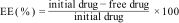Methicillin-resistant Staphylococcus aureus (MRSA) causes a wide range of infections and contributes to elevated morbidity, mortality, and healthcare costs. Herbal compounds combined with drug delivery systems could be an effective alternative option for treating resistant bacteria. This study evaluates the antimicrobial prowess of carvacrol-loaded niosomes against MRSA strains. In this study, six carvacrol–niosome formulations with different ratios of Span and Tween were prepared. The physicochemical attributes of the optimized synthesized niosomes were assessed using Fourier-transform infrared (FTIR) spectroscopy and scanning electron microscopy (SEM) as well as DLS Zetasizer. Encapsulation efficiency (EE) and in vitro drug release were studied. The antibacterial activity of the synthesized carvacrol–niosomes, in concentrations varying between 7.8 and 1000μg/ml, was evaluated using microdilution broth methods. The optimized niosomes, with a size of 207.3nm and an impressive EE of 91%, exhibited a spherical structure as confirmed by the electron microscopy analysis. Impressively, these carvacrol–niosomes demonstrated superior antimicrobial effectiveness against S. aureus, reducing MIC levels 4-fold to 62.5±0.0μg/ml and MBC to 125±0.0μg/ml, a significant improvement over the 250±0.0μg/ml MIC and 500±0.0μg/ml MBC of free carvacrol. Additionally, while empty niosomes showed minimal cytotoxicity with 88.32±1.32% cell viability at 100μg/ml, free carvacrol led to a marked reduction in viability to 39.46±1.26%. However, niosomes encapsulating carvacrol notably increased cell survival to 59.67±1.62% at this concentration. These findings underscore the enhanced antimicrobial potency of carvacrol when enclosed within niosomes, suggesting its potential as a potent herbal remedy for combating methicillin-resistant S. aureus.
Las infecciones por Staphylococcus aureus resistente a la meticilina (MRSA) causan una elevada morbimortalidad, y aumentan los costos de atención médica. Los compuestos a base de hierbas combinados con sistemas de administración de fármacos podrían ser una opción eficaz para combatir estas bacterias resistentes. En este estudio, se evaluó el desempeño antimicrobiano de niosomas cargados de carvacrol contra cepas de MRSA. Se prepararon 6 formulaciones de carvacrol-niosoma con diferentes proporciones de emulsificantes. Mediante las técnicas habituales (FTIR, SEM y DLS) se determinaron los atributos fisicoquímicos de los niosomas sintetizados y se calculó la eficiencia de encapsulación (EE); también se analizó la liberación del fármaco in vitro. La actividad antibacteriana de los niosomas de carvacrol sintetizados, en concentraciones de 7,8 a 1.000μg/ml, se evaluó por microdilución en caldo. Los niosomas optimizados (formulación 2) fueron esféricos (207,3nm), tuvieron una destacada EE, del 91%, y demostraron una eficacia antimicrobiana superior contra S. aureus, reduciendo en 4 veces la CIM (de 250 a 62,5μg/ml) y la CBM (de 500 a 125μg/ml) respecto del carvacrol libre. Mientras que los niosomas vacíos mostraron una citotoxicidad mínima (viabilidad celular: 88,32±1,32% a 100μg/ml), el carvacrol libre provocó una marcada reducción de la viabilidad, que fue del 39,46±1,26%. Sin embargo, los niosomas que encapsularon carvacrol aumentaron la supervivencia celular al 59,67±1,62% a esa concentración. Nuestros hallazgos subrayan la efectividad antimicrobiana mejorada del carvacrol cuando está encerrado dentro de niosomas, lo que lo perfila como un potente remedio de origen vegetal para combatir S. aureus resistente a la meticilina.
