosin staining was used. The ESC was defined as the presence of cancer cells outside the prostatic capsule, then we determined if the ESC was focal/microscopic (radial spread <0.5mm), or overt/macroscopic (when exceeding such spread). In some studies of the actual medical literature, focal ESC are defined as the identification of cancer cells beyond the capsule in a radial spread <0.5mm, and in other studies <2mm.20,21
Also, the Gleason score ranges were determined based on the most prevalent growth patterns in the surgical piece categorizing it within one of the five (5) groups-grades established by the medical literature.9
Statistical analysisFor the analysis of information the following softwares were used: STATA 13® (Stata Inc, College Station, Texas, USA) and SPSS 21® (SPSS, Chicago, Illinois, USA). One univariate analysis was conducted in order to establish the relative frequencies of categorical variables. The measurements of central tendency and dispersion of quantitative variables, including the age of the patients, the PSA, and the ADC and TCL values in patients classified into Gleason categories were taken. One bivariate analysis was conducted in order to assess the existing correlation using the Student's t test and eventually establish the differences between the measurements of ADC and TCL values in patients with and without ESC.
The operative characteristics of ADC maps and TCL were determined using the microscopic ESC described in pathology reports as a reference. Using the area under the ROC curve, the optimal cut-off points could be established.
One logistics regression analysis model was designed using as independent variables the values of ADC and TCL, and as the dependant variable the ESC defined using the specimen of pathology as a reference. The analysis of the ROC curves was conducted in order to assess the operative performance of the prediction model for microscopic ESCs. p<0.05 values were considered statistically significant.
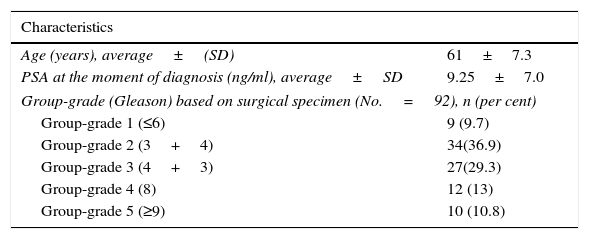
ResultsIn the total sample of 92 patients the average age was 61 years old (39–78 years old), the average value of the PSA, 9.25ng/ml (4.9 and 12ng/ml) and most patients (66 per cent) scored 7 in the Gleason grading system (groups-grades 2 and 3) as Table 1 shows.
Demographic and clinical characteristics of patients.
| Characteristics | |
|---|---|
| Age (years), average±(SD) | 61±7.3 |
| PSA at the moment of diagnosis (ng/ml), average±SD | 9.25±7.0 |
| Group-grade (Gleason) based on surgical specimen (No.=92), n (per cent) | |
| Group-grade 1 (≤6) | 9 (9.7) |
| Group-grade 2 (3+4) | 34(36.9) |
| Group-grade 3 (4+3) | 27(29.3) |
| Group-grade 4 (8) | 12 (13) |
| Group-grade 5 (≥9) | 10 (10.8) |
SD: standard deviation; PSA: prostate-specific antigen.
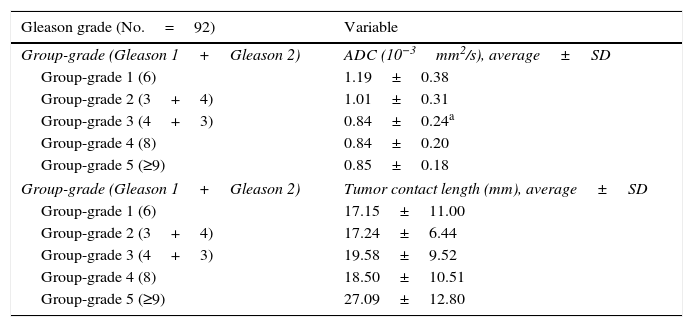
When comparing ADC values and Gleason scores, we found that patients with Gleason group-grade 3 (4+3) showed the most negative ADC values, with an average of 0.84±0.24×10−3mm2/s, followed in descending order, by the ADC values that corresponded to scores from groups-grades 4 and 5, with an average of 0.85±0.18×10−3mm2/s and 0.88±0.27×10−3mm2/s, respectively. One significant difference was identified (p=0.03) between the ADC values of Gleason group-grade 2 (3+4) and Gleason group-grade 3 (4+3), with values of 1.0±0.31×10−3mm2/s and 0.84±0.24×10−3mm2/s, respectively (Table 2).
