To compare two series of patients with breast cancer, one staged using preoperative MRI and the other staged using conventional techniques, analyzing the changes to treatment, the number of mastectomies, and the number of reinterventions due to involvement of the margins.
Material and methodsWe reviewed 600 patients divided into 300 patients with preoperative MRI (series 1) and 300 without preoperative MRI (series 2). We recorded the following variables: age, menopausal status, tumor size on pathological examination, multiplicity and bilaterality, surgical treatment and type of treatment, the administration of neoadjuvant chemotherapy, and reintervention for involved margins. We used Student's t-test and the chi-square test to compare the variables between the two series.
ResultsThe mean age of patients in the two series were similar (51.5 and 51.8 years, p=0.71). The mean size of the tumor was smaller in series 1 (16.9mm vs 22.3mm) (p<.001). More multiple tumors were detected in series 1 (28.7 vs 15.7%) (p<.001). The rate of mastectomies was lower in series 1 (25 vs 48%) (p<.001). Oncoplastic and bilateral surgeries were performed only in series 1. Neoadjuvant chemotherapy was administered more often in series 1 (30.7 vs 9.3%) (p<.001). The difference in the number of reinterventions for involved margins did not reach significance (7.2% in series 1 vs 3.2% in series 2) (p=.095).
ConclusionWhen MRI was used for staging, neoadjuvant chemotherapy and oncoplastic surgery were used more often and the mastectomy rate decreased. Despite the increase in conservative surgery in patients staged with MRI, the number of reinterventions for involved margins did not increase, although there was a trend toward significance.
Comparar 2 series de pacientes con cáncer de mama, una estadificadas mediante resonancia magnética (RM) preoperatoria y la otra con técnicas convencionales, y estudiar los cambios de tratamiento y el número de mastectomías y de reintervenciones por afectación de los bordes.
Material y métodosSe revisaron 600 pacientes divididas en 300 con RM preoperatoria (serie 1) y 300 sin RM (serie 2). Se valoraron: la edad, el estado menopáusico, el tamaño tumoral anatomopatológico, la multiplicidad y bilateralidad, el tratamiento quirúrgico y tipo de tratamiento, la administración de quimioterapia neoadyuvante y las reintervenciones por márgenes afectos. Las variables fueron comparadas con las pruebas t de Student y la Chi-cuadrado.
ResultadosLa edad media fue similar (51,5 y 51,8 años, p = 0,71). El tamaño tumoral medio fue menor (p < 0,001) en la serie 1 (16,9 vs 22,3mm). Se detectaron más tumores múltiples (p < 0,001) en la serie 1 (28,7 vs 15,7%). La tasa de mastectomías en la serie 1 (25%) fue menor (p < 0,001) que en la 2 (48%). Las técnicas de cirugía oncoplástica y bilaterales solo fueron realizadas en la serie 1. La quimioterapia neoadyuvante fue administrada más frecuentemente (p < 0,001) en la serie 1 (30,7 vs 9,3%). La diferencia no fue significativa (p = 0,095) en el número de reintervenciones por márgenes afectos (7,2% serie 1; 3,2% serie 2).
ConclusiónLas mastectomías disminuyen al emplear la RM, con disponibilidad de técnicas de cirugía oncoplástica y quimioterapia neoadyuvante. Pese al aumento de cirugías conservadoras en la serie con RM, no observamos un aumento significativo del número de reintervenciones por márgenes afectos, aunque existe una tendencia.
Magnetic resonance imaging (MRI) is more sensitive than conventional diagnostic techniques (mammography and ultrasound) to detect breast cancer, particularly in cases of invasive carcinomas.1–5 However, mammography remains the modality of choice for breast cancer screening, with an overall sensitivity rate of 63–98% decreasing to 30–48% in young patients with dense breast tissue.6 In contrast, ultrasound is more accurate than mammography in determining tumor size of invasive carcinomas in patients with dense breast tissue.7 Nevertheless, the combination of both techniques does not yield as accurate results as with MRI when determining tumor extent.
