Screening plays an important role in women with a high risk of breast cancer. Given this population's high incidence of breast cancer and younger age of onset compared to the general population, it is recommended that screening starts earlier. There is ample evidence that magnetic resonance imaging (MRI) is the most sensitive diagnostic tool, and American and the European guidelines both recommend annual MRI screening (with supplementary annual mammography) as the optimum screening modality. Nevertheless, the current guidelines do not totally agree about the recommendations for MRI screening in some subgroups of patients. The first part of this article on screening in women with increased risk of breast cancer reviews the literature to explain and evaluate the advantages of MRI screening compared to screening with mammography alone: increased detection of smaller cancers with less associated lymph node involvement and a reduction in the rate of interval cancers, which can have an impact on survival and mortality (with comparable effects to other preventative measures). At the same time, however, we would like to reflect on the drawbacks of MRI screening that affect its applicability.
En mujeres con alto riesgo de padecer cáncer de mama, la detección precoz tiene un importante papel. Debido a la alta incidencia de cáncer mamario y a edades más tempranas que en la población general, se recomienda que el cribado comience en edad más joven, y existe amplia evidencia de que la resonancia magnética es la herramienta diagnóstica más sensible: las principales guías americanas y europeas coinciden en la recomendación de realizar resonancia magnética anual (con mamografía anual suplementaria) como modalidad óptima de cribado. No obstante, no hay un total consenso actual entre las guías sobre algunos subgrupos de pacientes a incluir en la recomendación de cribado con resonancia magnética.
El objetivo de esta primera parte de nuestro trabajo es, mediante una revisión de la bibliografía, explicar y valorar las ventajas que este tipo de cribado con resonancia magnética proporciona respecto al cribado solo con mamografía, como son: mayor detección de cánceres de menor tamaño y con menor afectación ganglionar asociada y una reducción de los cánceres de intervalo, lo que puede tener repercusión en supervivencia y mortalidad, con efectos comparables a otras medidas de prevención. Pero, a su vez, también queremos reflejar los inconvenientes que el cribado con resonancia magnética conlleva, y que dificultan su aplicabilidad.
When establishing breast cancer (BC) prevention strategies, it must be borne in mind that the risk of suffering from the disease is not equal for the entire population. Certain factors increase the risk and modify the approach to prevention for women. Based on these factors, a woman can be considered to belong to one of three risk groups: normal, intermediate or high (corresponding to an absolute lifetime risk of suffering from BC of approximately ≤15%, 15–20% or ≥20%, respectively).
In patients at normal risk, with an absolute lifetime risk of 15% or less, screening is done with mammography (MG), the only technique that has been proven to significantly reduce mortality.1,2 There are ongoing debates with respect to the optimal frequency of mammograms (every year versus every other year), the age at which screening should start (40–50 years of age), and the age at which screening should stop (70 versus >70 years); these debates are reflected in the different recommendations of guidelines from different countries and continents.
In patients at high risk (HR), with an absolute lifetime risk of 20–25% or greater, in recent years there has been a generalised agreement among the main European and American radiology associations (the European Society of Breast Cancer Specialists [EUSOMA], European Society of Breast Imaging [EUSOBI], Society of Breast Imaging [SBI], American College of Radiology [ACR] and American Cancer Society [ACS]) with regard to recommendations for screening with magnetic resonance imaging (MRI) and MG, with a yearly frequency, and inclusion of the following patients: women with a genetic predisposition, risk due to family history equal to or greater than 20–25% or a history of chest radiotherapy at a young age3–7 (Table 1A). These are patients with a higher incidence of BC at younger ages than in the general population (mammographic density is greater at a younger age) and in whom faster-growing tumours develop. In them, MG detects cancers in a suboptimal stage, and MRI detects a greater number of cancers in an earlier stage.
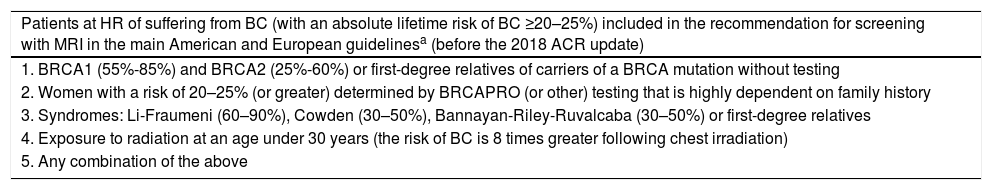
Patients at HR included in the recommendation for screening with MRI.
| Patients at HR of suffering from BC (with an absolute lifetime risk of BC ≥20–25%) included in the recommendation for screening with MRI in the main American and European guidelinesa (before the 2018 ACR update) |
| 1. BRCA1 (55%-85%) and BRCA2 (25%-60%) or first-degree relatives of carriers of a BRCA mutation without testing |
| 2. Women with a risk of 20–25% (or greater) determined by BRCAPRO (or other) testing that is highly dependent on family history |
| 3. Syndromes: Li-Fraumeni (60–90%), Cowden (30–50%), Bannayan-Riley-Ruvalcaba (30–50%) or first-degree relatives |
| 4. Exposure to radiation at an age under 30 years (the risk of BC is 8 times greater following chest irradiation) |
| 5. Any combination of the above |
Agreement of the SBI, ACR, ACS, EUSOMA and EUSOBI, prior to the 2018 ACR update (1 and 2 based on evidence; 3 and 4 based on expert consensus opinion).
Additional note: among the different tests for measuring BC risk, in Spain the one that is most widely used by oncologists is the BOADICEA test.7
Patients with a personal history of BC; a history of prior biopsy with a histology result of atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH) or lobular carcinoma in situ (LCIS); or risk due to family history of BC of 15–20%, used to be considered to belong to the intermediate risk group (elevated compared to the general population, but with insufficient evidence for adding MRI to screening with yearly MG in the absence of other added risk factors).
However, in 2018, the ACR8 updated its recommendation to include screening with MRI for some women who up to then had been considered to be at “intermediate risk”, specifically, “women with personal histories of breast cancer and dense tissue, or those diagnosed by age 50”. They also indicated that additional MRI should be considered in all other women with a personal history of BC or a personal history of prior biopsy with a result of ADH or lobular neoplasm (LCIS or ALH), especially if other risk factors are present. (Table 1B).
| The ACR, in its 2018 update,8 included other patients in its recommendation for screening with MRI: |
| Women with a personal history of BC and dense breasts |
| Women with a personal history of BC, if the BC was diagnosed by age 50 |
| They indicated that all other women with a personal history of BC or a history of biopsy with a result of ADH or lobular neoplasia (LCIS or ALH) should be considered for additional MRI, especially if other risk factors are present |
They included, in their recommendation for screening with MRI in patients at increased risk, several new patient subgroups.
Additional note: among the different tests for measuring BC risk, in Spain the one that is most widely used by oncologists is the BOADICEA test.7
They argued in support of this change that women treated for BC have a risk of recurrence of 19.3% at 10 years and 21.4% at 15 years.9 Specifically in women with dense breasts, the combination of the two risk factors would entail a lifetime risk of suffering from BC in excess of 20% and, in women diagnosed by age 50, the risk of suffering from a second BC would also exceed 20%.10 Women with a personal history of BC are at higher risk of suffering from interval BC than women without said personal history (rate of interval cancer 3.6‰ versus 1.4‰ of screened women). In addition,11,12 the sensitivity of screening with MG in women with a personal history of BC is lower than in women without a personal history of BC (65.4% versus 76.5%) and is even lower in the first five years. Early detection of a second BC improves its prognosis.
