Medicinal plants are known as a prolific source of secondary metabolites which have important function both in vivo and in vitro during the ovarian folliculogenesis and steroidogenesis in many animal species. Some secondary metabolites can act as antioxidants generally through their ability to scavenge reactive oxygen species (ROS) or can regulate ovarian hormonal production. In general, these properties are responsible for the medicinal functions to treat woman infertility disorder. Some plants are constituted of biological actives substances which have been used to treat reproductive dysfunction. However, until recently, little was known about the implication of plants and/or their secondary metabolites on in vitro folliculogenesis and steroidogenesis. With the development of the technology, there is an increase implication of those substances in assisted reproductive technology (ART). The present review highlights some medicinal plants used in the treatment of woman disorders related to infertility. In addition, it provides an in vivo and in vitro overview of herbs and their active compounds with claims for improvement of ovarian activity thus showing their implication in female reproductive health care.
Sabe-se que as plantas medicinais são uma fonte abundante de metabólitos secundários que têm função importante tanto in vivo quanto in vitro durante a foliculogênese e a esteroidogênese ovarianas em muitas espécies animais. Alguns metabólitos secundários podem atuar como antioxidantes, geralmente através de sua capacidade de eliminar espécies reativas de oxigênio (ROS) ou podem regular a produção hormonal ovariana. Em geral, essas propriedades são responsáveis pelas funções medicinais usadas para tratar distúrbios da infertilidade feminina. Algumas plantas contêm substâncias biológicas ativas que têm sido utilizadas para tratar a disfunção reprodutiva. No entanto, até recentemente, pouco se sabia sobre o efeito das plantas e/ou seus metabólitos secundários na foliculogênese e na esteroidogênese in vitro. Com o desenvolvimento da tecnologia, há uma implicação crescente dessas substâncias na tecnologia de reprodução assistida (TRA). A presente revisão destaca algumas plantas medicinais utilizadas no tratamento de distúrbios femininos relacionados à infertilidade. Além disso, fornece uma visão in vivo e in vitro de ervas e seus compostos ativos com alegações de melhora da atividade ovariana, mostrando assim seu envolvimento nos cuidados de saúde reprodutiva feminina.
Infertility is a disease of the reproductive system which affects both men and women with almost equal frequency. It is a global phenomenon affecting an average of 10% of human reproductive age population.1 Many conditions can be associated to this problem, including intrinsic (anatomic, genetic, hormonal and immunological disorders) and extrinsic factors such as sexually transmitted infections (STIs), infections after parturition or surgery, tuberculosis of the pelvis, and obesity.2,3
There are a range of medical treatment options for infertility, such as the use of commercial treatments to stimulate “superovulation” which correspond to the development and release of more than one egg per ovulatory cycle. In addition, ART is commonly applied to solve infertility problems, including procedures to bring about conception without sexual intercourse. Among the available techniques, in vitro maturation (IVM), in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI) and intrauterine insemination (IUI)4 are frequently applied. As an alternative, medicinal plants can also be used to solve part of the reproductive problems. Due to their chemical composition, many plants have showed beneficial properties in the folliculogenesis and steroidogenesis through their antioxidant properties and regulation of some enzyme of the steroidogenesis.5–8
For a better understanding of the medicinal properties of crude plant extract or secondary metabolites on the regulation of reproductive function (folliculogenesis and steroidogenesis), many in vivo studies have been performed.5,6,8,9 Several studies showed that the plant secondary metabolites act either directly on ovarian cells to eliminate the ROS or through action on several enzymes such as catalase, glutathione, superoxide dismutase and glutathione peroxidase.10–12 On the other hand, plants (infusion, decoction, beverages, crude extracts) showed their implication during the steroidogenesis through their capacity to mimic the biologic effects of endogenous hormones. These plant medicine derivatives can act by binding to their nuclear receptor or regulating the activities of key enzymes of their metabolisms.6,13
The present review is an attempt to consummate the available scientific information on various medicinal plants, which have been evaluated for their effect on female reproduction. Among all the female reproductive organs, only the ovary is discussed on this review since it is the site of the folliculogenesis and steroidogenesis.14 The review also includes known evidences collected for the involvement of plant extracts in vivo and in vitro. A number of plants and/or secondary metabolites have been discussed in detail and a few others were only tabulated; a major criterion for this arrangement was the ethnopharmacological relevance of the plant.
Mammalian ovary, folliculogenesis and ovarian folliclesThe mammalian ovary is the female gonad which contains germ cells responsible for the perpetuation of the species. Furthermore, it is also the reproductive gland controlling many aspects of female development and physiology.15 That is why it is important for the reproductive biologists to understand not only the normal functioning of the ovary but also the pathophysiology and genetics of diseases such as infertility.