Methicillin-resistant Staphylococcus aureus (MRSA) leads to a range of infections including endocarditis, skin and soft tissue infections, osteomyelitis, and respiratory illness, as well as bacteremia. Elevated morbidity, higher fatality rates, extended hospitalizations, and increased healthcare costs are all outcomes of MRSA infections7,19. Moreover, the World Health Organization (WHO) has categorized this resistant strain as a high-priority pathogen due to the risks related to antibiotic resistance18. The recent prevalence trends of nosocomial MRSA infections vary globally, with rates of 26.0% reported in Italy (2017–2021), 63% in Egypt (2022), and 43.44% in Pakistan (2020)4,16,31,35. The resistance process to methicillin in MRSA is conferred by the mecA gene, obtained through horizontal transfer of the staphylococcal cassette chromosome mec (SCCmec). The mentioned genetic segment is responsible for encoding PBP2a (penicillin-binding protein 2), an enzyme with low affinity for β-lactams, leading to resistance across this antibiotic class34.
Numerous studies indicate that while herbal products exhibit milder antimicrobial activity compared to commercial antibiotics, they are promising in inhibiting MRSA, either used alone or alongside antibiotics2,30,37. Carvacrol, a monoterpenoid found in Labiatae family plants, is valued for its wide-ranging properties including anti-inflammatory, antitumor effects, and antimicrobial properties27. The antibacterial attributes of carvacrol against Escherichia coli, Listeria monocytogenes, Salmonella spp., S. aureus, and Pseudomonas aeruginosa have been demonstrated previously9,21,22. Carvacrol exerts antibacterial effects by disrupting cell homeostasis, increasing cell membrane fluidity and permeability15. Wijesundara et al. demonstrated through microscopic monitoring of cell death triggered by carvacrol that it led to the release of cytoplasmic components, including lactate dehydrogenase (LDH) enzymes and nucleic acids, by altering the cell membrane39. Moreover, carvacrol inhibits ATPase activity and reduces proton motive force, ultimately resulting in cell death. Additionally, it may impact biofilm formation by downregulating quorum sensing gene expression in various bacteria15,33. Encapsulating carvacrol in nanocarriers could enhance their solubility and antibacterial effectiveness, overcoming their inherent volatility, instability, and hydrophobicity15. Furthermore, the nanocarrier showed a unique ability to penetrate bacterial membranes for targeted delivery36. Niosomes, as a drug delivery system, are bilayer assemblies containing non-ionic surfactants. Their solubility in water enables the transportation of drugs specifically into pathogen cells24,29. These drug nanocarriers are favored for treating infectious diseases owing to their excellent stability, bioavailability, poisonousness, and versatility in carrying hydrophilic or hydrophobic molecules. Furthermore, niosomal carriers not only minimize adverse effects but also need a smaller amount of drug, thanks to their ability to achieve controlled drug release3. Hence, this study explores the antibacterial impact of carvacrol on MRSA by developing six distinct formulations of carvacrol-loaded niosomes.
Materials and methodsMaterialsCarvacrol, sorbitan monostearate (Span 60), Tween 60, cholesterol, dimethyl sulfoxide (DMSO), chloroform, Mueller-Hinton broth, Mueller-Hinton agar, sulfuric acid (H2SO4), and barium chloride (BaCl2) were obtained from Merck, Germany. Moreover, trypsin–EDTA, trypan blue, penicillin and streptomycin (100×), RPMI-1640 medium, Dulbecco's modified Eagle's medium (DMEM), and also fetal bovine serum (FBS) were sourced from Gibco, Germany. MTT reagent and dialysis membrane (12000 molecular weight cut off) were purchased from Sigma-Aldrich, St. Louis, MO, USA. Moreover, ethanol (96%) (Zakaria Jahrom, Iran), PBS (Bio Basic, Canada), Milli-Q water (Merck Millipore, Germany), and acetone (Arvin Chemistry, Iran) were used in this study.