Many studies on screening with MRI in women with a personal history of BC have shown high rates of detection (mean: 10–29‰ MRIs performed), with equivalent sensitivity, fewer false positives (FPs) and greater specificity than in patients with a genetic or familial risk, with a similar main benefit: finding invasive cancers (IC) in early stages.13–17
Although in practice it may not be feasible due to lack of resources to screen all patients with a personal history of BC with MRI, the ACR recommendation indicates that MG may be insufficient for purposes of their follow-up. By contrast, most other guidelines recommend follow-up with MG only if the patient does not meet HR criteria, with the exception of the European Society for Medical Oncology (ESMO) recommendation (which recommends MG and ultrasound, and even MRI in special situations such as young women with dense breasts and a genetic or familial predisposition).11,12,18
In patients with a personal history of BC, it may be suitable to perform an additional evaluation of characteristics related to the first cancer, including those depending on the patient (age of presentation, clinical presentation, mammographic density), the primary tumour itself (histological grade, molecular subtype) and the type of treatment received (conservative, radiotherapy). This is because a better understanding of the factors that influence cancer risk and detection on imaging could guide the clinician in preparing more specific follow-up regimens at different intervals and/or using some modality supplementary to MG such as MRI or ultrasound.19 Research must be done to determine which technique is most cost-effective. Obviously, MRI offers greater detection, but due to its cost and accessibility, ultrasound may be more feasible (as in women with a personal history of BC, it also offers detection of other cancers in addition to those detected by MG, 2.8–5‰ in cases of dense breasts20,21; it offers the same when added in cases of dense breasts in general22). The harm done by adding MRI or ultrasound to MG (both generate callbacks and biopsies with FPs) is acceptable in view of the benefit of finding smaller cancers and cancers with negative lymph nodes (the treatment of which is easier than curative treatment).
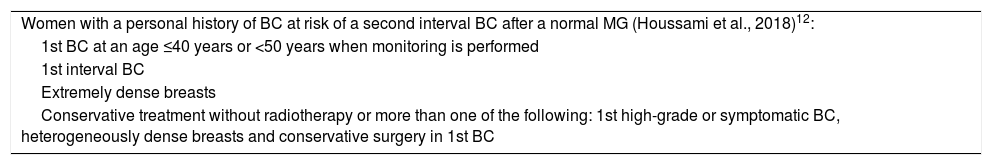
A study published by Houssami et al. in 201812 on screening in women with a personal history of BC specifies those patients in whom a second additional technique should be added to MG to increase sensitivity, considering significant predictive factors for interval IC a year after a normal MG based on the evidence reported (women at higher risk of a second cancer, such as interval cancer, and women in whom the sensitivity of MG is estimated to be less than 55%). Ultrasound might seem more feasible, available and acceptable to the patient (Table 2).
| Women with a personal history of BC at risk of a second interval BC after a normal MG (Houssami et al., 2018)12: |
| 1st BC at an age ≤40 years or <50 years when monitoring is performed |
| 1st interval BC |
| Extremely dense breasts |
| Conservative treatment without radiotherapy or more than one of the following: 1st high-grade or symptomatic BC, heterogeneously dense breasts and conservative surgery in 1st BC |
They indicated which patients should have a second technique added to MG to increase sensitivity. In those women, the sensitivity of MG should be less than 55% (suboptimal), and it was estimated that they are at greater risk of a second cancer such as interval cancer.
They reported that ultrasound might seem more feasible, available and acceptable to the patient.
In this context, abbreviated MRI, with protocols that must be standardised and validated with prospective multicentre studies before they are implemented in routine clinical practice, might render MRI more cost-effective and more accessible in screening in the near future.23
Another technique to consider, which added to conventional MG significantly increases sensitivity, and which also has shown special usefulness in women with a personal history of BC, is MG with contrast (with sensitivity close to that of MRI, such that it could be proposed as another alternative24).
Recent updates to guidelines from Spain recommend adding additional techniques to MG in women with a personal history of BC. The Sociedad Española de Senología y Patología Mamaria [Spanish Society of Senology and Breast Pathology] (SESPM) recommends follow-up with MG and ultrasound (or MRI) in patients with dense breasts or at HR of relapse.25 The Sociedad Española de Oncología Médica [Spanish Society of Medical Oncology] (SEOM) recommends follow-up with MG and ultrasound (and MRI may be indicated in young patients, especially those with dense breasts and a genetic or familial predisposition),26 in line with the ESMO.18
The trend in women with a personal history of BC should be towards personalised post-treatment imaging follow-up, aimed at detecting small, aggressive BCs; decreasing interval BC; and decreasing mortality.23
In the 2018 update to the ACR recommendations in patients at increased risk, Monticciolo et al.8 indicated that something similar happens in, on the one hand, patients with a prior histology diagnosis of ADH or lobular neoplasia (ALH or LCIS) with dense breasts and, on the other hand, patients with a personal history of BC with dense breasts: their lifetime risk of BC is equal to or greater than 20%.27 In this group of patients with ADH or lobular neoplasia there are also published studies on screening with MRI with rates of detection comparable to those for patients with a genetic mutation27–30 (with the similar benefit of finding IC in early stages).
Among patients at increased risk, it is therefore evident that at present there are discrepancies between guidelines with respect to those subgroups of women for whom MRI is to be recommended.
This study seeks, through a literature review, to explain and assess the advantages of screening with MRI in order to justify its use in patients at increased risk, since compared to screening “with MG only” it offers greater detection of smaller-sized cancers and cancers with less associated lymph node involvement as well as a reduction in interval cancers, which could have repercussions for survival and mortality with effects comparable to other preventive measures. The study also seeks in turn to reflect on the drawbacks of screening with MRI that limit its potential for implementation, including its increased cost and limited availability, especially when expanding its use to subgroups of patients up to now included in the intermediate risk group in whom MG is proving insufficient.
Pros of screening with MRIThe recommendation to add MRI to MG in HR screening is based on the fact that it offers significant benefits:
- 1.
Higher rate of cancer detection
The first prospective studies on screening with MRI in HR patients31–39 found that the sensitivity of MRI was twice that of MG; this benefit was confirmed in subsequent studies.40–45 In fact, currently, with more experienced readers and improved standards for acquisition and reports, the sensitivity of MRI is as high as 90%39 whereas that of MG in HR patients is around 25–59%.8 Its specificity is also high, with published figures ranging from 78% to 97%44 (mean 85%-90%), and increases following the first round to 90–95%.43,45
The BC detection rate for MRI in HR is 16–30‰ (versus approximately 7.6–7.9‰ for MG).
- 2.
Detection of cancers in an earlier stage
The use of MRI allows cancers to be detected in an earlier stage compared to when screening is done with MG only:
- •
On the one hand, these are cancers with a smaller size (mean size 7–18mm; around 50% of cancers detected with MRI are ductal carcinoma in situ (DCIS) or T <1cm).39 A prospective study in a population with BRCA comparing detection in screening with MRI to a control group with MG only reported differences in mean size in the ICs detected of 9mm versus 18mm, and in detection of tumours with a T>2cm of 3% versus 29%,46 respectively.
- •
On the other hand, these are cancers with less axillary involvement (11–29%39,41,42,46,47 of cancers detected with MRI have positive axillary lymph nodes, with a mean of 15–16%, versus 30–45% when screening is performed with MG only).
In summary, with the use of MRI there is a significant reduction in large ICs and ICs with positive lymph nodes (stages II–IV).
- 3.
Lower rate of interval cancers
With the use of MRI, the mean rate of interval cancers is around 5.4%, less than 10%,39,46 versus a mean of practically 50% when screening is done with MG only.
Both point 2 and point 3 determine screening efficacy.