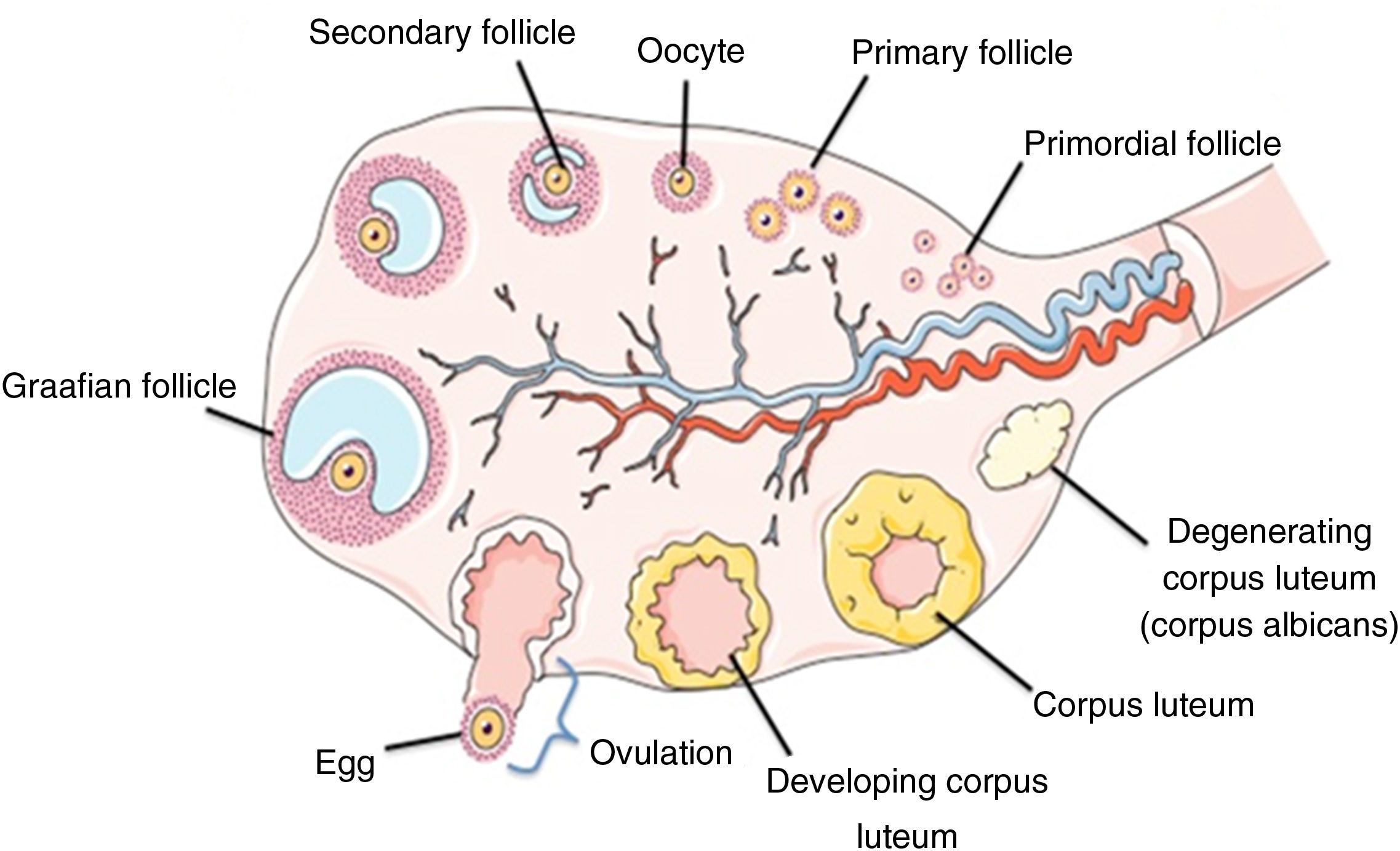
The ovary consists of many types of differentiated cells, which work together, promoting an ideal environment to perform the endocrine and exocrine functions. Those functions are performed by different factors such as autocrine, paracrine, juxtacrine and endocrine are essential for ovarian folliculogenesis.16 Folliculogenesis is the result of a complex and closely integrated series of events which start generally soon after conception. This process can be defined as the formation, growth and maturation of follicle, starting with the formation of the oocyte surrounding by the granulosa cells which formed the primordial follicles.17
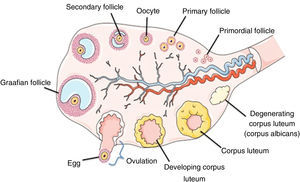
Besides the granulosa cells, the thecal cells are recruited to the oocyte and are directly or indirectly necessary for the oocyte development, physiology and survival. The dynamic of the ovarian folliculogenesis is classified in different stages known as: (a) formation of the primordial follicles; (b) recruitment into the growing pool to form a primary, secondary, and tertiary follicles; (c) lastly ovulation and subsequent formation of a corpus luteum.18 In most species, the mammalian ovary shows extensive variation mainly in relation to the interstitial tissue of the organ, the so-called interstitial gland, and the degree of gonad regionalization, which implies the existence of a cortex and a medulla.15 The internal part consists of fibroelastic connective tissues, nerve and vascular tissues (medula) whereas the external part called the cortex is located at the outer layer and is surrounded by the germinal epithelium, which contains the ovarian follicles and corpora lutea in various stages of development or in regression16 (Fig. 1).
Schematic representation of the ovarian structure. Adapted from http://faculty.southwest.tn.edu/rburkett/A&P2_reproductive_system_lab.htm.19
The follicle which represents the morphological and functional unit of the mammalian ovary consists of an oocyte surrounded by somatic cells (granulosa and/or theca) organized or demarcaded by the basement membrane.
Before formation of an ovarian follicle, oocytes are present within germ cell clusters. Primordial follicle formation occurs when oocytes that survive the process of germ cell cluster breakdown are individually surrounded with squamous pre-granulosa cells. This process takes place during the latter half of fetal development in humans and in the days immediately following birth in mice.18,20 In mammals, the population of primordial follicles serves as a resting and finite pool of oocytes available during the female reproductive life span. Germ cell cluster breakdown, primordial follicle formation, and subsequent recruitment remain the least understood steps of folliculogenesis, that is why key regulators of these initial stages of follicle development continue to be identified. Furthermore, despite many unanswered questions during this crucial period, the concept of ovarian cross talk between oocytes and somatic cells is apparent from the formation of primordial follicles onward.21,22 After differentiation of the primordial germ cells, oogonia undergo mitotic proliferation with incomplete cytokinesis, leaving daughter cells connected by intercellular bridges. The majority of germ cells in a cluster divide synchronously such that a single germ cell cluster contains 2n germ cells.23 Germ cells subsequently enter meiosis, becoming oocytes. Individual oocytes within these nests lack surrounding somatic cells, and the majority of the oocytes will undergo apoptosis as the germ cell clusters break down to give rise to primordial follicles.
The primordial follicles represent the first category of follicles. After their formation, the granulosa cells stopped multiplying and enter a period of quiescence. Throughout the life of the female, a small group of follicles is stimulated to grow gradually, forming the activation follicular phase. The first sign of activation of primordial follicles is the resumption of proliferation of granulosa cells. Upon activation, a series of events that increases the number of granulosa cells, formation of the zona pellucida and oocyte diameter increased leading to the formation of other categories of preantral follicles, primary and secondary follicles occurs. Once activated, the follicles enter a pre-programmed course of development and maturation which is necessary for successful ovulation and fertilization or alternatively are lost through the process of atresia.24 The second category of follicles is characterized by the organization of granulosa cells in several layers and formation of a cavity filled with follicular fluid called antrum. This follicular fluid consists of water, electrolytes, serum proteins and high concentrations of steroid hormones secreted by granulosa cells.25 However, throughout the life of the female, only a small group of follicles, approximately 0.1%, reached ovulation,26 thus reducing the reproductive potential of the female. In several pathological conditions, the woman can suffer of premature ovarian failure (POF) caused by different factors: endocrine, paracrine, genetic and metabolic factors such as high production of ROS.27,28
ROS production in the ovaryOxidative metabolism is indispensable for energy production of ovarian follicle, which in turn results in generation of ROS (oxygen hydroxide, superoxide ion, heavy metals and free radicals). Although a critical amount of ROS is essential for their physiological activities, excessive amount of them causes oxidative stress,29 damage to mitochondria and also to cellular structures such as the membrane lipids, damage to nucleic acids and proteins.27 It does become necessary to use antioxidants to counteract this overproduction of ROS.30
Prevention of oxidative stress is vital in order to maintain normal reproductive function.31 Sources of ROS during ART procedures could either be endogenously from gametes or via exogenous environmental factors.32 However, unless measures are taken to curb ROS production, both the endogenous and exogenous sources of ROS will ultimately lead to the development of oxidative stress, which would then have negative impact on follicles development, oocyte maturation, fertilization rates and pregnancy outcome. Valorization of natural compounds of plants could improve and be an alternative to reduce the cost of ART.