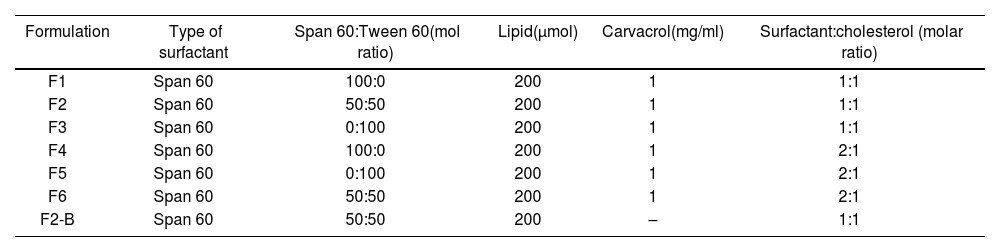
Preparation of carvacrol–niosomesWe synthesized carvacrol–niosomes employing the thin-film hydration procedure13, using various formulations with different molar ratios of Span 60, Tween 60, and cholesterol (Table 1). Precise quantities of cholesterol, Span 60, and Tween 60 were solubilized in a chloroform solvent. Then, the solution was subjected to evaporation using a rotary evaporator (150rpm, 60°C) until the solvent had completely evaporated and a thin lipid layer was formed. During the hydration stage, carvacrol was added to a thin lipid layer in a phosphate buffer (PBS with pH=7.2) and hydrated using a rotary evaporator (120rpm, 60°C, 30min). Finally, sonication was performed for 7min to reduce particle size effectively.
Formulations of carvacrol–niosomes.
| Formulation | Type of surfactant | Span 60:Tween 60(mol ratio) | Lipid(μmol) | Carvacrol(mg/ml) | Surfactant:cholesterol (molar ratio) |
|---|---|---|---|---|---|
| F1 | Span 60 | 100:0 | 200 | 1 | 1:1 |
| F2 | Span 60 | 50:50 | 200 | 1 | 1:1 |
| F3 | Span 60 | 0:100 | 200 | 1 | 1:1 |
| F4 | Span 60 | 100:0 | 200 | 1 | 2:1 |
| F5 | Span 60 | 0:100 | 200 | 1 | 2:1 |
| F6 | Span 60 | 50:50 | 200 | 1 | 2:1 |
| F2-B | Span 60 | 50:50 | 200 | – | 1:1 |
Mean nanoparticle size, zeta potential, and particle dispersion index (PDI) of the carvacrol–niosomes were evaluated using a Zetasizer Nano ZS device (ZEN3600, Malvern Instrument Ltd., Malvern) employing a dynamic light scattering (DLS) method at 25°C. The structural morphology of the nanoparticles was further analyzed employing a scanning electron microscope (SEM). The nanoparticle suspension was thinned by diluting it in deionized water at a proportion of 1:100. Subsequently, a drop of this diluted sample was evenly distributed on a conductive surface, such as aluminum, and allowed to dry at room temperature. Fourier-transform infrared spectroscopy or FTIR spectroscopy was employed to identify the chemical composition and molecular structure of the carvacrol–niosomes. FTIR spectrum was obtained using the Spectrum Two FT-IR Spectrometer from Perkin-Elmer, USA.
Drug encapsulation efficiency (EE)Free drug separation from the drug-loaded niosomes was achieved by centrifugation. In this process, the niosomes were centrifuged at 14000rpm at 4°C for 45min. The drug-loaded nanoparticles precipitated, while the supernatant retained the free drug and any substances that did not participate in the reaction. The absorbance of the supernatant sample at 278nm was measured using the colorimetric method (Folin method). The quantity of free drug was determined using the calibration curve, and the encapsulation efficiency of carvacrol–niosomes was computed using the following formula11:
In vitro drug releaseThe release of the drug (carvacrol) from the nanocarrier (niosomes) at specific time intervals was examined using a dialysis bag. Carvacrol–niosomes (2ml) and carvacrol solution (2ml) were individually enclosed in separate dialysis bags. Each dialysis bag was immersed in a flask containing PBS (50ml) at 37°C, positioned on a stirrer. Flasks holding the bags with carvacrol–niosomes and the niosomes without drug were then placed on a stirrer. Samples were collected from the flask at various times including 1, 2, 4, 8, 24, 48, and 72h. A fresh 1ml of PBS was substituted for each collected 1ml sample. Ultimately, the absorption of the samples was assessed using a UV–vis spectrophotometer (Shimadzu, Japan) at 760nm using the colorimetric method (Folin method). Utilizing the standard curve of the drug, the drug concentration in 1ml was calculated and related to the total formulation amount. Subsequently, the percentage of drug release was determined. Finally, a graph was drawn to show the cumulative percentage of drug release from the niosomes over 72h.