Distribution of the apparent diffusion coefficient and tumor contact length according to Gleason's grading system.
| Gleason grade (No.=92) | Variable |
|---|---|
| Group-grade (Gleason 1+Gleason 2) | ADC (10−3mm2/s), average±SD |
| Group-grade 1 (6) | 1.19±0.38 |
| Group-grade 2 (3+4) | 1.01±0.31 |
| Group-grade 3 (4+3) | 0.84±0.24a |
| Group-grade 4 (8) | 0.84±0.20 |
| Group-grade 5 (≥9) | 0.85±0.18 |
| Group-grade (Gleason 1+Gleason 2) | Tumor contact length (mm), average±SD |
| Group-grade 1 (6) | 17.15±11.00 |
| Group-grade 2 (3+4) | 17.24±6.44 |
| Group-grade 3 (4+3) | 19.58±9.52 |
| Group-grade 4 (8) | 18.50±10.51 |
| Group-grade 5 (≥9) | 27.09±12.80 |
ADC: apparent diffusion coefficient; SD: standard deviation.
When assessing the average TCL and Gleason scores we found the lowest value in Gleason group-grade 1 (17.15±11.00mm), and the highest value in Gleason group-grade 5 (27.09±12.80mm); however, we did not find any significant differences in these values like Table 2 shows.
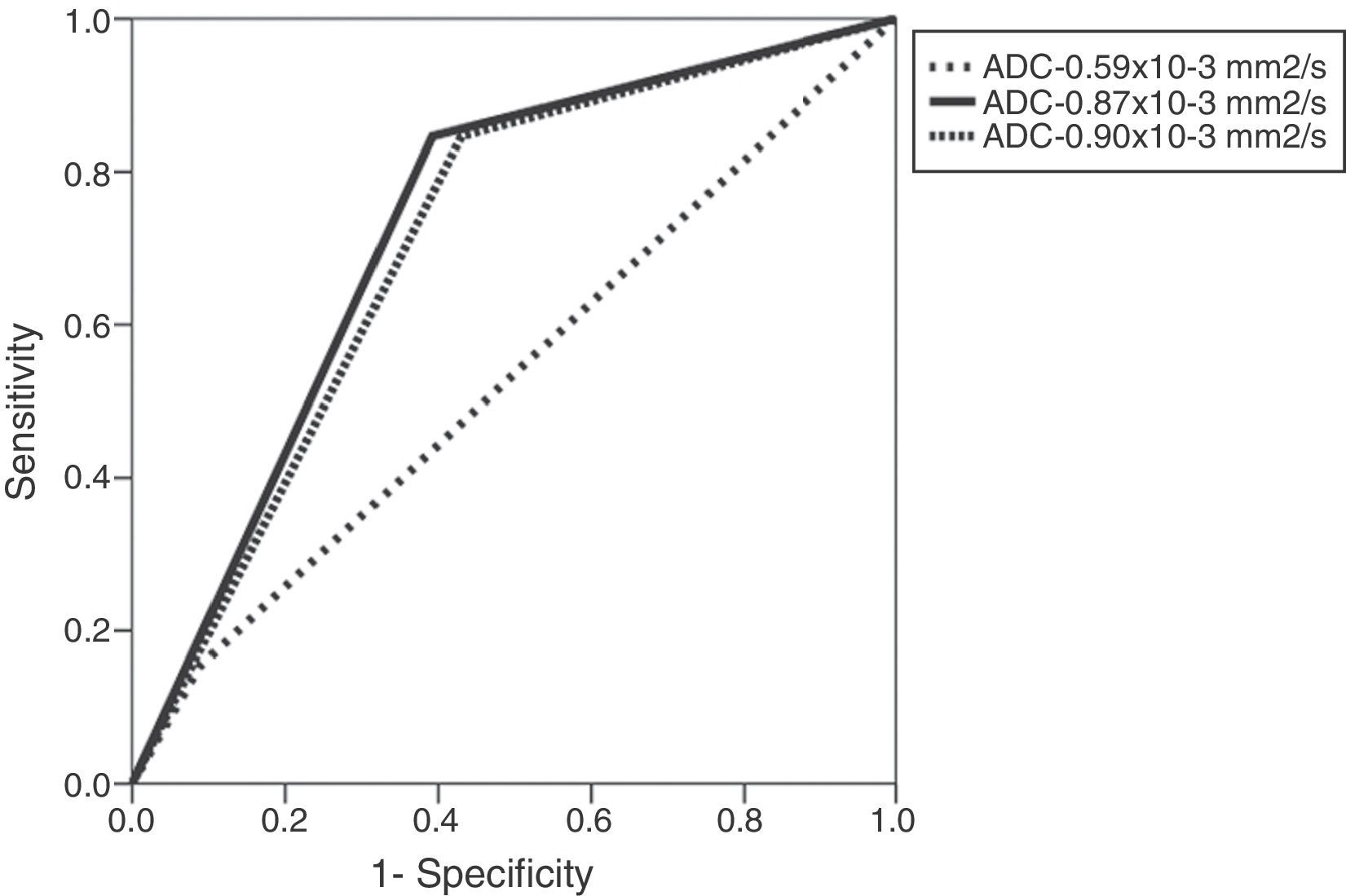
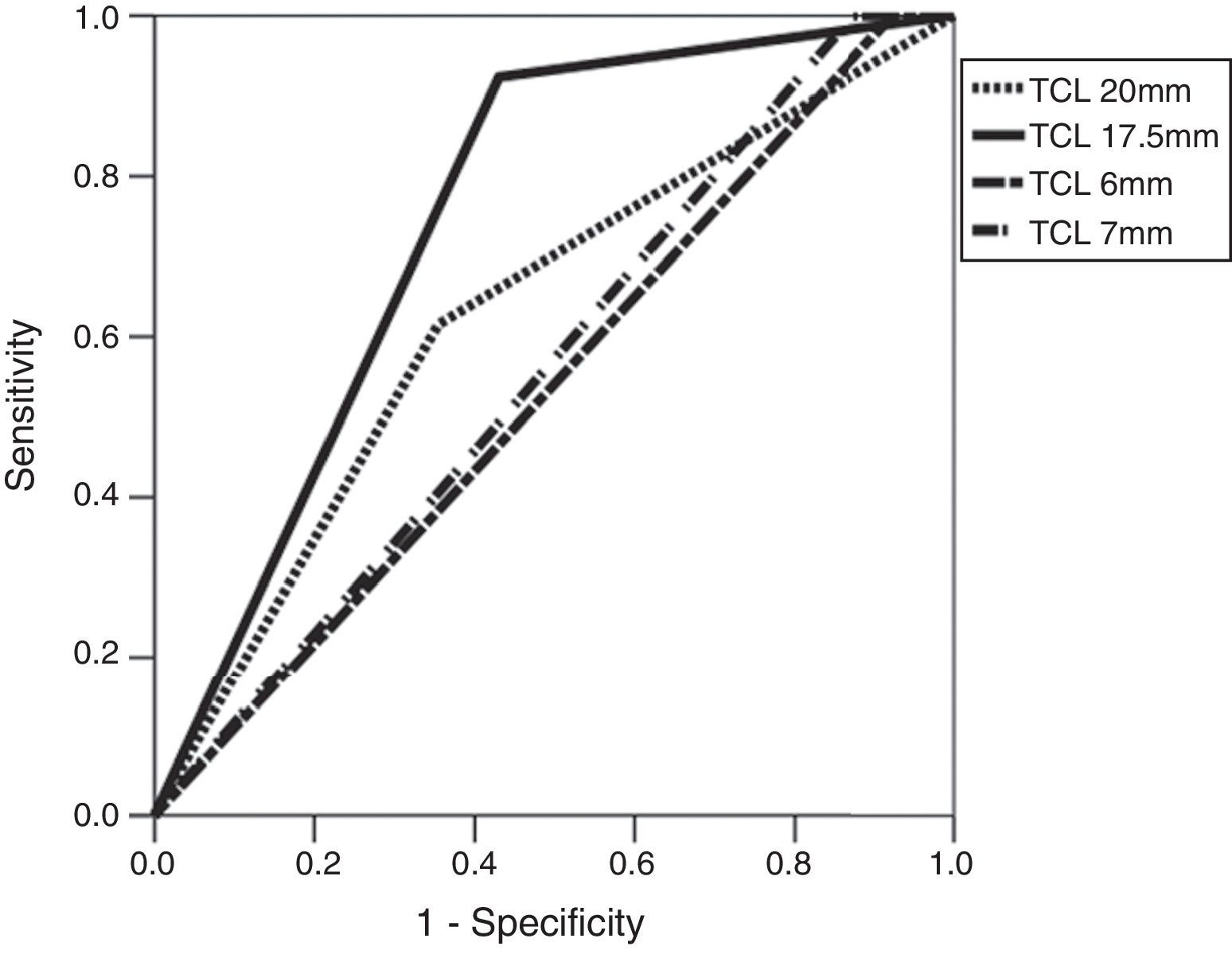
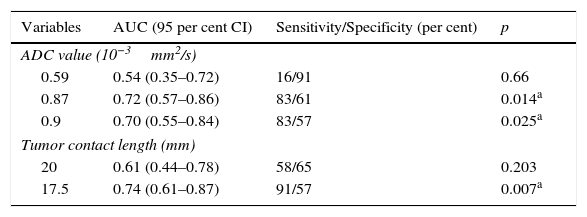