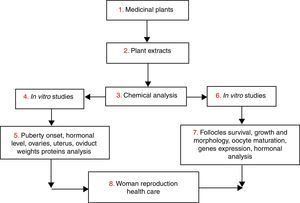
PhytotherapyPhytotherapy can be defined as the use of medicinal plants in the prevention, relief or cure of diseases. A plant can be considered as medicinal when the whole plant or at least one of its parts has one or more medical properties.33 Medicinal plants are used by the people to treat several diseases, including to solve infertility problems. In this context, some plants are rich in compounds which exhibit regulator effect on reproductive function acting directly or indirectly on the hypothalamic–pituitary–ovarian axis by induction or inhibition of ovulation and steroidogenesis disrupting hormonal functioning of the hypothalamus and pituitary gland.5 Their use can bring direct answers to some health problems such as reproductive disorders. The use of medicinal plants (Fig. 2) in response to reproductive problems can be seen as an alternative to manufactured drugs, especially in developing countries where they are expensive and/or inaccessible.34
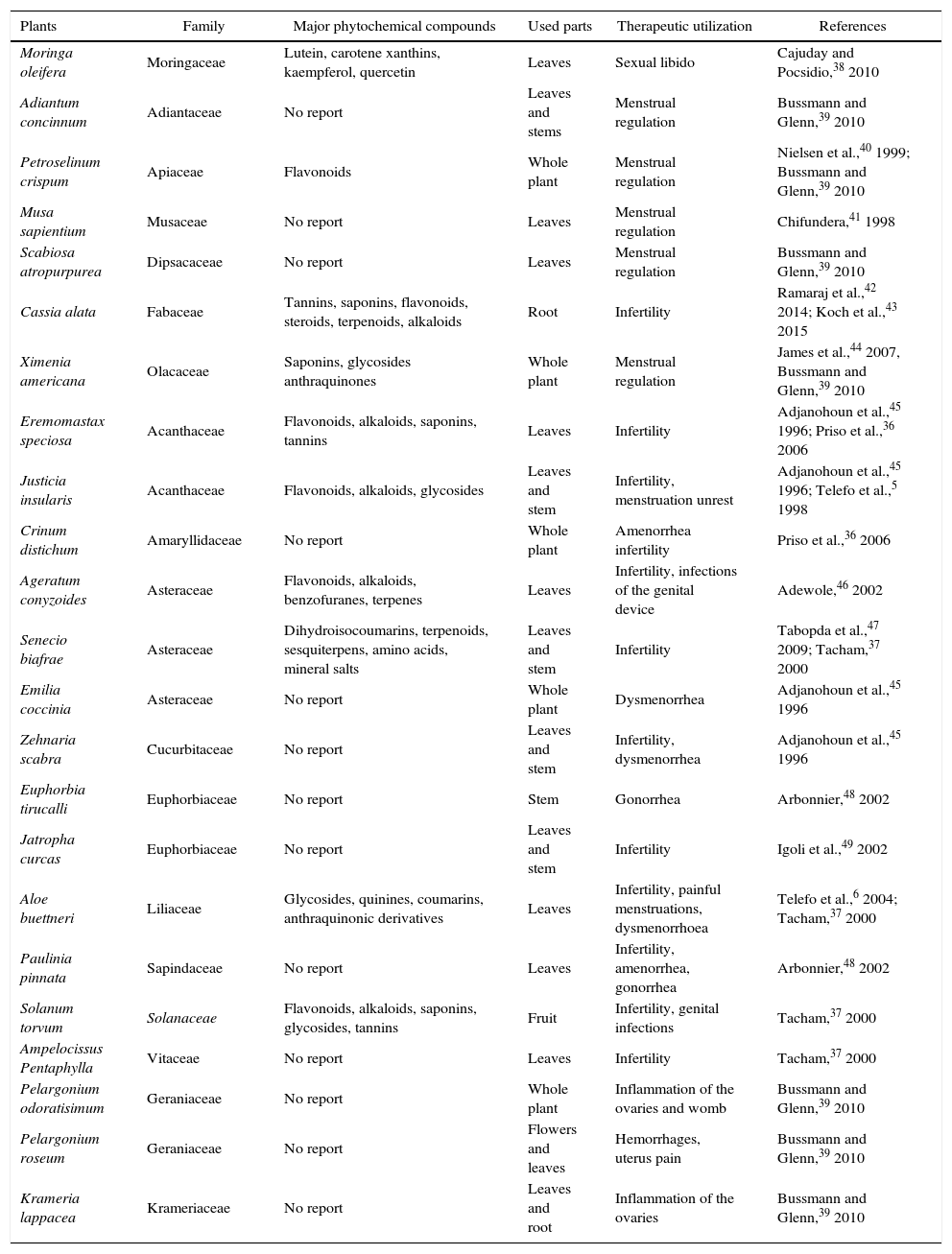
Several studies have shown the beneficial implication of natural compounds on the woman reproduction acting directly on the reproductive organs or indirectly regulated physiological process. For example, studies by Telefo et al.35 showed that the aqueous extract of the mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis, is used in traditional medicine to normalize the menstrual cycle increasing female fertility. In addition, Acanthus montanus, Aloe vera, Carica papaya, Citrus aurantifolia, Elaeis guineensis, and Panax quiquefolius, Eremomastax speciosa are used in Nkam (Littoral region in Cameroon) with the same purpose.36Asystasia vogeliana, Crinum distichum, Crinum jagus, Crassocephalum biafrae, Scoparia dulcis, Solanum torvum, Aframomum letestuanum, Aloe buettneri and Eremomastax speciosa make part of the cast of plants most widely used to treat diseases of the reproductive system.37 Generally, medicinal plants used for the improvement of the reproductive functions have more than one property. Table 1 illustrates some traditional medicinal plants used in the treatment of female reproductive disorders.