A number of studies have demonstrated that MRI may detect more multifocal and multicentric neoplasms (10–30% of patients with diagnosed breast cancer) and more bilateral tumors (3–9%) than conventional techniques.2,3,7,8 Therefore, MRI often leads to a change in therapeutic protocol in many of the patients with a diagnosis of breast cancer.9–17 However, the clinical significance of MRI findings is currently a matter of controversy, and this technique is not established in the protocol of all hospitals.18–22 The detection of more multiple tumors could lead to an increased number of mastectomies, which is a backward step if we consider breast-conserving surgery to be the standard treatment of breast cancer. Some clinical trials have demonstrated that breast-conserving surgery enables local management of the disease, provides a good cosmetic outcome, and yields a very similar survival rate to that of mastectomy.2,10
This study retrospectively compares two consecutive series of patients with preoperative diagnosis of breast cancer. The patients were staged by MRI in one series and with conventional techniques in the other. Our aim was to evaluate the changes of treatment between both series and particularly assess the differences in mastectomy rate and rate of tumors with uninvolved or negative margins at surgery.
Materials and methodsWe reviewed a sample of 600 women divided into two series of 300 who had consecutively been diagnosed with breast cancer by percutaneous biopsy in our institution. Patients from population-based screening programs are not treated in our hospital, since our institution essentially provides second opinion oncology services. Ethics committee approval and the requirement for informed consent were waived after consultation with the institutional ethics committee.
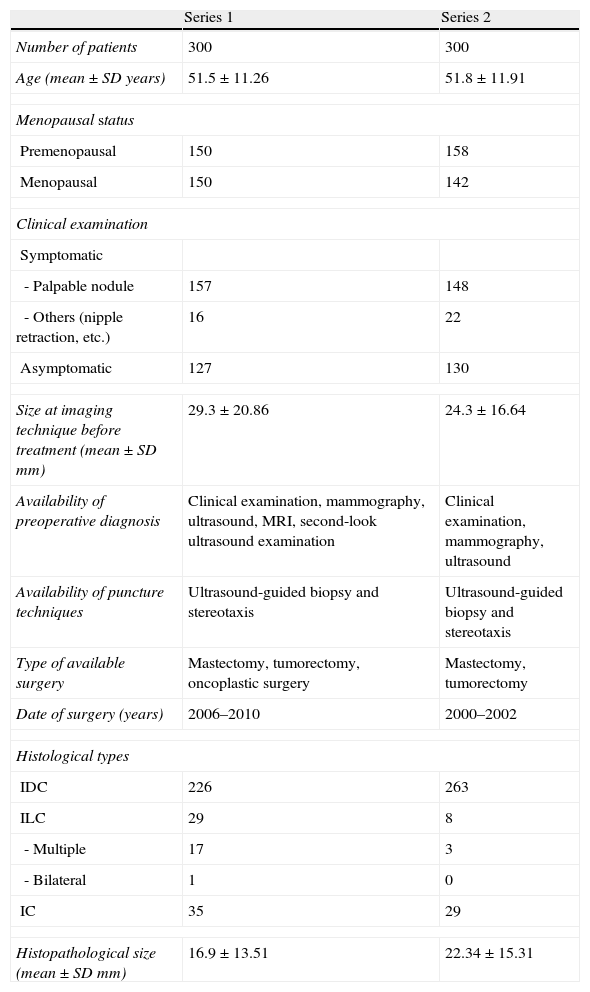
Table 1 shows the description of the two series. Series 1 included 300 patients who underwent surgery between April 2006 and June 2010, and were staged by conventional techniques (mammography and ultrasound) and MRI. Oncoplastic surgery techniques were introduced in our institution during this period. Series 2 included 300 patients who underwent surgery between January 2000 and December 2002, and had been staged by conventional techniques only because MRI was not part of the protocol at that time.