- 4.
Increased survival and decreased mortality
One of the major limitations of most studies that have compared screening with “MRI and MG” to screening with “MG” is that they have not shown their repercussions for mortality. Undoubtedly, arguments in favour of decreased mortality must stem from recurrence and survival data from long-term observational studies.
Currently there are observational studies with follow-up periods of 6–10 years that have shown how screening with MRI supplementary to MG, along with some changes in treatment protocols, achieve increased survival and decreased mortality in HR patients47–53 as they detect invasive cancers in early stages.
However, several factors must be borne in mind: (a) mortality in HR should include long, complete follow-up periods of 10 or more years, and more results in this regard must be awaited, and (b) there have been lower survival values than expected in the subgroup with BRCA1. In patients who are carriers of the BRCA1 mutation, greater numbers of interval and later-stage cancers are detected at the time of diagnosis. Annual MRI screening is less effective, and more deaths occur. This is due to a higher frequency of tumours with a triple-negative molecular subtype. There is evidence on the relationship between tumour size and prognosis, and triple-negative tumours at the time of diagnosis in this subgroup of patients are larger in size; this, added to the fact that a substantial portion of them metastasise despite being diagnosed when they are less than 10–15mm, contributes to that worse clinical course.
Survival outcomes from studies by Evans et al.47 (United Kingdom) and Rijnsburger et al.48 (Netherlands) with follow-up periods of 10 years and six years, respectively, showed increases in survival when MRI is added: 95.3% survival with screening with MRI and MG versus 87% (screening with MG only) or 73.7% (no screening) in the study by Evans et al., and 92.7% survival versus 74.5% (screening with MG only) in the study by Rijnsburger et al.
Passaperuma et al.49 (Canada) reported good long-term outcomes in patients with BRCA1/2 for: (a) mortality (annual mortality rate 0.5% after eight years of follow-up), compared to data from the Netherlands48 (1.2% after six years); and (b) distant recurrence (only 3.6% after eight years), also compared to data from the Netherlands48 (which reported that 84% of patients were disease-free after six years).
Although by subgroups survival figures are worse in BRCA1, if “five-year survival” in BRCA1 is compared across different decades, a gradual increase may be discerned: this survival was 50% in the 1980s and 1990s (when there was neither screening with MRI nor the cancer chemotherapies that exist today), whereas in the first couple of decades of the 21st century, this survival is around 90% (with figures of 89±7%, after 9.7 years of follow-up in an Italian study by Podo et al.50, similar to those in the above-mentioned British study47 and Dutch study48). Möller et al.51 (Norway) achieved worse outcomes, with five-year survival in BRCA1 of 75%, possibly because a lower proportion of their patients received adjuvant chemotherapies (65% versus 89% of the study by Podo et al.). Podo et al.50 highlighted the decreased difference in survival between “triple-negative” and “non-triple-negative”: they found a non-significant difference in survival with five years of follow-up (86±9% in “triple-negative” versus 93±5% in “non-triple-negative”) despite the more aggressive characteristics of “triple-negative”, influenced by early detection thanks to screening with MRI and more effective treatments (adjuvant chemotherapy and therapeutic and prophylactic mastectomy). Survival figures are better in BRCA2 and all other HR subgroups; some studies speak of 100% of patients, and in studies with some deaths in patients with BRCA2, these are lower than in patients with BRCA1.47,48,52
Saadatmand et al.53 (Netherlands) compared metastasis-free survival in patients with BRCA or a familial predisposition screened with MRI versus unscreened patients under age 50 and versus patients over age 50 screened with MG every other year. At 10 years of follow-up they developed three times fewer metastases, and the difference in women with a familial risk was more pronounced than in women with BRCA1; differences in women with BRCA2 were not significant.
- 5.
Effect on survival comparable to the other main preventive measure (prophylactic mastectomy)
Grann et al.54 used an analytical model to compare preventive measures in BRCA1/2 and found that preventive surgeries and chemoprevention are more cost-effective than screening with MRI and MG (because screening with MRI is very costly). They deduced that:
- 1.
Prophylactic oophorectomy and mastectomy is the cheapest strategy and the one with the greatest survival “in years of life”, and therefore is more cost-effective, but has lower rates of preference by women.
- 2.
Screening with MRI and MG is the most effective strategy due to its high rate of preference by women and because its high sensitivity is associated with high survival, with the most quality-adjusted life years (QALYs); however, it is the most expensive strategy, it is recurring and it leads to many biopsies/FPs and possible overdiagnosis.
Kurian et al.,55 using a computed simulation model incorporating data from the scientific literature, conducted a study to estimate probabilities of survival and causes of death at ages 70 and 80 for 25-year-old women with BRCA1/2. They compared different strategies to reduce mortality due to cancer in these women. They deduced that replacing prophylactic mastectomy with screening with MRI and MG and keeping prophylactic oophorectomy at age 40 only decreases survival by an estimated 3–5% and has a high rate of preference by women: screening with MRI and MG is expensive, but effective.
Subsequently, a major prospective study by Heemskerk-Gerritsen et al.56 that compared the efficacy of prophylactic mastectomy versus follow-up with MRI and MG found that prophylactic mastectomy decreased BC incidence as well as deaths due to BC or any cause, but after 10 years of follow-up the reduction in mortality found due to BC or any cause “was not significant”. Overall survival was 99% in the group with prophylactic mastectomy and 96% in the group with MRI and MG; this is comparable to the 3–5% increase in survival reported in the study by Kurian et al.
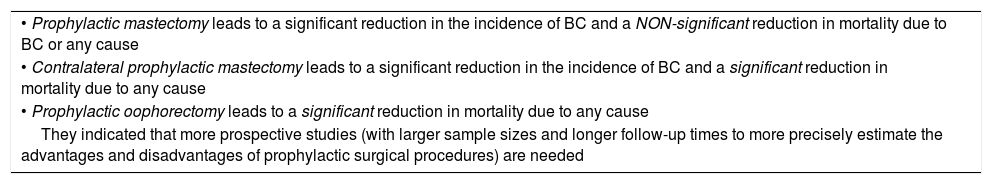
A meta-analysis by Li57 et al. arrived at similar conclusions. In their study, they assessed the effectiveness of prophylactic surgical procedures in patients with BRCA1/2 by reviewing the most representative literature. According to their study, it does seem that mortality reduction could be significant when prophylactic mastectomy is contralateral, in patients already diagnosed with BC (Table 3).
| • Prophylactic mastectomy leads to a significant reduction in the incidence of BC and a NON-significant reduction in mortality due to BC or any cause |
| • Contralateral prophylactic mastectomy leads to a significant reduction in the incidence of BC and a significant reduction in mortality due to any cause |
| • Prophylactic oophorectomy leads to a significant reduction in mortality due to any cause |
| They indicated that more prospective studies (with larger sample sizes and longer follow-up times to more precisely estimate the advantages and disadvantages of prophylactic surgical procedures) are needed |
Screening with MRI entails many drawbacks, listed below.
- 1.
Additional investigation generated by the result of screening with MRI
It does harm in three main ways:
- •
Callbacks/additional imaging (post-MRI targeted ultrasound): (4.1–28%; mean 16–17%).
- •
Additional biopsies: (3–15%).
- •
Short-term follow-up: (7–16%).
However, that harm decreases after the first round, following which the specificity and positive predictive value of callbacks and biopsies decreases, reaching acceptable values in the following rounds.39,44
Although the rate of cancer detection decreases after the first round45 (showing the fraction of prevalent cancers at the start of screening), the continuous yield of the MRI technique in cancer detection is maintained in subsequent rounds. No differences have been detected in T stage, N stage or tumour grade between the first round of screening and successive rounds.44,45
- 2.