Medicinal plants and their in vivo therapeutic utilization on female reproductive function.
| Plants | Family | Major phytochemical compounds | Used parts | Therapeutic utilization | References |
|---|---|---|---|---|---|
| Moringa oleifera | Moringaceae | Lutein, carotene xanthins, kaempferol, quercetin | Leaves | Sexual libido | Cajuday and Pocsidio,38 2010 |
| Adiantum concinnum | Adiantaceae | No report | Leaves and stems | Menstrual regulation | Bussmann and Glenn,39 2010 |
| Petroselinum crispum | Apiaceae | Flavonoids | Whole plant | Menstrual regulation | Nielsen et al.,40 1999; Bussmann and Glenn,39 2010 |
| Musa sapientium | Musaceae | No report | Leaves | Menstrual regulation | Chifundera,41 1998 |
| Scabiosa atropurpurea | Dipsacaceae | No report | Leaves | Menstrual regulation | Bussmann and Glenn,39 2010 |
| Cassia alata | Fabaceae | Tannins, saponins, flavonoids, steroids, terpenoids, alkaloids | Root | Infertility | Ramaraj et al.,42 2014; Koch et al.,43 2015 |
| Ximenia americana | Olacaceae | Saponins, glycosides anthraquinones | Whole plant | Menstrual regulation | James et al.,44 2007, Bussmann and Glenn,39 2010 |
| Eremomastax speciosa | Acanthaceae | Flavonoids, alkaloids, saponins, tannins | Leaves | Infertility | Adjanohoun et al.,45 1996; Priso et al.,36 2006 |
| Justicia insularis | Acanthaceae | Flavonoids, alkaloids, glycosides | Leaves and stem | Infertility, menstruation unrest | Adjanohoun et al.,45 1996; Telefo et al.,5 1998 |
| Crinum distichum | Amaryllidaceae | No report | Whole plant | Amenorrhea infertility | Priso et al.,36 2006 |
| Ageratum conyzoides | Asteraceae | Flavonoids, alkaloids, benzofuranes, terpenes | Leaves | Infertility, infections of the genital device | Adewole,46 2002 |
| Senecio biafrae | Asteraceae | Dihydroisocoumarins, terpenoids, sesquiterpens, amino acids, mineral salts | Leaves and stem | Infertility | Tabopda et al.,47 2009; Tacham,37 2000 |
| Emilia coccinia | Asteraceae | No report | Whole plant | Dysmenorrhea | Adjanohoun et al.,45 1996 |
| Zehnaria scabra | Cucurbitaceae | No report | Leaves and stem | Infertility, dysmenorrhea | Adjanohoun et al.,45 1996 |
| Euphorbia tirucalli | Euphorbiaceae | No report | Stem | Gonorrhea | Arbonnier,48 2002 |
| Jatropha curcas | Euphorbiaceae | No report | Leaves and stem | Infertility | Igoli et al.,49 2002 |