When assessing the ADC values for the prediction of microscopic ESCs three (3) cut-off points were considered (0.59, 0.87, and 0.9×10−3mm2/s) and the area under the curve (AUC) was identified at 72 per cent of the cut-off point 0.87×10−3mm2/s (Table 3 and Fig. 3). Similarly, for that cut-off point (0.87×10−3mm2/s) the sensitivity and specificity for the prediction of microscopic ESCs were 83 per cent and 61 per cent, respectively, which was statistically significant (p=0.014). Similarly, we confirmed that with a cut-off point of 17.5mm for the TCL, the AUC for the prediction of microscopic ESC is larger (74 per cent), and when comparing it to 20mm2 cut-off point, a much smaller AUC of 61 per cent was found. With the 17.5mm2 cut-off point we identified high sensitivity (91 per cent) and specificity (57 per cent)–also statistically significant (p=0.007) (Table 3 and Fig. 4).
Receiver–operator characteristics and areas under the curve for the diagnosis of microscopic extracapsular spread.
| Variables | AUC (95 per cent CI) | Sensitivity/Specificity (per cent) | p |
|---|---|---|---|
| ADC value (10−3mm2/s) | |||
| 0.59 | 0.54 (0.35–0.72) | 16/91 | 0.66 |
| 0.87 | 0.72 (0.57–0.86) | 83/61 | 0.014a |
| 0.9 | 0.70 (0.55–0.84) | 83/57 | 0.025a |