Description of the series.
| Series 1 | Series 2 | |
| Number of patients | 300 | 300 |
| Age (mean±SD years) | 51.5±11.26 | 51.8±11.91 |
| Menopausal status | ||
| Premenopausal | 150 | 158 |
| Menopausal | 150 | 142 |
| Clinical examination | ||
| Symptomatic | ||
| - Palpable nodule | 157 | 148 |
| - Others (nipple retraction, etc.) | 16 | 22 |
| Asymptomatic | 127 | 130 |
| Size at imaging technique before treatment (mean±SD mm) | 29.3±20.86 | 24.3±16.64 |
| Availability of preoperative diagnosis | Clinical examination, mammography, ultrasound, MRI, second-look ultrasound examination | Clinical examination, mammography, ultrasound |
| Availability of puncture techniques | Ultrasound-guided biopsy and stereotaxis | Ultrasound-guided biopsy and stereotaxis |
| Type of available surgery | Mastectomy, tumorectomy, oncoplastic surgery | Mastectomy, tumorectomy |
| Date of surgery (years) | 2006–2010 | 2000–2002 |
| Histological types | ||
| IDC | 226 | 263 |
| ILC | 29 | 8 |
| - Multiple | 17 | 3 |
| - Bilateral | 1 | 0 |
| IC | 35 | 29 |
| Histopathological size (mean±SD mm) | 16.9±13.51 | 22.34±15.31 |
IDC: Infiltrating ductal carcinoma; IC: Intraductal carcinoma; ILC: Infiltrating lobular carcinoma); SD: Standard deviation.
Oncoplastic surgery was used in tumors whose location, size or multiplicity did not enable breast conservation with conventional techniques. This was especially so for large tumors with atypical or multiple locations. However, decision on oncoplastic surgery was made on an individual basis because this surgery depends, among other factors, on breast size and patient preference. Oncoplastic surgery was not performed in series 2 patients because this technique was not available at our institution at that time.
In series 1 patients, the preoperative breast MRI was performed in a 1.5T unit (Symphony, Siemens, Erlangen, Germany) with axial T2-weighted STIR sequences (TR 5600ms; TE 59ms; and slice thickness 2mm) and coronal T1-weighted gradient echo with fat saturation sequences (TR 4.93ms; TE 1.53ms; slice thickness 1.5mm; matrix 512×512; FOV 340; 96 slices per acquisition; and bandwidth of 320Hz/pixel). One non-contrast image was acquired and five were acquired after intravenous administration of 0.15mmol/kg gadolinium (Magnevist®, Bayer, Germany). The total acquisition time of the dynamic study was 6.55min.
All lesions detected on MR imaging were classified according to the BI-RADS categories. Additional lesions detected on MRI were examined by second-look ultrasound with a dedicated system (MyLab™ 60, Esaote, Florence, Italy) and a multi-frequency transducer (7.5–13MHz). All the lesions visualized by ultrasound and classified as BI-RADS 3, 4, and 5 on MRI were biopsied. Because of lack of MRI-guided biopsy, lesions detected on MRI but not visualized on second-look ultrasound examination were included in the same surgical specimen if in close proximity to one another (closer than 20mm) or underwent follow-up if distant from one another.
In series 2, an intraoperative biopsy was performed in patients with infiltrating carcinomas in order to reduce the risk of involved margins after surgery. With respect to intraductal tumors, a specimen radiograph was obtained during surgery, but without intraoperative biopsy.
The parameters assessed in both series were age and menopausal status (clinical parameters); tumor size on histopathological examination as well as multifocality, multicentricity or bilaterality (staging) evaluated throughout the whole case series, especially in infiltrating lobular carcinomas (ILCs); and finally, surgical treatment, which included tumorectomy, oncoplastic surgery techniques, mastectomy or bilateral surgery, neoadjuvant chemotherapy, and rate of repeat surgery when surgical margins are involved.
The data collected were analyzed with SPSS software for Windows™ version 15.0 (Chicago IL, USA). The means were considered normally distributed because normality is assumed when the sample size is sufficiently large (n>30), even if the initial distribution is not normal (central limit theorem). The Student's t test was performed to compare quantitative variables, such as age and histopathological size, and Pearson's chi-square test was used for qualitative variables, with values p<0.05 considered statistically significant.