| Aloe buettneri | Liliaceae | Glycosides, quinines, coumarins, anthraquinonic derivatives | Leaves | Infertility, painful menstruations, dysmenorrhoea | Telefo et al.,6 2004; Tacham,37 2000 |
| Paulinia pinnata | Sapindaceae | No report | Leaves | Infertility, amenorrhea, gonorrhea | Arbonnier,48 2002 |
| Solanum torvum | Solanaceae | Flavonoids, alkaloids, saponins, glycosides, tannins | Fruit | Infertility, genital infections | Tacham,37 2000 |
| Ampelocissus Pentaphylla | Vitaceae | No report | Leaves | Infertility | Tacham,37 2000 |
| Pelargonium odoratisimum | Geraniaceae | No report | Whole plant | Inflammation of the ovaries and womb | Bussmann and Glenn,39 2010 |
| Pelargonium roseum | Geraniaceae | No report | Flowers and leaves | Hemorrhages, uterus pain | Bussmann and Glenn,39 2010 |
| Krameria lappacea | Krameriaceae | No report | Leaves and root | Inflammation of the ovaries | Bussmann and Glenn,39 2010 |
As can be seen from Table 1, various medicinal, plants belonging to different families showed therapeutic effects. Traditionally, rural women have used plant medicines rather than modern medicine for their personal ailments due to lack of modern facilities in the regions. Bannu region in Pakistan was ranked first having large number of gynecological plant to medical treatment of female reproductive system: uterus, vagina, and ovaries.50 Moreover, many women in Costa Rica consider menopause as a natural phenomenon and treat the symptoms with herbs. Interestingly, Latina women in the United States also tend not to use hormone therapy (estrogenic and/or progesteronic compounds) opting for natural remedies for menopause such as diet, exercise and herbal remedies.51 More detailed scientific studies are desperately needed to evaluate the efficacy and safety of the remedies employed traditionally.
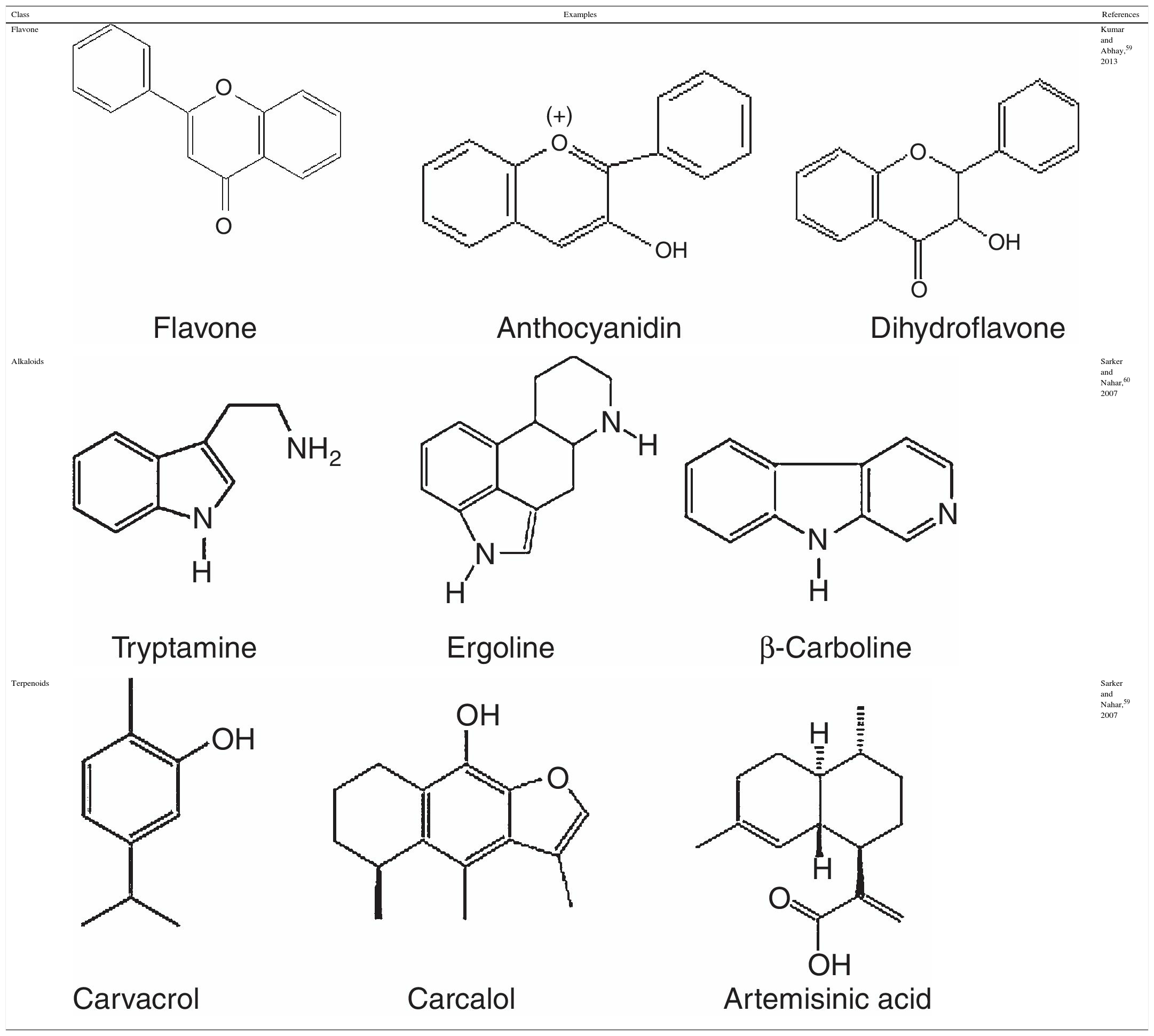
Secondary metabolites of plants: description and medicinal propertiesDescriptionSecondary metabolites are structurally diverse; their classification is mainly derived from their biosynthetic pathways. In pharmacognosy, secondary metabolites are classifying in: (a) phenolic; (b) alkaloids and (c) terpenoids compounds,52 as described below.
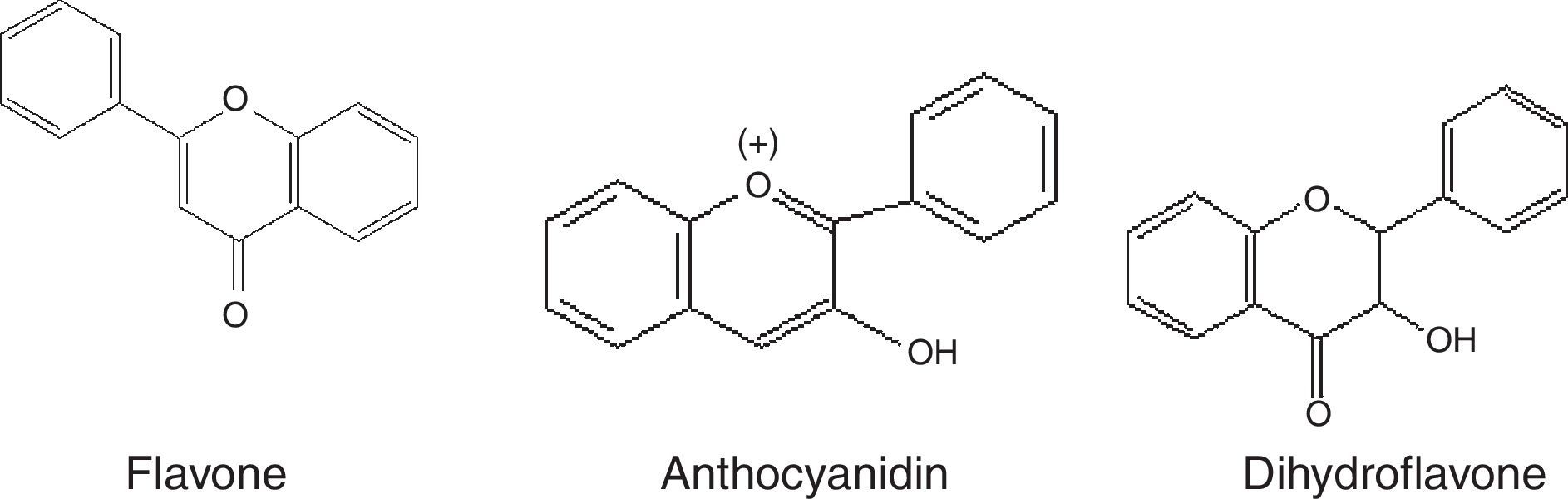
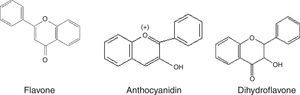