High cost
Screening with MRI has a high cost, but it is cost-effective in patients with BRCA and in HR women who are not carriers.58–62
The benefits of early detection in that population (BCs in an early stage cost less to treat) outweigh the costs and FPs, but the cost-effectiveness is highly dependent on the price of MRI. The cost-effectiveness of screening with MRI depends on: (a) the incidence of cancer in the study population, and this is a population with a very high incidence/prevalence of BC, and (b) the cost of the examination, not just the examination in itself but also derivative examinations such as MRI-guided biopsies.
- 3.
Time to perform the study (occupation of the MRI equipment) and time to interpret the report
One way to increase efficiency/cost-effectiveness is to decrease the time to acquire images and to interpret the radiology report for the examination using abbreviated MRI protocols. Using a smaller number of sequences or rapid acquisition sequences may render screening with MRI cost-effective for a wider population range without significantly decreasing detection.23,63
“Abbreviated protocols” are based on the fact that cancers are better observed in the arterial phase, in which there is more contrast between tumour and tissue. The number of sequences is decreased relative to the standard complete protocol: baseline T1 and early post-contrast sequences are performed and then subtracted and reconstructed (maximum intensity projection, MIP); these are essential for detection. They provide morphological information on lesions that enhance in an early phase. Late post-contrast sequences and additional T2 and STIR sequences, which serve to better characterise lesions and the absence of which leads to less diagnostic certainty and a slight increase in follow-ups and callbacks, are omitted.64–66 However, some articles have proposed “combined abbreviated protocols”67–70 which do include, at the expense of a very slight time penalty, at least one T2 sequence, STIR sequence, intermediate sequence or later sequence from the dynamic post-contrast study, which aid in characterising lesions. Some of those articles have stressed70 the importance of performing the final post-contrast phase for preparing kinetic curves and not omitting dynamic information. The sequence protocol would have to be standardised and more studies must be conducted to confirm the benefit of abbreviated MRI and validate it as a viable alternative to standard MRI, but the results to date are promising. Also being studied are short MRI protocols that employ rapid acquisition dynamic imaging techniques using “ultrarapid” sequences that obtain diagnostic images with high spatial resolution while capturing the entry of contrast into breast lesions. This imaging is done with 3-Tesla MRI equipment that combines morphological and dynamic information and offers high spatial and temporal resolution.71–74 These sequences may be added to a conventional abbreviated protocol.75
- 4.
Availability of MRI equipment in a diagnostic imaging department
There are multiple other sections in diagnostic imaging departments with which MRI equipment must be shared; hence, the time slots required for MRI and MRI-guided biopsies are not always available.
- 5.
Availability of an MRI-guided biopsy system
Post-MRI targeted ultrasound and subsequent ultrasound-guided biopsy is known to be the most cost-effective and efficient step in evaluating lesions detected on MRI. However, 30–40% of lesions found on MRI cannot be located on post-MRI ultrasound, and, of them, 11–22% (mean 15%) are malignant: the absence of translation to ultrasound does not rule out the need for (MRI-guided) biopsy76–80 (Fig. 1).
- 6
Use of gadolinium
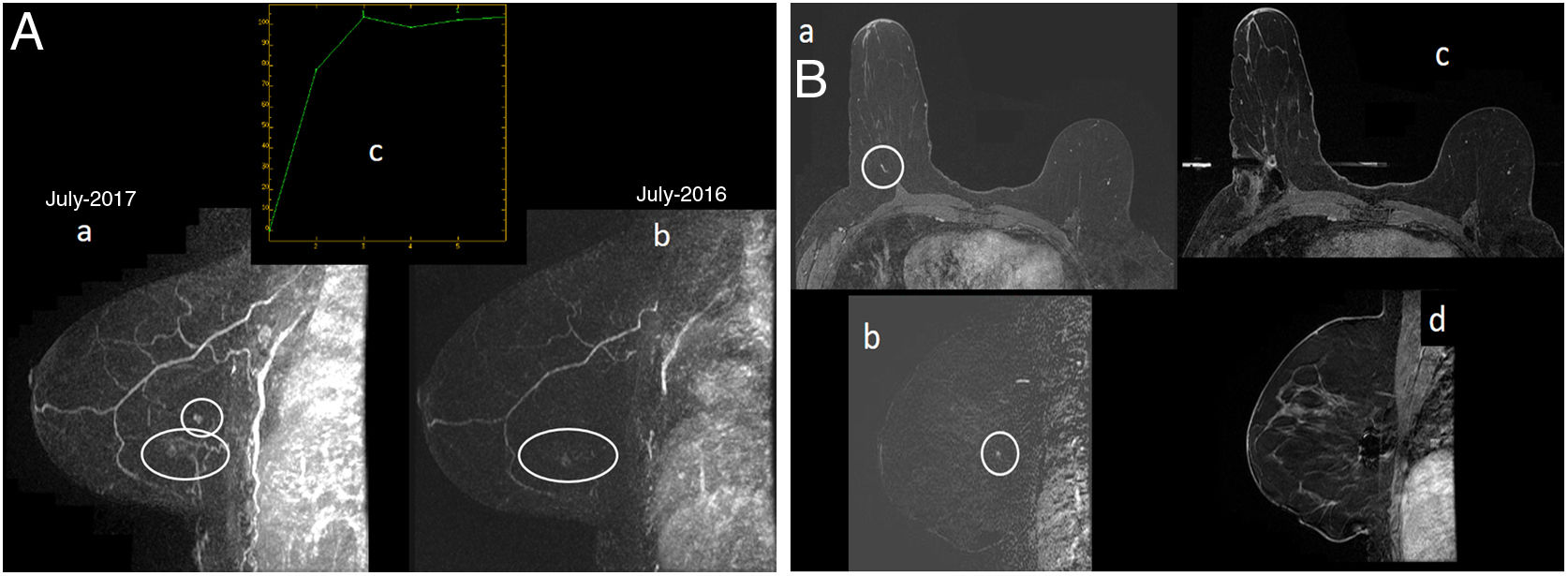
A 53-year-old patient. Follow-up with MRI and MG as she was a carrier of the BRCA1 mutation and had a history of BC in her left breast (infiltrating ductal carcinoma in August 2002, with mastectomy and reconstruction with skin flap and abdominal fat). Prophylactic bilateral oophorectomy in 2010. On right unilateral MG “ACR B” (not shown), only the hamartoma in the lower outer quadrant of the right breast, known and stable, could be visualised. (A) MRI with intravenous contrast. Early sagittal maximum-intensity projection (MIP) reconstruction of the right breast (image “a” in 2017 and image “b” in 2016): enhancing focus of new onset in the outer interquadrants of the right breast on MRI in July 2017 (circle in “a”) adjacent to another focal enhancement in the lower outer quadrant corresponding to a harmatoma, stable compared to her prior MRI in July 2016 (ellipse in “a” and “b”). The focus had a type 2 uptake curve (image “c”). The focus in the outer interquadrant was considered a suspicious finding as it was new. (B) MRI with intravenous contrast and MRI-guided vacuum-assisted biopsy. No translation to ultrasound of the outer interquadrant focus of the right breast was demonstrated; therefore, an MRI-guided biopsy was performed. Delayed 3D axial images are shown with fat subtraction (“a”) and early sagittal subtraction of the right breast (“b”), where the new enhancement focus in the outer interquadrants of the right breast are visualised (circle). In addition, 3D axial images are shown with fat subtraction (“c”) and early sagittal subtraction of the right breast (“d”), both post-biopsy, with signal void related to a metal marker deposited post-biopsy. Result of MRI-guided vacuum-assisted biopsy: grade III infiltrating ductal carcinoma (1mm) + high-grade DCIS (Van Nuys group 3). Molecular subtype: triple-negative. The sentinel lymph node biopsy was negative.