| Tumor contact length (mm) | |||
| 20 | 0.61 (0.44–0.78) | 58/65 | 0.203 |
| 17.5 | 0.74 (0.61–0.87) | 91/57 | 0.007a |
AUC: area under the curve; ADC: apparent diffusion coefficient; 95 per cent CI: 95 per cent confidence interval.
We did not find any significant differences in ADC values between patients with microscopic ESCs (average: 0.82; standard deviation [SD]: 0.26×10−3mm2/s) and those without microscopic ESCs (average: 0.94; DE: 0.29×10−3mm2/s) (p=0.11), but we could identify significant differences in the TLC values between patients with microscopic ESCs (average: 23.48; DE: 6.96mm) and those without microscopic ESCs (average: 18.4; SD: 9.68mm) (p=0.03). However, when conducting one binominal logistics regression analysis we confirmed that when it comes to ADC values, one cut-off point of 0.87×10−3mm2/s is a predictor of microscopic ESC with statistically significant results (p=0.02) (coefficient: 1.89; 95 per cent confidence interval [95 per cent IC]: 0.27±3.52); when it comes to TLC values, one cut-off point of 17.5mm is also a predictor of microscopic ESC with statistically significant results (p=0.037) (coefficient: 2.26; 95 per cent IC: 0.13±4.39).