Phenolic compoundsPhenolic compounds are among the most widespread class of secondary metabolites in nature. This class of compounds are synthesized from a common precursor: the amino acids phenylalanine or tyrosine. Phenolic compounds consist of flavonoids, tannins, coumarins, quinones and anthocyanins and are regarded as the widest spread phytochemicals. Phenolic compounds may assume a wide range of structures from simple ones containing one aromatic ring only, to very complex polymeric forms.53 The term flavonoid is a collective name for plant pigments, mostly derived from benzo-δ-pyrone.54 They are widely distributed in plants contain free hydroxyl groups attached to the aromatic rings (Table 2). Flavonoids such as rutin, present in certain buckwheat (Fagopyrum esculentum) species are known to inhibit lipid oxidation by radicals scavenging.55
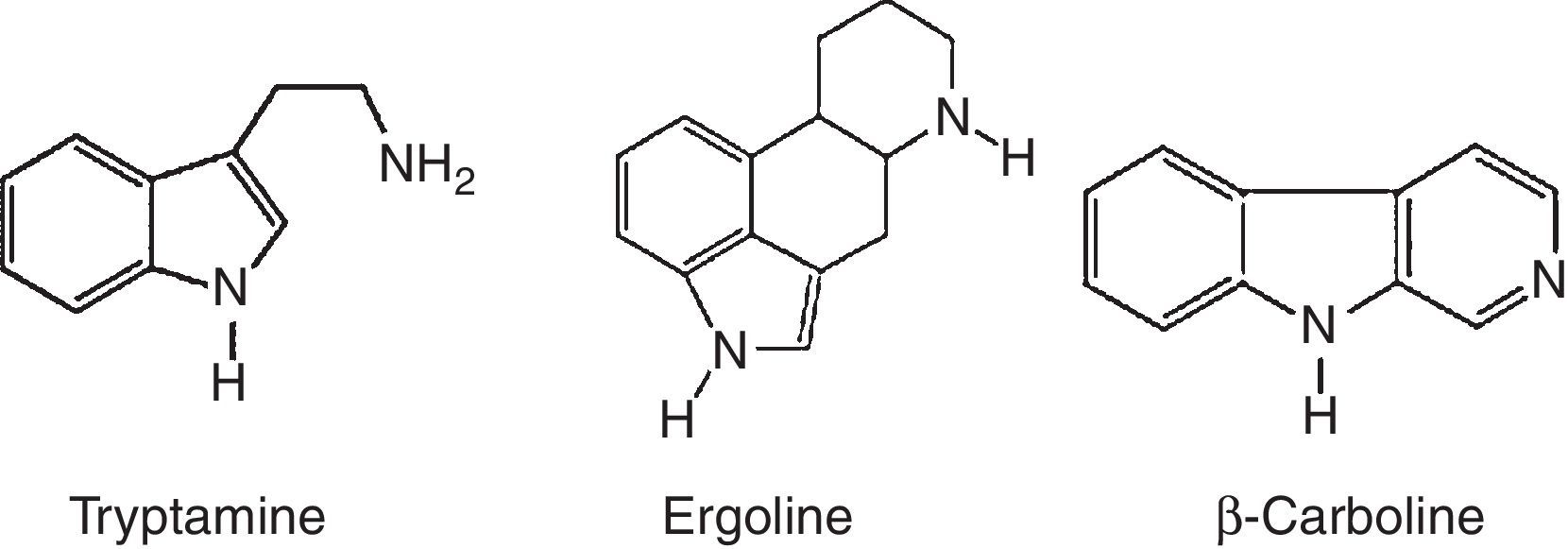
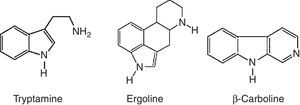
Alkaloids compoundsAlkaloids are nitrogenated compounds synthesized by living organisms. In general, they contains heterocyclic rings (Table 2) and due the presence of one or more nitrogen atoms, they present basic properties. The name alkaloid is directly related to the fact that nearly all alkaloids are basic (alkaline) compounds. Derived from amino acids, in general, they are pharmacologically active. Alkaloids constitute ‘a very large group of secondary metabolites, with more than 12,000 substances isolated. A huge variety of structural formulas, coming from different biosynthetic pathways and presenting diverse pharmacological poperties.56
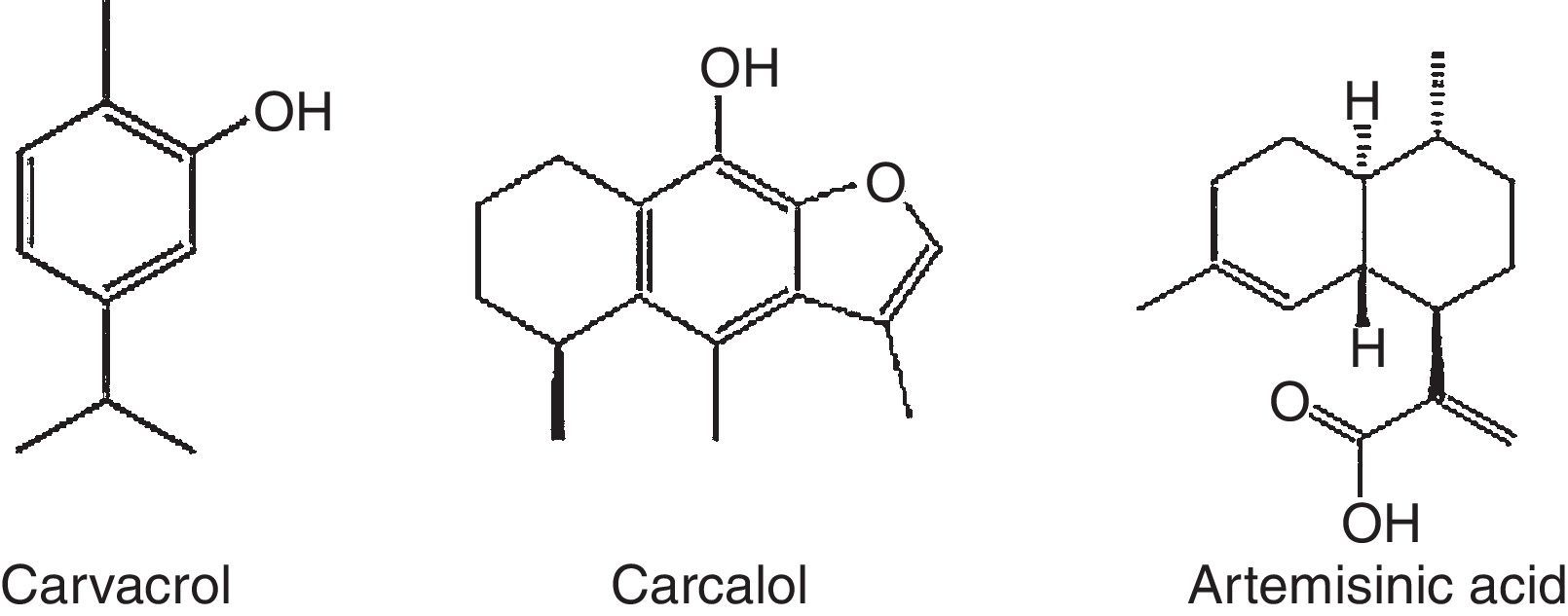
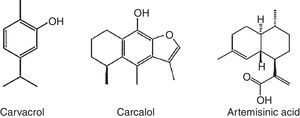
Terpenoids compoundsThe terpenoids comprising monoterpenes, sesquiterpenes, sesterterpenes and triterpenes, besides steroids, saponins and cardiac glycosides. They are considered be the phytochemicals having the most diverse chemical structures.57 Terpenoids are the largest and most diverse family of natural products, ranging in structure from linear to polycyclic molecules and in size from the five carbon hemiterpenes to natural rubber, comprising thousands of isoprene units (Table 2). The monoterpenes and sesquiterpenes are common in essential oils produced by plants.58
Medicinal propertiesCrude extracts or secondary metabolites from medicinal plants can act as antioxidant agents generally through their ability to scavenge ROS or as a regulator of ovarian hormonal production. These properties can be responsible for their medicinal functions.