ResultsClinical parametersNo statistically significant differences were found between the two series regarding patients’ age (p=0.717) and menopausal status (p=0.414).
Staging parametersMean histological tumor size in series 1 (16.9±13.51mm) was significantly lower than in series 2 (22.34±15.31mm) (p<0.001).
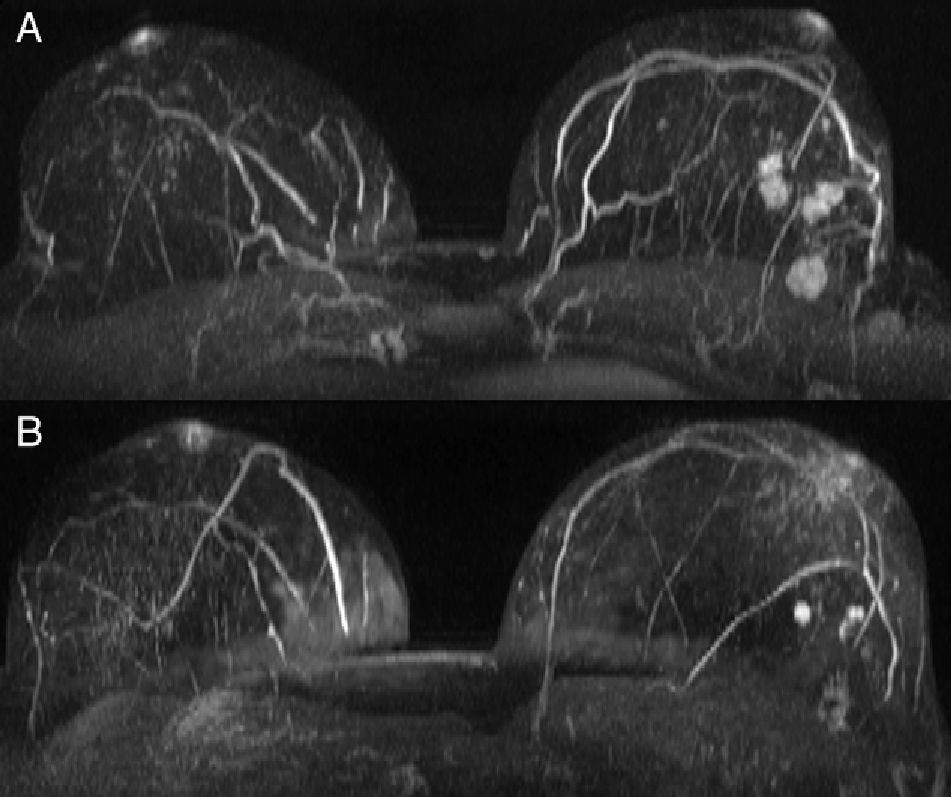
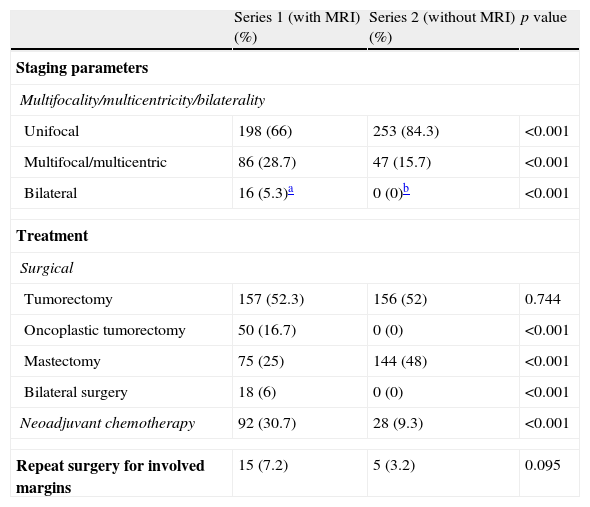
Statistically significant differences were also found in unifocality, multiplicity (multifocal/multicentric) or bilaterality (p<0.001). Series 1 showed a higher rate of multiple tumors (Fig. 1) with a total number of 86 patients (28.7%) in contrast to 47 patients (15.7%) in series 2 (Table 2). Bilateral tumors were only diagnosed in series 1 (16 cases, 5.3%). However, in series 2, 2 patients developed contralateral tumors during the first year after surgery and 4 patients in less than five years after surgery. Thus, as many as 6 patients (3%) developed contralateral carcinomas in less than 10 years. Upon closer review of ILCs in both series (39 cases), 20 of them (51.3%) turned out to be multiple tumors and one (2.6%) a bilateral tumor.