Gadolinium can cause adverse reactions, is contraindicated in patients with impaired kidney function, accumulates in the body and has been shown to lead to signal changes in the dentate nuclei (although the clinical repercussions of this deposition are not well known).63,81 In addition, the cost of the test is increasing.
- 7.
Overdiagnosis
MG is well known to lead to unnecessary biopsies and overdiagnosis of indolent lesions and DCIS. Overdiagnosis resulting from MRI, on the other hand, has not been evaluated.44,61 However, in favour of overdiagnosis from screening with MRI compared to screening with MG, it must be emphasised that ICs are detected more by MRI, whereas most BCs detected with MG are DCIS. MRI detects almost all ICs, as well as biologically significant DCIS82 — i.e. intermediate- and high-grade, potentially invasive and potentially metastatic DCIS — the detection of which would not amount to overdiagnosis. By contrast, few BCs are not detected by MRI and they almost always correspond to low-grade DCIS34; therefore, it is to be expected that there are no significant repercussions for mortality.
In addition, FPs that include “atypias” in screening with MRI are more numerous (at least one study has even indicated that there are twice as many83) compared to findings in screening with MG/tomosynthesis. This carries prognostic significance and clinical repercussions: not all FPs are alike!83
- 8.
False negatives in diagnosis
A considerable number of cancers, approximately 42.5% on average if the literature from the past decade is analysed,84–89 are retrospectively visible on prior rounds (previous MRIs). False negatives (FNs) may occur due to non-detection (cancer not detected for technical reasons), incorrect interpretation (a finding detected is interpreted to be benign whereas in reality it is malignant), or improper management (generally a poor correlation between MRI and post-MRI ultrasound).
It is important to be aware of the main causes of FNs and to know what measures can be taken to attempt to prevent errors and delays in the diagnosis of some cancers. These are analysed below.
- 1.
Technically inadequate examination:
- •
Proper technical quality is necessary and essential for detecting small ICs and DCIS.
- •
A slice thickness of 1–3mm and a pixel size of less than a millimetre must be used,88 movement artefacts must be prevented87 and it must be made certain that the lesion is included in the field87 (care must be taken to include pre-axillary lesions in the antenna).
- 2.
Small lesion size: “foci”, pseudo-nodular enhancements ≤4mm, are common and usually benign findings.
- •
Biopsy of a focus should be considered if the focus enlarges, has suspicious characteristics or is of new onset (there is debate with regard to the latter assumption)84,86,90 (Fig. 2).
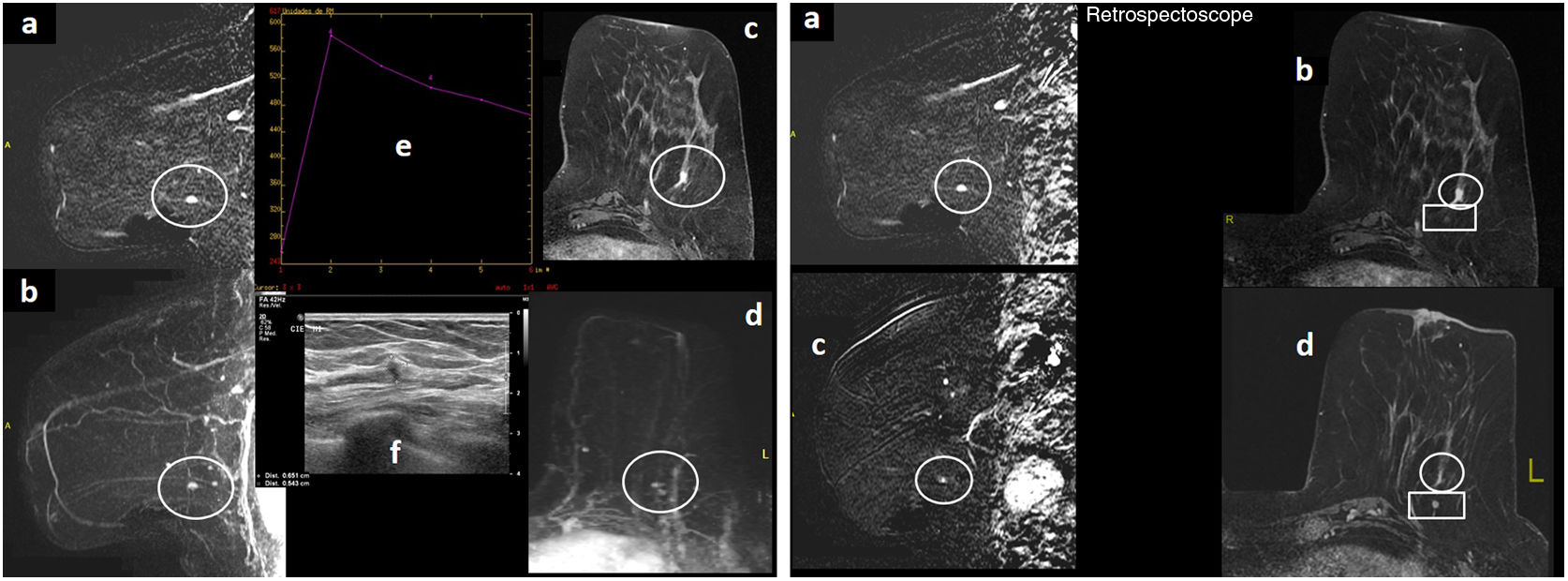
Figure 2.A 68-year-old patient who was a carrier of the BRCA2 mutation with a history of right mastectomy due to BC, follow-up with MRI and MG. Left unilateral MG “ACR A” (not shown), with no significant findings. (A) MRI with intravenous contrast. Early sagittal subtraction of the left breast (“a”) and early sagittal MIP reconstruction of the left breast (“b”), delayed 3D axial images with fat saturation (“c”) and early axial MIP reconstruction (“d”): a nodular focus/enhancement measuring 5mm may be visualised (circle) in the lower outer quadrant of the left breast. It had a type 3 uptake curve (“e”), and on post-MRI ultrasound (“f”) it was seen as a hypoechoic solid nodule with irregular margins measuring 5.5mm. This was a suspicious lesion on MRI. (B) MRI with intravenous contrast. Delayed 3D early sagittal and axial subtraction with fat saturation at the time of diagnosis (“a” and “b”), with a suspicious focus (circle) and identical MRI sequences from the previous year/prior round (“c” and “d”), where a posteriori (with a “retrospectoscope”) a subtle punctiform enhancement focus that had gone unnoticed due to its very small size (false negative) was seen in that location (circle). The suspicious focus was located in front of a circumscribed nodule of a millimetre corresponding to an intramammary lymph node (rectangle), a stable benign finding. The result of the ultrasound-guided core-needle biopsy of the suspicious focus of the lower outer quadrant of the left breast was: infiltrating lobular carcinoma. Molecular subtype: luminal A. The sentinel lymph node biopsy showed 1500 copies (micrometastasis).