Gouveia et al.61 identified and quantified five substances from Amburana cearensis, namely: protocatechuic acid (PCA), epicatechin, p-coumaric acid, gallic acid and kaempferol, which were identified by high performance liquid chromatography (HPLC) from the crude ethanolic extract of A. Cearensis. Gallic acid is a well known antioxidant compound, was the main compound found in A. cearensis.62 Gallic acid prevents in vivo and in vitro DNA oxidative increasing the activities of antioxidant enzymes (superoxide dismutase, GPx and glutathion-S-transferase-π) and decreasing the intracellular ROS concentrations.63 Another compound found in the A. cearensis extract was the PCA, commonly found in several vegetables and fruits.64 PCA acts in vitro by increasing the activities of antioxidant enzymes such as superoxide dismutase, scavenging ROS or inhibiting their formation, and consequently reducing oxidative stress damage.64–66 One of the prominent and most useful properties of the flavonoids is their ability to scavenge ROS.67 They are considered more efficient antioxidants than vitamins C and E.68 Coumarins, another group of phenolic compound (1,2-benzopyrone) are natural antioxidant compounds widely distributed in plants.69,70 Studies have reported that coumarins inhibit lipid peroxidation, decreasing the injury caused by oxidative stress and decreasing the levels of ROS in different types of cells.71,72 Likewise, anethole a constituent of essential oil (terpenoids) from Croton-zehntneri, a medicinal plant popularly known as “canela de cunhã” or “canelinha” in the Northeast of Brazil, decreased the levels of ROS both in vivo73,74 using mice model, and in vitro during culture of cell lines isolated from the peripheral blood of male patient with acute myeloblastic leukemia.75 Sá et al.76 showed that addition of anethole (300 and 2000μg/mL) to the in vitro culture medium was able to improve the development of goat preantral follicles by reducing concentrations of ROS and increasing the percentage of oocytes able to resume meiosis. In addition to their antioxidant activity, compounds from plant can have an important role during the steroidogenesis.
Many compounds (flavonoids, lignans, coumestans) derived from plants have ability to mimic the biologic effects of endogenous hormones by binding to their nuclear receptor or regulating the activities of key enzymes of their metabolisms such as cytochrome P450 aromatase, and 17β-hydroxysteroid dehydrogenase which is a key enzyme of the steroidogenesis.13 The estrogenic effects of some compounds are often related to the stimulation of the hypothalamus–pituitary complex increasing the follicle stimulating hormone (FSH), which will thereafter induce ovarian steroidogenesis. Flavanoids with estrogenic potential have been reported to inhibit aromatase activity in various tissues.77
The best-described property of almost plants is their capacity to act as antioxidants. For instance, flavones and catechins seem to be the most powerful flavonoids for protecting the body against ROS. Follicular cells are continuously threatened by the damage caused by free radicals and ROS, which are produced during normal oxygen metabolism or are induced by exogenous damage.78 The mechanisms and the sequence of events by which free radicals interfere with cellular functions are not fully understood, but one of the most important events seems to be the lipid peroxidation, which results in cellular membrane damage. This cellular damage causes a shift in the net charge of the cell, changing the osmotic pressure, leading to swelling and eventually cell death.79 The ROS produced during the metabolism are made inactive according the following equation, where R• is a free radical and O• is an oxygen free radical.
Nowadays, medicinal plants are widely used around the world as an alternative to pharmaceutical drugs. Although herbal products are considered to have fewer adverse effects compared with synthetic drugs, they are not completely free of indesejable effects. The volatile terpenoids camphor, a compound of the essential oil of Artemisia kopetdaghensis crosses the placenta and may lead to abortion.80 In another study, Linjawi81 reported that camphor induces significant structural changes on uterus of pregnant rats. Therefore, it is rational to assume that camphor is involved in the abortifacient effect of A. kopetdaghensis. Results from Oliaee et al.82 using female rats as animal model showed that continuous consumption of 800μg/mL of A. kopetdaghensis in pregnancy may increase the risk of abortion and also may have toxic effect on some cells of body. Therefore, its continuous use is not recommended in pregnancy.
Implication of plant extracts or compounds in ovarian cells cultured in vitroWith the aim to elucidate their properties, plant extracts or its secondary metabolites have been used in the culture of various types of cells including follicles and granulosa cells. Hsia et al.83 demonstrated that the partitioned fractions of Coix lachryma-jobi (Adlay), a traditional Chinese medicine used for the dysfunction of endocrine system extracts possess hypogonadal effect in vitro conditions. This plant shows a great capacity to reduce the production of progesterone (P4) and estradiol (E2) by decreasing the activity of cholesterol side-chain cleavage enzyme (P450scc) and 3beta-Hydroxysteroid dehydrogenase (3β-HSD). In contrary, the use of leaves mixture of Aloe buettneri, Justicia insularis, Dicliptera verticillata and Hibiscus macranthus (ADHJ) in vitro attest the direct effects of some chemical components on rat ovarian steroidogenesis. Indeed, alkaloids, coumarins glycosides, flavonoids and quinones from ADHJ are more effective when the plant extract (130g/ml) is combined to 0.1IU/mL of human chorionic gonadotropin (hCG) during 2h of incubation. In these conditions, estradiol production increased by 13-fold compared to the medium without hCG and the plant extract; and by 5-fold compared to the medium containing only the plant extract (130g/ml) or hCG (0.1IU/mL).6
Studies with quercetin, a flavonoid present in several plants, as well as nonsteroidal compounds known as phytoestrogens84 affects porcine granulosa cell function by interfering with steroidogenic activity and redox status as well as by inhibiting vascular endothelial growth factor output.85 This phytoestrogen represents a potential modulator of ovarian functions through inhibition of steroidogenic enzymes.77,86
Suppressive action of phytoestrogen on cytochrome P450 (enzyme that catalyzed the conversion of cholesterol to pregnenolone) represents a rate-limiting step in the steroidogenic pathway. Several studies87,88 showed that phytoestrogen induced decrease of P4 production in granulosa cells. This decrease is due to the inhibition of 3β-hydroxysteroid enzyme. Furthermore, Santini et al.89 revealed the inhibitory effect of quercetin on aromatase activity. It has been suggested that the action of phytoestrogen on aromatase activity could be mediated by nitric oxide (NO). In fact, this free radical seems to represent an autocrine regulator of granulosa cells E2 production.90 However, molecular studies should be done to better understand the mechanism of action of phytoestrogen on steroidogenic enzymes.
Implication of plants extract or compounds on oocyte maturationDuring in vitro oocyte culture, the levels of antioxidants are lower than in vivo because the oocytes are divorced from the donor body and do not benefit from the maternal antioxidant protection.90 The addition of an antioxidant to the medium, therefore, may be important for in vitro oocyte maturation.