Parameters analyzed.
| Series 1 (with MRI) (%) | Series 2 (without MRI) (%) | p value | |
| Staging parameters | |||
| Multifocality/multicentricity/bilaterality | |||
| Unifocal | 198 (66) | 253 (84.3) | <0.001 |
| Multifocal/multicentric | 86 (28.7) | 47 (15.7) | <0.001 |
| Bilateral | 16 (5.3)a | 0 (0)b | <0.001 |
| Treatment | |||
| Surgical | |||
| Tumorectomy | 157 (52.3) | 156 (52) | 0.744 |
| Oncoplastic tumorectomy | 50 (16.7) | 0 (0) | <0.001 |
| Mastectomy | 75 (25) | 144 (48) | <0.001 |
| Bilateral surgery | 18 (6) | 0 (0) | <0.001 |
| Neoadjuvant chemotherapy | 92 (30.7) | 28 (9.3) | <0.001 |
| Repeat surgery for involved margins | 15 (7.2) | 5 (3.2) | 0.095 |
Fifty oncoplastic (16.7%) and 18 bilateral (6%) surgical procedures were performed in series 1, whereas no surgical procedures were performed in series 2 (Table 2). In addition, the number of mastectomies in series 1 (75 cases, 25%) was significantly lower than in series 2 (144 mastectomies, 48%) (p<0.001). Two of the bilateral interventions were elective because the patients had unilateral tumor with high-risk features, and thus, they opted for bilateral surgery.
MRI findings changed the management in 91 patients (30.3%). In 89 patients (97.8%) surgery was more extensive than initially planned and less extensive in two patients (2.1%). In 68 out of the 89 patients, conservative surgery was replaced by oncoplastic surgery or mastectomy, and in 14 patients, unilateral surgery was replaced by bilateral surgery. In the two patients who underwent minor surgery based on the MRI findings, the shift was from mastectomy to oncoplastic surgery. The change in management based on MRI findings was a wrong choice in four of the 91 patients (4.4%) due to MRI false positives. Only one false negative result was obtained with the second-look ultrasound in the 10 patients with additional lesions detected on MRI and ultrasound examination was not able to detect such lesions subsequently. This was not a case of late diagnosis since the tumor was identified upon contralateral symmetrization during the same surgical procedure.
Neoadjuvant chemotherapy was significantly more extensively used in series 1 patients’ (92 cases, 30.7%) than in series 2 patients (28 patients, 9.3%) (p<0.001).
Finally, there were no significant differences—although there was a tendency toward significance (p=0.095)—in the number of repeat surgery due to positive margins, with a rate of 7.2% in series staged using MRI in contrast with a rate of 3.2% in the series staged by conventional techniques.
DiscussionThis study demonstrates that the combination of MRI and oncoplastic surgery techniques as well as a greater use of neoadjuvant chemotherapy has led to a decrease in the number of mastectomies with no significant increase in the number of repeat surgery for involved margins, in spite of the higher number of multiple tumors detected.
In our study, the histopathological size of the tumor was significantly smaller in the series staged by MRI, despite the fact that the size determined by MRI in this series (29.3±20.86mm) was larger than that in the other series (24.3±16.64mm). This difference in histopathological size may primarily be due to the fact that a higher rate of patients (30.7%) underwent neoadjuvant chemotherapy in series 1 than in series 2 (9.3%). This smaller histopathological size may have had an impact on the decrease in mastectomy rate.