(0.15MB). - •
Suspicious morphological characteristics of foci include being isolated, being more intense than other foci, having type 3 curves, changing the curve or the initial slope of the curve, or simply having an initial maximum slope.86,89
- •
At least one author has claimed that, with foci, curves are often not very helpful, because some small ICs (and DCIS) do not have type 3 curves and assessing their morphology is difficult.84,89
- •
There is a certain amount of debate around the management of foci of new onset. Some authors recommend follow-up in 6–12 months90 in view of the low frequency of malignant lesions. Others advocate for biopsying them84,91 considering that some cancers present slow growth, that stability does not always rule out malignancy and that it may be difficult to perceive growth in small lesions in short periods of time. Some authors have reported, for example, a focus with a type 2 or type 3 uptake curve that subsequently appears as interval cancer, especially in patients with BRCA1.88
- 3.
Stability in size:
- •
Stability does not rule out malignancy. Although in HR patients cancers are more aggressive, their growth rate varies and they may present periods of stability.84 Hence, many authors prefer biopsy to follow-up in cases of foci of new onset (Fig. 3).
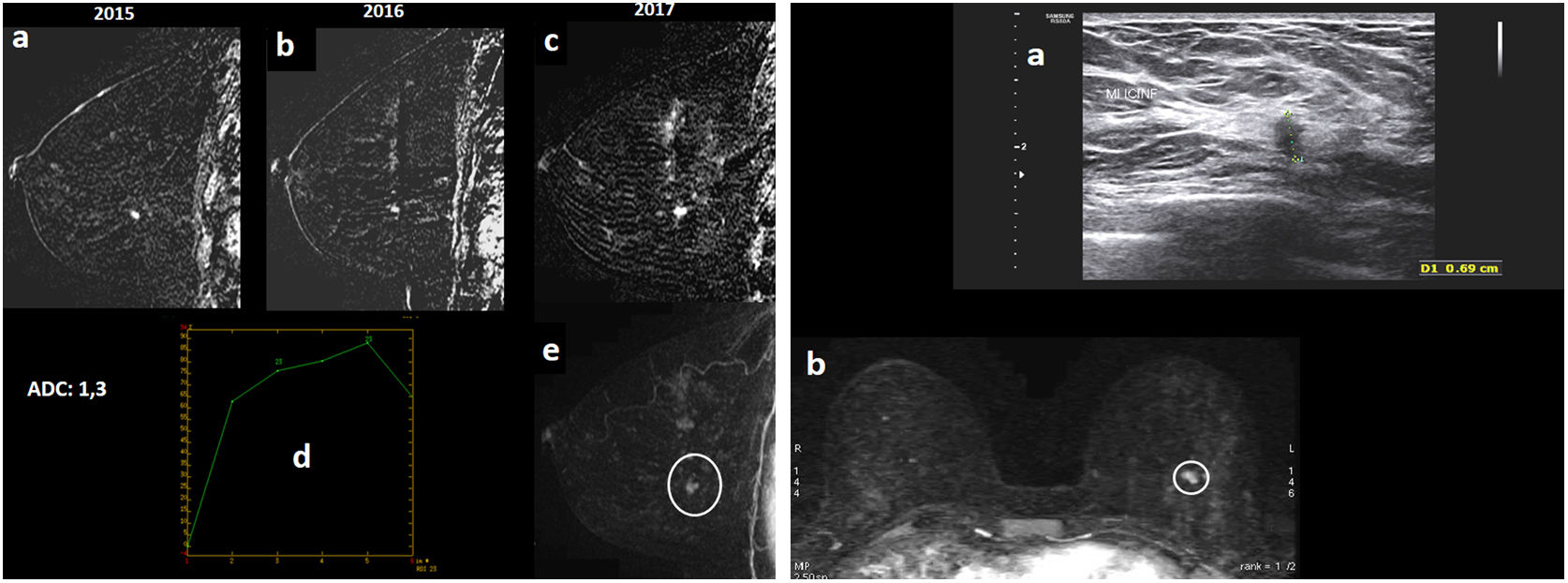
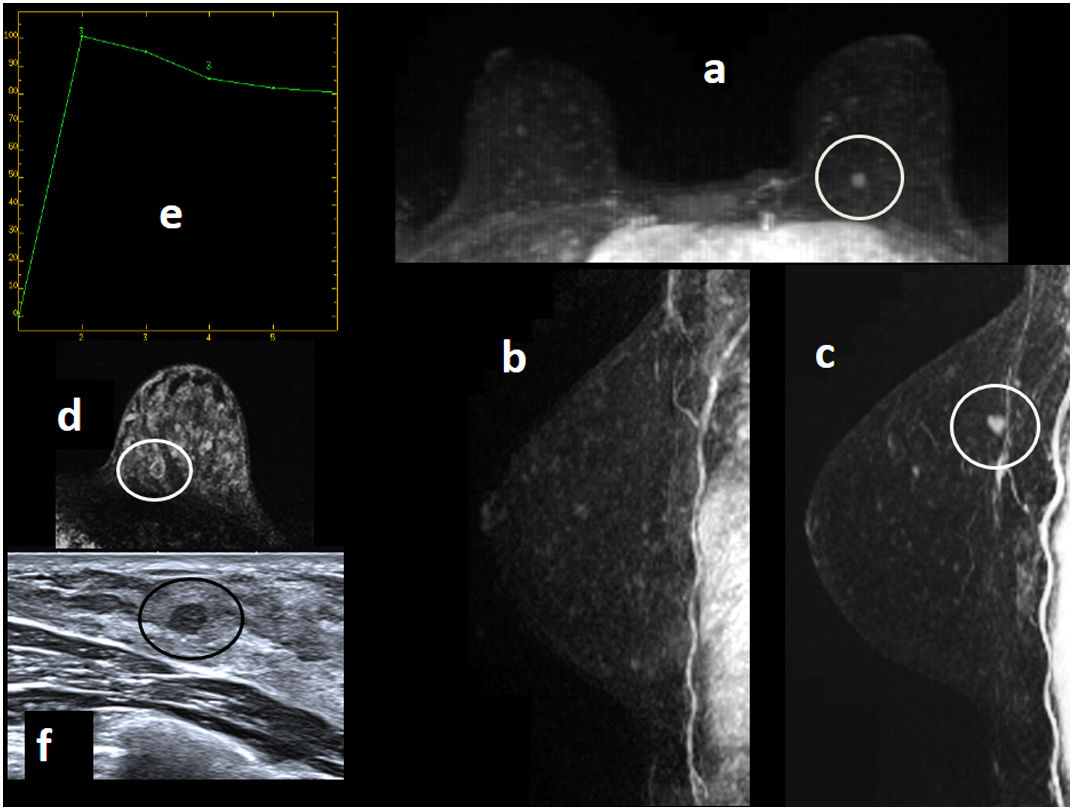
Figure 3.A 46-year-old patient who was a carrier of the BRCA1 mutation went in for annual screening (regular follow-up with yearly MRI and MG due to HR). Bilateral MG “ACR C” (not shown), with no findings. (A) MRI with intravenous contrast. Early sagittal subtractions of the left breast from 2015, 2016 and 2017 (“a”, “b” and “c”) are shown. The study had artefacts due to patient movement which decreased image quality. It was seen that an enhancement focus in the lower outer quadrant of the left breast, which remained stable on her prior MRI in 2016 compared to 2015, showed growth on her MRI in 2017 (nodular enhancement of around 6–7mm). Its apparent diffusion coefficient (ADC) value is transcribed (ADC=1.3×10−3mm2/s, diffusion map not shown); its enhancement curve (“d”), which is gradual with its slope peaking in the first minute and gradually descending and washing out, is shown; and early sagittal MIP reconstruction of the left breast (“e”), with nodular enhancement reported in the lower outer quadrant of the left breast (circle), is shown. (B) Post-MRI ultrasound imaging (“a”) is shown: the nodular enhancement on ultrasound corresponded to a hypoechoic solid nodule with irregular margins measuring 6.9mm. Early axial MIP reconstruction (“b”) is also shown, with nodular enhancement of a millimetre reported in the lower outer quadrant of the left breast (circle): this was considered a suspicious and potentially malignant finding, and an ultrasound-guided core needle biopsy was performed with a result of grade I infiltrating ductal carcinoma. Molecular subtype: luminal A. The sentinel lymph node biopsy was negative.