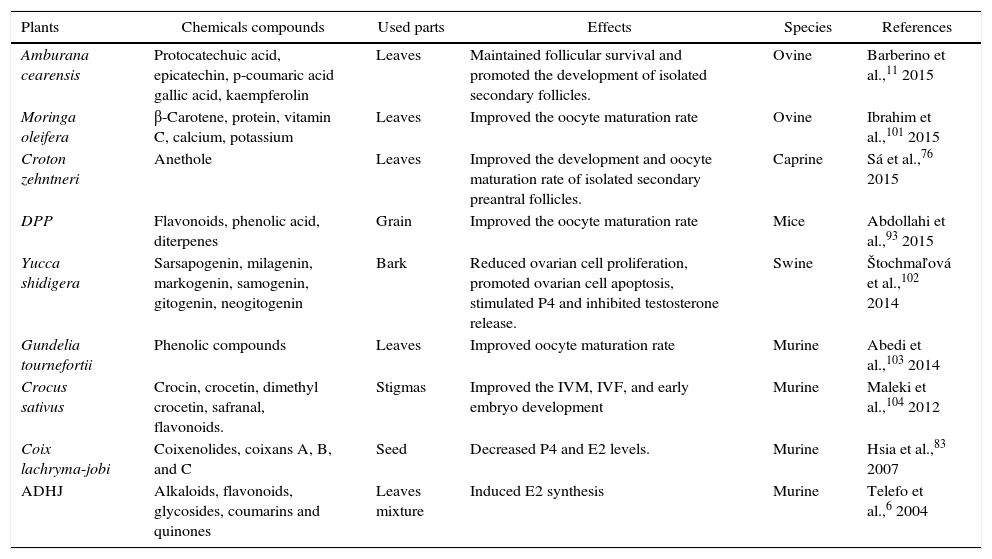
Reports from Rajabi-Toustani et al.91 shows that supplementation of appropriate concentrations of Papaver rhoeas extract (50μg/mL) in maturation medium (bicarbonate-buffered TCM 199 supplemented with 10% FBS, 0.2mM sodium pyruvate 0.1IU/mL hMG, 100IU/mL penicillin and 100μg/mL streptomycin) improve the sheep oocyte maturation rate. Similar results have been obtained when maturation medium of mouse oocyte was supplemented with 5μg/mL of Crocus sativus92 or when supplemented with 20μg/mL of Phoenix dactylifera pollen grain.93 Improvement maturation rate of oocytes treated with P. rhoeas extract may be partly due to increase of intracellular glutathione (GSH) levels in oocytes91 or super oxide dismutase (SOD) activity.94 Anthocyanins protect cells against free radicals by gamma-glutamylcysteine synthetase (γ-GCS) activation, while of γ-GCS elevates GSH levels in medium.95 Increased GSH levels through oocyte maturation are associated with improvement in subsequent embryo development.96 On the other hand, the reduction on in vitro maturated oocytes to metaphase II stage might be due to deleterious effects of excessive concentrations (200μg/ml of P. rhoeas extract), because some flavonoids displayed toxic effects91,97 by changing the cell membrane structure and consequently damage cell polarization.98 Locklear et al.99 demonstrated that extract of Justicia pectoralis acts as an E2 and P4 agonists on the cellular membrane and inhibits the activity of the cyclooxygenase 2 (COX-2) enzyme in vitro. The COX-2, is the rate-limiting enzyme in the biosynthesis of prostaglandins (PGs) which are considered to participate in follicular rupture during ovulation.100J. pectoralis is a medicinal plant commonly used by women in Costa Rica to treat symptoms associated with premenstrual syndrome (common forms of hormonal imbalance affecting women) and menopause. Table 3 shows some in vitro implications of medicinal plants from different species on folliculogenesis and steroidogenesis process.
In vitro implication of some medicinal plants on folliculogenesis and steroidogenesis in different species.
| Plants | Chemicals compounds | Used parts | Effects | Species | References |
|---|---|---|---|---|---|
| Amburana cearensis | Protocatechuic acid, epicatechin, p-coumaric acid gallic acid, kaempferolin | Leaves | Maintained follicular survival and promoted the development of isolated secondary follicles. | Ovine | Barberino et al.,11 2015 |
| Moringa oleifera | β-Carotene, protein, vitamin C, calcium, potassium | Leaves | Improved the oocyte maturation rate | Ovine | Ibrahim et al.,101 2015 |
| Croton zehntneri | Anethole | Leaves | Improved the development and oocyte maturation rate of isolated secondary preantral follicles. | Caprine | Sá et al.,76 2015 |
| DPP | Flavonoids, phenolic acid, diterpenes | Grain | Improved the oocyte maturation rate | Mice | Abdollahi et al.,93 2015 |
| Yucca shidigera | Sarsapogenin, milagenin, markogenin, samogenin, gitogenin, neogitogenin | Bark | Reduced ovarian cell proliferation, promoted ovarian cell apoptosis, stimulated P4 and inhibited testosterone release. | Swine | Štochmaľová et al.,102 2014 |
| Gundelia tournefortii | Phenolic compounds | Leaves | Improved oocyte maturation rate | Murine | Abedi et al.,103 2014 |
| Crocus sativus | Crocin, crocetin, dimethyl crocetin, safranal, flavonoids. | Stigmas | Improved the IVM, IVF, and early embryo development | Murine | Maleki et al.,104 2012 |
| Coix lachryma-jobi | Coixenolides, coixans A, B, and C | Seed | Decreased P4 and E2 levels. | Murine | Hsia et al.,83 2007 |
| ADHJ | Alkaloids, flavonoids, glycosides, coumarins and quinones | Leaves mixture | Induced E2 synthesis | Murine | Telefo et al.,6 2004 |
IVM, in vitro maturation; IVF, in vitro fertilization; ADHJ, mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis; DPP, Phoenix dactylifera pollen grain.
Female reproductive problems continue to be a major health challenge worldwide. An impressive number of plant species is traditionally used to remedy such disorder. Those plants mainly constituted of secondary metabolites have been used over decades for the treatment of diseases which affect woman reproduction leading to infertility. These substances widely distributed over the world are constituted of compounds whose concentrations and compositions vary among plants and between the same genus. Several factors affect the plants composition among which the season, site and time of harvest. With the development of technology, an interest of plant is reported due to their antioxidant capacity and their ability to mimic the effect of steroidogenic enzymes. But little remain unknown about their implication in vitro which represents alternative studies of the in vivo studies. Finally, further studies should be performed to better understand the mechanism of action of plant and/or secondary metabolites. The discovery may also help to reduce the cost and improve the results of treatments normally applied.
Conflicts of interestThe authors declare no conflicts of interest.