Breast MRI is regarded as the most sensitive technique for infiltrating cancer detection,23 and can be of help in young women with dense breast tissue.24 MRI is able to detect a higher number of multiple tumors (multifocality and multicentricity) than conventional techniques.8 This implies that the initial surgical planning is changed by a more extensive surgery in up to 30% of patients staged by conventional techniques.3,20,21 MRI is able to detect additional lesions in up to 34% of patients.25,26 In our study, MRI detected a higher number of multiple tumors (28.7 vs 15.7% in the series without MRI), and changed the surgical management in 30.3% of patients, almost always entailing more extensive surgery (97.8% of cases) than initially planned. This change of treatment based on the MRI findings was a wrong choice in four out of the 91 patients (4.4%). This was due to MRI false positives, either because the additional lesions detected turned out to be benign (a more extensive surgery was performed, but no mastectomy) or because size was overestimated.
A probability of at least 2–3% is estimated for bilateral synchronous tumors to develop in patients with diagnosed breast cancer. These tumors go unnoticed by mammography in 75% of cases.7 MRI is able to detect them in 3–9% of patients with diagnosed breast cancer.3,7,20 Some studies have demonstrated that the ILC histologic subtype and age <55 years are found to increase the risk of developing bilateral tumors.27 Therefore, preoperative MRI may be advisable for all patients with diagnosed breast cancer, and particularly, for young patients with dense breast tissue and histologic diagnosis of ILC.8 In our study, bilateral synchronous tumors were detected in 5.3% of patients staged by MRI but no such tumors were detected when the patients were staged only by conventional techniques. However, six patients in this series later developed cancer in their contralateral breast. Accordingly, 3% of patients in this series developed contralateral breast cancer within less than 10 years. When multiplicity/multicentricity and bilaterality were considered, it was noteworthy that 20 (51.%) of the 39 cases of ILC featured multiple tumors and one (2.6%) featured a bilateral tumor. One of the limitations of MRI is its relatively low specificity, so that this technique detects both benign and malignant lesions.2,5 In fact, approximately 20% of additional lesions detected on MR are benign.11,25 For this reason, any MR examination should be followed by second-look ultrasound and by a review of mammograms targeting additional lesions. If there is the slightest suspicion of malignancy, an ultrasound-, MRI-guided or stereotactic biopsy, should be performed if ultrasound and mammography yield negative results.25 According to some authors, between 10 and 14% of additional lesions detected on MRI with no second-look ultrasound correlation are finally malignant.1,28–31 In our study, second-look ultrasound examinations yielded one false negative out of the 10 cases that showed no ultrasound correlation. Second-look ultrasound was performed on those women showing new suspicious MRI enhancing lesions that had not been previously detected by conventional imaging. This resulted in a change of treatment in 30.3% of patients. All these lesions were tracked down by guided ultrasound after review of the mammograms, and were biopsied under ultrasound guidance when visible. When the lesions could not be visualized at ultrasound, the lesions were resected if in close proximity to the primary tumor or, if they were distant from it, follow-up examinations were advised because MRI-guided biopsies were not available. It is very important to biopsy lesions detected on preoperative MRI to avoid more extensive surgery associated with overestimation of tumor extent.2
With respect to the treatment used, one of the goals of preoperative staging is to be as accurate as possible in the size of tumor resection. Oncoplastic surgery techniques are useful to resect large tumors with atypical or multiple locations in one single surgical specimen, which reduces the number of mastectomies. MRI is an accurate technique to measure tumor size,2,12,21,29,30 which conventional techniques mostly underestimate, especially with tumors larger than 2cm.32 Underestimation may lead to incomplete resection of the tumor, and thus, to an increased rate of repeat surgery due to involved margins or early recurrences.33–35 In contrast, other studies have demonstrated that MRI overestimates tumor size in 2–39% of infiltrating tumors.4,31