(0.13MB). - •
Non-mass enhancements that remain stable or exhibit mild growth may be cancers.84 If they are DCIS, they may have microinfiltration in surgery. Therefore, with non-mass enhancements, short-term follow-up must be limited to areas with very low suspicion. It would probably be appropriate to perform follow-up in three months and, if unresolved in different menstrual cycles, do a biopsy. New, persistent and suspicious non-mass enhancements should be biopsied.
- 4.
Extensive background parenchymal enhancement:
- •
Background parenchymal enhancement appears to be associated with greater risk92–94 and lesser detection of BC94,95; however, this matter is disputed in the scientific literature, with articles arguing for and against both. Background parenchymal enhancement may cause a cancer masking effect. Several studies on screening with MRI have pointed to background parenchymal enhancement as one of the causes of their FNs.86–88
- 5.
Circumscribed margins:
- •
The presence of circumscribed margins does not rule out malignancy. Cancers in HR patients may be circumscribed in approximately 17% of cases and there may also be septa without enhancement, classically reported in fibroadenomas.87,96,97 However, in a recent article, Marino et al.98 indicated that the cancer phenotypes detected in screening in HR women using the 5th edition of the BI-RADS (with MG, ultrasound and MRI) have at least one characteristic of malignancy in all modalities (Fig. 4).
Figure 4.A 38-year-old patient with familial HR went in for her first round of annual screening with MRI and MG (she had been classified as a patient at HR as her family history had been identified in genetic counselling). MG: dense breast “ACR D” (not shown) with no findings. MRI with intravenous contrast: early axial MIP reconstruction (“a”), early sagittal MIP reconstruction of the right breast (“b”) and of the left breast (“c”), and delayed 3D axial images with fat saturation of the left breast (“d”) are shown: a prepectoral circumscribed nodular enhancement in the upper inner quadrant of the left breast close to the upper interquadrants, measuring around 6–7mm (circle); a type 3 enhancement curve (“e”); and a post-MRI ultrasound (“f”) corresponding to a circumscribed isoechoic/slightly hypoechoic solid nodule measuring around 6.7mm (circle) are seen. This was considered a suspicious finding. An (ultrasound-guided) post-MRI core-needle biopsy was performed with the following result: grade I infiltrating ductal carcinoma + DCIS. Molecular subtype: luminal B, HER 2+. The sentinel lymph node biopsy was negative.
(0.1MB).
- 6.
Improper correlation between MRI and post-MRI ultrasound:
- •
Correlation is essential to ensure sampling of the lesion of interest.87,91 It is appropriate to use metal markers to confirm that the lesion biopsied by (post-MRI) ultrasound-guided biopsy corresponds to the enhancement of interest on MRI, either with follow-up in six months, or preferably at the same time since, if there is no correlation, then proper diagnosis of potential cancer would not be delayed by six months99 (Fig. 5). It has been reported in the scientific literature that inappropriate sampling is done in up to 8–12% of cases.91
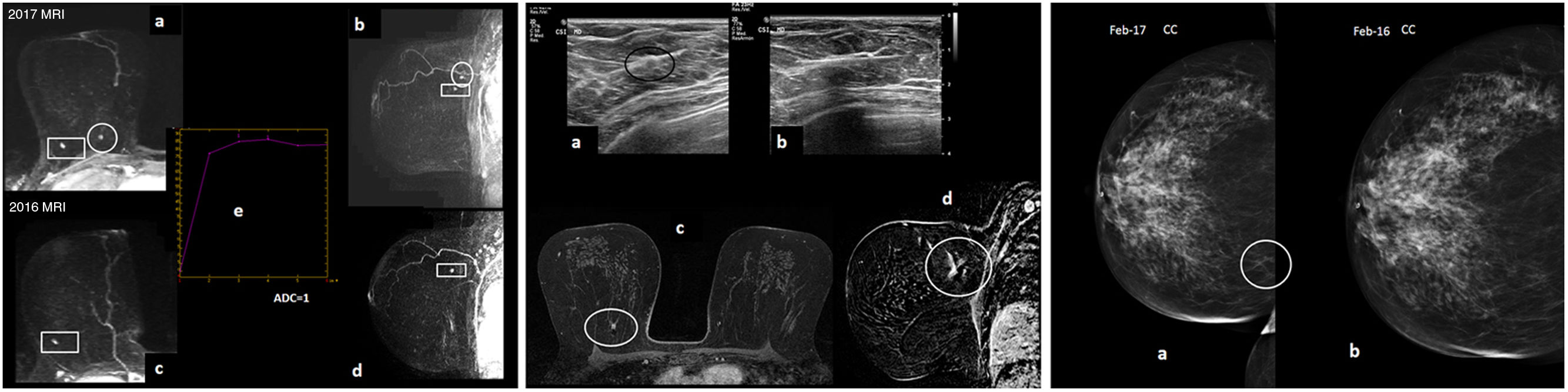
Figure 5.A 49-year-old patient who was a carrier of the BRCA1 mutation went in for annual screening (regular follow-up with yearly MRI and MG due to HR). (A) MRI with intravenous contrast. Early sagittal and axial MIP of the right breast from 2017 (“a” and “b”) and 2016 (“c” and “d”) are shown. An enhancement focus in the upper inner quadrant of the right breast was seen; being of new onset on MRI in 2017 compared to the previous round in 2016 (circle), it was considered suspicious. It had a type 2 enhancement curve (“e”) and its ADC value was transcribed (ADC=1×10−3mm2/s; ADC map not shown). There was another stable nodular enhancement of a millimetre in the upper outer quadrant of the right breast corresponding to an intramammary lymph node, a benign finding (rectangle). (B) Post-MRI ultrasound (“a”’): the new enhancement focus in the upper inner quadrant of the right breast corresponded to an apparently circumscribed isoechoic nodule (so subtle that, in such a large breast, doubts may arise as to whether it corresponded to the enhancement of interest on MRI). An ultrasound-guided post-MRI core-needle biopsy (“b”) was performed and a metal marker was deposited; subsequently, post-biopsy MRI monitoring (delayed 3D axial images with fat saturation [“c”] and sagittal subtraction [“d”] are shown) confirmed that the signal void corresponding to the marker deposited in the nodule biopsied by ultrasound-guided biopsy exactly matched the suspicious enhancement on MRI. Biopsy result: nuclear grade III infiltrating ductal carcinoma with foci of necrosis. Molecular subtype: triple-negative. The sentinel lymph node biopsy was negative. (C) The craniocaudal mammographic projection of the right breast (from the time of diagnosis in 2017 [“a”] and from a year earlier, in 2016 [“b’]) is shown: “ACR B” pattern, a slight translation to MG of the cancer may be visualised as subtle focal asymmetry of new onset in the inner quadrants in 2017 (circle), which was assessed a posteriori, since at that stage synchronous MRI and MG were still performed (and the MRI had already been assessed when the MG was performed, such that MG efforts were targeted).
(0.2MB). - •
If the ultrasound-guided biopsy is not representative or the marker does not correspond to the suspicious lesion on MRI: an MRI-guided biopsy should be performed.
- •
If the MRI-guided biopsy is not representative or the sampling was not appropriate: it should be repeated or surgical removal should be performed.
- 7.