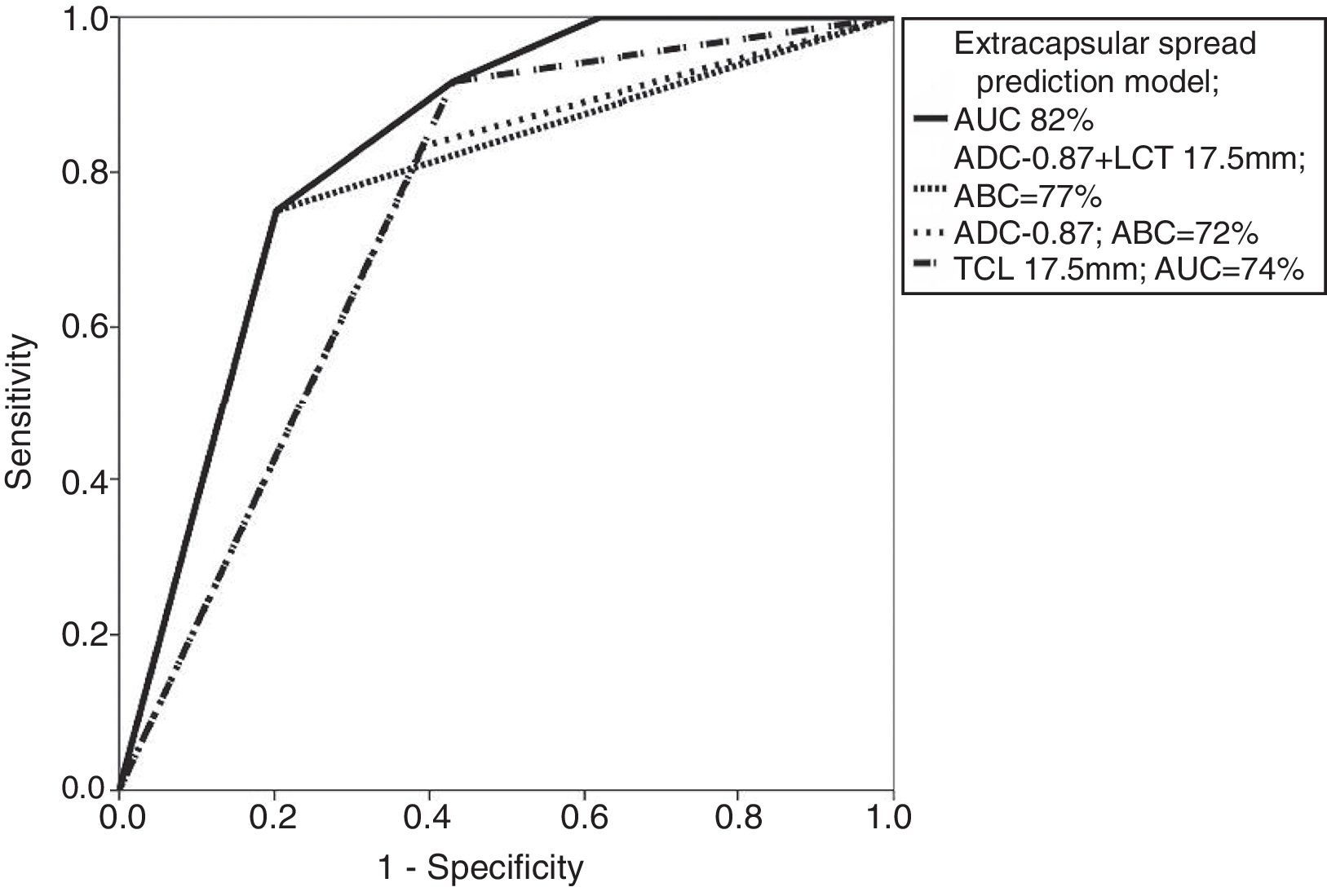
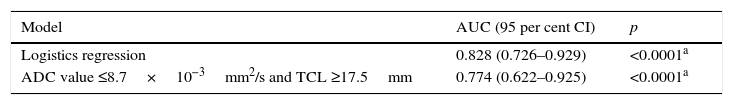
When combining the cut-off points of ADC (0.87×10−3mm2/s) and TLC (17.5mm) values, one AUC larger than the one found independently was obtained: of 77 per cent (77 per cent sensitivity; 61 per cent specificity). However when these cut-off points were assessed together using one logistics regression model, the AUC reached 82 per cent. These values were statistically significant with p<0.0001, like Table 4 and Fig. 5 show.