The use of preoperative MRI has become a matter of controversy in the literature because it may lead to an increase in the number of mastectomies. A number of studies argues for the use of preoperative MRI, whereas others argue against it.3,9,15,16,18,19,22,36 Additionally, a recent clinical trial (COMICE trial) has evaluated the clinical efficacy of MRI, finding no statistically significant differences between the rate of repeat surgery for local recurrences in patients staged by conventional techniques only and those staged by MRI as well.37 In our study, the number of mastectomies was significantly lower in patients staged by MRI (25%) than in the series of patients staged by conventional techniques (48%). Since MRI is the most sensitive technique for preoperative staging, we suggest its use in patients who are to undergo breast-conserving surgery, thus enabling an adequate surgery planning and avoiding local recurrences. A number of studies have demonstrated that up to a third of patients treated with breast conservation undergo reexcision to obtain clear margins.38
In our study, although breast-conserving surgery rates increased in series 1, no statistically significant differences were reported in the rate of repeat surgery for involved margins between both series (7.2% in series 1 vs 3.2% in series 2) although there was a tendency toward significance (p=0.095). This rate is substantially lower than those reported in the literature.38,39 Our results may have been influenced by the use of intraoperative biopsy of the infiltrating tumors and intraoperative radiography of tumors with microcalcifications.40
Our study has some limitations. First, it is a retrospective study, and thus, subject to more bias. Second, histopathological and radiological tumor sizes varied between series, with larger sizes and a higher number of mastectomies in series 2 and larger radiological sizes in series 1, which justifies a greater use of neoadjuvant chemotherapy in this series. In the third place, the type of surgery performed was different in each series. The MRI-staged series not only involved conventional tumorectomies and mastectomies, but also oncoplastic surgery techniques and bilateral procedures. In contrast, surgery in the series staged by conventional techniques was exclusively conventional tumorectomies and mastectomies. Therefore, the remarkably high number of mastectomies in this series (49%) may in part be due to the fact that no oncoplastic surgery techniques were implemented or that neoadjuvant chemotherapy was administered in a much lower number of patients than in series 1 (9.3 vs 30.7%).
In conclusion, our study demonstrates a decline in the overall number of mastectomies in the series staged by conventional techniques and preoperative MRI, in spite of a higher rate of multifocal and multicentric tumors diagnosed in this series. Oncoplastic surgery techniques and neoadjuvant chemotherapy treatments have had a pivotal role in the decline in the number of mastectomies. In spite of the increase in the number of breast-conserving procedures, no significant differences were found in the number of repeat surgery for involved margins, although there was a tendency toward significance. Therefore, preoperative MRI is recommended for preoperative staging of breast cancer, but further prospective or retrospective research is required to provide additional evidence supporting this conclusion.
Authorship- 1.
Responsible for the integrity of the study: L. Pina and A. García-Lallana.
- 2.
Conception of the study: L. Pina, F. Martínez-Regueira, N. Rodríguez-Spiteri and A. García-Lallana.
- 3.
Design of the study: L. Pina, A. Elizalde and A. García-Lallana.
- 4.
Acquisition of data: A. García-Lallana.
- 5.
Analysis and interpretation of data: I. Antón.
- 6.
Statistical analysis: I. Antón.
- 7.
Bibliographic search: R. Saiz-Mendiguren and A. García-Lallana.
- 8.
Drafting of the paper: L. Pina and A. García-Lallana.
- 9.
Critical review with intellectually relevant contributions: L. Pina, A. García-Lallana, I. Antón and R. Saiz-Mendiguren.
- 10.
Approval of the final version: L. Pina, A. García-Lallana, I. Antón, R. Saiz-Mendiguren, A. Elizalde, F. Martínez-Regueira and N. Rodríguez-Spiteri.
The authors declare not having any conflict of interest.
Please cite this article as: García-Lallana A, et al. La estadificación con resonancia magnética puede cambiar el manejo terapéutico en el cáncer de mama. Radiología. 2012. doi:10.1016/j.rx.2011.12.007.