Location in a postoperative area:
- •
A suspicious enhancement should not be assumed to be benign merely because it is found in the postoperative area; it should be biopsied.69
- 8.
Absence of dual reading or computer-aided diagnosis (CAD):
- •
Both the use of dual reading84,88 and the use of computer support systems such as CAD85 increase detection of BC.
A very recently published study indicated that most cancers diagnosed after follow-up at six months from lesions categorised as BI-RADS 3 in screening with MRI in the HR population are diagnosed in an early stage, and that short-term follow-up (six months) is safe, which allows unnecessary biopsies to be avoided.100 They reflect a rate of malignancy of BI-RADS 3 lesions of 6%, but they stress the fact that the percentage of malignancy is greater in lesions categorised as BI-RADS 3 in subsequent rounds (9%) compared to those categorised in the first round (2%); therefore, caution must be exercised in the use of BI-RADS 3 in rounds subsequent to the baseline round.
ConclusionScreening with MRI in women at HR of BC allows ICs to be detected in early stages. This is among the factors that allow patients in this population subgroup not having opted for a prophylactic mastectomy to have a better chance of not dying from BC. The data on repercussions for mortality are still based on studies with follow-up periods equal to or less than 10 years.
The overdiagnosis to which it leads compared to screening with MG has different clinical repercussions with prognostic significance.
The biggest problems are its high cost (although cost-effectiveness in patients with BRCA and HR patients who are not carriers has been demonstrated) and, above all, its applicability. This could improve if abbreviated protocols currently being studied are eventually validated.
All this suggests that the balance between pros and cons when screening with MRI leans towards the pros side.
Authorship- 1.
Responsible for study integrity: SAR.
- 2.
Study conception: SAR.
- 3.
Study design: SAR.
- 4.
Data acquisition: SAR, ABD, JAL, BCC and SSJ.
- 5.
Data analysis and interpretation: SAR, ABD, JAL, BCC and SSJ.
- 6.
Statistical processing: SAR and JAL.
- 7.
Literature search: SAR.
- 8.
Drafting of the article: SAR.
- 9.
Critical review of the manuscript with intellectually significant contributions: SAR, ABD, JAL and BCC.
- 10.
Approval of the final version: SAR, ABD, JAL, BCC and SSJ.
This review received no specific grants from public agencies, the commercial sector or non-profit organisations.
Patient informed consent and dataThis study has the approval of the ethics committee at our centre.
We have written informed consent forms for patients whose images from different imaging tests are shown in this article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to express our gratitude to all the other departments at our hospital centre that play a role in the management of breast pathology (Gynaecology, Oncology, Pathology and Nuclear Medicine) for their efforts and close, continuous collaboration with us (because the fruits of our professional and scientific labour are rooted in the great work done by each one of us and in strong multidisciplinary ties and cohesion, as well as in always trying to do our best with the resources that we have, without ever losing sight of the main objective: the benefit of the patients).
As the first author, I would like to thank my family for their patience and for the time that my dedication to science takes from my dedication to them.
I would also like to thank Ann Marsden for her kind help in translating the abstract into English.
Please cite this article as: Alonso Roca S, Delgado Laguna AB, Arantzeta Lexarreta J, Cajal Campo B, Santamaría Jareño S. Cribado en pacientes con riesgo incrementado de cáncer de mama (parte 1). Pros y contras del cribado con resonancia magnética. Radiología. 2020. https://doi.org/10.1016/j.rx.2020.01.007









![A 49-year-old patient who was a carrier of the BRCA1 mutation went in for annual screening (regular follow-up with yearly MRI and MG due to HR). (A) MRI with intravenous contrast. Early sagittal and axial MIP of the right breast from 2017 (“a” and “b”) and 2016 (“c” and “d”) are shown. An enhancement focus in the upper inner quadrant of the right breast was seen; being of new onset on MRI in 2017 compared to the previous round in 2016 (circle), it was considered suspicious. It had a type 2 enhancement curve (“e”) and its ADC value was transcribed (ADC=1×10−3mm2/s; ADC map not shown). There was another stable nodular enhancement of a millimetre in the upper outer quadrant of the right breast corresponding to an intramammary lymph node, a benign finding (rectangle). (B) Post-MRI ultrasound (“a”’): the new enhancement focus in the upper inner quadrant of the right breast corresponded to an apparently circumscribed isoechoic nodule (so subtle that, in such a large breast, doubts may arise as to whether it corresponded to the enhancement of interest on MRI). An ultrasound-guided post-MRI core-needle biopsy (“b”) was performed and a metal marker was deposited; subsequently, post-biopsy MRI monitoring (delayed 3D axial images with fat saturation [“c”] and sagittal subtraction [“d”] are shown) confirmed that the signal void corresponding to the marker deposited in the nodule biopsied by ultrasound-guided biopsy exactly matched the suspicious enhancement on MRI. Biopsy result: nuclear grade III infiltrating ductal carcinoma with foci of necrosis. Molecular subtype: triple-negative. The sentinel lymph node biopsy was negative. (C) The craniocaudal mammographic projection of the right breast (from the time of diagnosis in 2017 [“a”] and from a year earlier, in 2016 [“b’]) is shown: “ACR B” pattern, a slight translation to MG of the cancer may be visualised as subtle focal asymmetry of new onset in the inner quadrants in 2017 (circle), which was assessed a posteriori, since at that stage synchronous MRI and MG were still performed (and the MRI had already been assessed when the MG was performed, such that MG efforts were targeted). A 49-year-old patient who was a carrier of the BRCA1 mutation went in for annual screening (regular follow-up with yearly MRI and MG due to HR). (A) MRI with intravenous contrast. Early sagittal and axial MIP of the right breast from 2017 (“a” and “b”) and 2016 (“c” and “d”) are shown. An enhancement focus in the upper inner quadrant of the right breast was seen; being of new onset on MRI in 2017 compared to the previous round in 2016 (circle), it was considered suspicious. It had a type 2 enhancement curve (“e”) and its ADC value was transcribed (ADC=1×10−3mm2/s; ADC map not shown). There was another stable nodular enhancement of a millimetre in the upper outer quadrant of the right breast corresponding to an intramammary lymph node, a benign finding (rectangle). (B) Post-MRI ultrasound (“a”’): the new enhancement focus in the upper inner quadrant of the right breast corresponded to an apparently circumscribed isoechoic nodule (so subtle that, in such a large breast, doubts may arise as to whether it corresponded to the enhancement of interest on MRI). An ultrasound-guided post-MRI core-needle biopsy (“b”) was performed and a metal marker was deposited; subsequently, post-biopsy MRI monitoring (delayed 3D axial images with fat saturation [“c”] and sagittal subtraction [“d”] are shown) confirmed that the signal void corresponding to the marker deposited in the nodule biopsied by ultrasound-guided biopsy exactly matched the suspicious enhancement on MRI. Biopsy result: nuclear grade III infiltrating ductal carcinoma with foci of necrosis. Molecular subtype: triple-negative. The sentinel lymph node biopsy was negative. (C) The craniocaudal mammographic projection of the right breast (from the time of diagnosis in 2017 [“a”] and from a year earlier, in 2016 [“b’]) is shown: “ACR B” pattern, a slight translation to MG of the cancer may be visualised as subtle focal asymmetry of new onset in the inner quadrants in 2017 (circle), which was assessed a posteriori, since at that stage synchronous MRI and MG were still performed (and the MRI had already been assessed when the MG was performed, such that MG efforts were targeted).](https://static.elsevier.es/multimedia/21735107/0000006200000004/v2_202009020633/S2173510720300422/v2_202009020633/en/main.assets/thumbnail/gr5.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