Receiver–operator characteristics and areas under the curve for the diagnosis of microscopic extracapsular spread using one logistics regression model and the presence of an apparent diffusion coefficient ≤0.87×10−3mm2/s and a tumor contact length ≥17.5mm.
| Model | AUC (95 per cent CI) | p |
|---|---|---|
| Logistics regression | 0.828 (0.726–0.929) | <0.0001a |
| ADC value ≤8.7×10−3mm2/s and TCL ≥17.5mm | 0.774 (0.622–0.925) | <0.0001a |
AUC: area under the curve; ADC: apparent diffusion coefficient; 95 per cent CI: 95 per cent confidence interval.
The election of treatment and prognosis of patients with prostate cancer depend on the presence or absence of ESC, being the radical prostatectomy reserved for patients with localized prostate cancer (without ESC). Currently, preoperative diagnoses using clinical criteria only in moderate and high-risk prostate cancers show 59 per cent substaging,11 while, postoperatively, in 20–50 per cent of the cases findings of microscopic ESCs have been reported.12,13 Even the use of mp-MRI conventional criteria (T2-weighted images) for the diagnosis of ESC has shown great variability in the sensitivity and specificity of different studies. One meta-analysis (30 studies) showed low sensitivity (53 per cent) (95 per cent CI: 44–63 per cent), and high specificity (91 per cent) (95 per cent CI: 88–93 per cent).19 Other studies have shown greater variability, with sensitivities ranging from 13 per cent to 95 per cent and specificities ranging from 49 per cent to 97 per cent.23 The diagnosis of microscopic (focal) ESCs using T2-weighted imaging conventional criteria is much lower than the diagnosis of established or macroscopic ESCs (50 per cent vs 69 per cent)24; this is why it is very important to improve the preoperative diagnosis in the local staging of these patients when using new mp-MRI quantitative tools.
In this study two (2) quantitative criteria for the prediction of microscopic ECS, TCL, and quantification of the ADC map were assessed, since we have been able to see that adding these quantitative measures to T2-weighted imaging conventional criteria improves the diagnosis of ECS as recent studies have confirmed.20–22,25 This is why in the update of the 2015 PI-RADS criteria (version 2) it has been included that the use of TCL and the quantification of the ADC map may be used as good predictors of ECS.18
In this study significant differences were found in the TCL averages between patients with and without microscopic ECS (p=0.03), but not in the ADC averages (p=0.11). The optimal cut-off point for the ADC value was 0.87×10−3mm2/s, with an AUC of 72 per cent (83 per cent sensitivity; 61 per cent specificity) while the optimal cut-off point for the TCL was 17.5mm, with an AUC of 74 per cent (91 per cent sensitivity; 57 per cent specificity)–both statistically significant (p=0.014 and p=0.007, respectively).
The results from our study are similar to those from the medical literature. Baco et al.’s study22 (111 patients, 1.5T machine and pelvic antenna) found a similar cut-off point for the TCL (20mm), with AUCs of 88 per cent (79 per cent sensitivity; 85 per cent specificity). However, in another study conducted by Rosenkrantz et al.20 (90 patients, 3T machine and pelvic antenna), the cut-off point for the TCL (6mm) was lower than the cut-off point from our study and also lower than Baco et al.’s study,22 with AUCs of 81.3 per cent (80.4–89.1 per cent sensitivity; 73.1–74.6 per cent specificity)20–a finding that seems hard to replicate in a clinical setting yet despite the differences reported in the image acquisition machines. The reasons for these different cut-off points for the TCL are not quite clear.20
The ADC values are also similar to the ones reported in the medical literature. In a study conducted by Woo et al.,25 one cut-off point of 0.893×10−3mm2/s presents a 75 per cent ADC (92 per cent sensitivity; 55 per cent specificity). Another study conducted by Chong et al.,26 confirmed similar ADC values with an average 0.883±0.18×10−3mm2/s, with significant differences (p<0.001) between patients with ECS (0.729±0.15×10−3mm2/s) and those without ECS (0.985±0.23×10−3mm2/s).
When comparing the characteristics of this study with other studies from the aforementioned medical literature we realized that the studies are similar. They were all retrospective studies. When it comes to the number of patients, this study included 92 patients while other studies ranged from 69 to 117 patients. Similarly, in these studies the pelvic antenna was used the same way it was used in this study. When it comes to the mp-MRI used, Baco et al.’s study22 also used a 1.5T MRI scanner while the other three (3) studies used a 3T MRI scanner.20,25,26 The benefits derived from using 3T MRI scanners instead of 1.5T MRI scanner, and the pelvic antenna instead of the endorectal antenna have been widely discussed. The last PI-RADS consensus from 2015 (versión 2) claimed that although the 3T MRI scanner is superior to the 1.5T MRI scanner, both machines provide an adequate diagnosis when used with the appropriate optimized parameters. Nevertheless, the systematic use of the endorectal antenna is not recommended but it is suggested in 1.5T MRI scanners for image quality enhancement.18
No studies assessing these quantitative criteria have ever been published (TCL+ADC). In this study we found that the sum of both criteria improved the AUC (77 per cent), and that the use of one logistics regression model improved the AUC even more (to 82 per cent), which is a larger (and statistically significant) percentage than the AUCs obtained using each criterion separately.
With the evidence presented it may be deemed possible to use ADC and TCL for their systematic measurement in mp-MRIs in patients with prostate cancer for a better preoperative staging and more precise surgical planning in an effort to reduce local and postoperative side effects such as erectile dysfunction and incontinence.
As a strong point here we have to say that we did not find any studies in the medical literature assessing TCL or ADC values as quantitative values for the prediction of microscopic ECS, so in this regard, our study would be the first one to make correlations between these parameters.
Some of the limitations of this study are that it is retrospective and that the number of patients was low. Future research should include larger populations and prospective studies; also image assessment should be performed by more than just one radiologist.
We think that the results coming from this study allow us to apply new quantitative criteria in mp-MRIs for the diagnosis of ECS, in particular ECS microscopic affectation, for a better surgical planning and prognosis of these patients with moderate and high-risk prostate cancers.
Ethical disclosuresProtection of people and animalsThe authors declare that the procedures followed abide by the ethics and regulations of the Human Research Committee, the World Health Organization and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols from their centres on the disclosure of data from patients.
Right to privacy and informed consentThe authors have obtained prior written informed consent from the aforementioned patients. This document belongs to the corresponding author.
Authors- 1.
Manager of the integrity of the study: DCFC, DAA.
- 2.
Study idea: MFGS, CMPA, DCFC, DAA and JR.
- 3.
Study design: MFGS, CMPA, DCFC and DAA.
- 4.
Data mining: DCFC, DAA, JR and MP.
- 5.
Data analysis and interpretation: MFGS, DAA and CMPA.
- 6.
Statistical analysis: MFGS, DAA and CMPA.
- 7.
Reference: DAA.
- 8.
Writing: MFGS, CMPA, DCFC, DAA, JR and MP.
- 9.
Critical review of the manuscript with intellectually relevant remarks: MFGS, CMPA, DCFC, DAA, JR and MP.
- 10.
Approval of final version: MFGS, CMPA, DCFC, DAA, JR and MP.
The authors declare that they have no conflicts of interest.
Please cite this article as: Granja MF, Pedraza CM, Flórez DC, Romero JA, Palau MA, Aguirre DA. Predicción de la extensión extracapsular en el cáncer de próstata mediante la longitud del contacto tumoral y el coeficiente de difusión aparente. Radiología. 2017;59:313–320.