Stability studiesThe stability of the niosomes was evaluated following ICH guidelines14, with tests carried out under both 4°C and 25°C conditions. The assessment included EE% and size evaluations at specific time intervals.
Bacterial strainsTwo hundred and fifty clinical specimens were obtained from several hospitals in the Tehran province of Iran, namely Imam Hossein, Sarem, and Atiyeh hospitals, during 2022–2023. These samples were isolated from urine, blood, skin, and wounds. Fifty samples of S. aureus were isolated using a combination of microbial tests, including Gram staining, growth on mannitol salt agar medium, and Baird-Parker medium. Biochemical tests such as catalase, coagulase, and deoxyribonuclease (DNase) test were also conducted.
Antimicrobial susceptibility testThe susceptibility of the S. aureus isolates to antibiotics was assessed using the Kirby-Bauer disk diffusion method, adhering to the guidelines set by the Clinical and Laboratory Standards Institute (CLSI)8. The antibiotic profile evaluated included cefoxitin (10μg), vancomycin (10μg), ciprofloxacin (5μg), penicillin (10μg), erythromycin (15μg), trimethoprim (12.5μg), amikacin (15μg), ampicillin (10μg), gentamicin (10μg), amoxicillin (10μg), chloramphenicol (15μg) and clindamycin (10μg). The S. aureus ATCC 25923 strain served as the positive control.
Antimicrobial activity of carvacrol–niosomes compared to the free drugMeasurement of MIC and MBCTo evaluate the antibacterial efficacy of the carvacrol–niosome formulation and its free form, the study utilized minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) procedures. Therefore, the MIC assessment, conducted in accordance with CLSI standards, involved a microbroth dilution approach and was executed in triplicate for precision. Concentrations spanning from 7.8 to 1000μg/ml were used to determine the MIC for the samples.
Cell culture and cell viability assayThe human foreskin fibroblasts (HFF) cell line (NCBI461 code) was sourced from the Pasteur Institute of Iran. These cells were cultured in RPMI, along with 10% FBS and 1% penicillin and streptomycin. Cultivation occurred at 37°C under a 5% CO2 atmosphere.
The cytotoxic effects of niosomes, carvacrol–niosomes, and free carvacrol on the HFF cell line were evaluated with the MTT colorimetric assay. Initially, 1×106 cells were seeded in 96-well plates. These cells were then introduced at various concentrations (100, 50, 25, 12.5, 6.25, and 3.125μg/ml) of niosomes, carvacrol–niosomes, and carvacrol for 24h. Post-treatment, the 96-well plates were emptied, and the MTT dye was added to each well. The plates were then incubated for 4h in a 5% CO2 atmosphere at 37°C. Subsequently, the MTT dye was discarded, and the formazan crystals made by viable cells were liquified in isopropanol. The absorbance of the resulting solution was assessed at 570nm with an ELISA reader from BioTek, USA.
Statistical analysisThe entire assay conducted in this study was simulated three times, and the statistical analysis was performed using the SPSS software, version 16. The results were evaluated with a one-way ANOVA. Data are expressed as mean±standard deviation, with a significance level set at 0.05 for all tests.
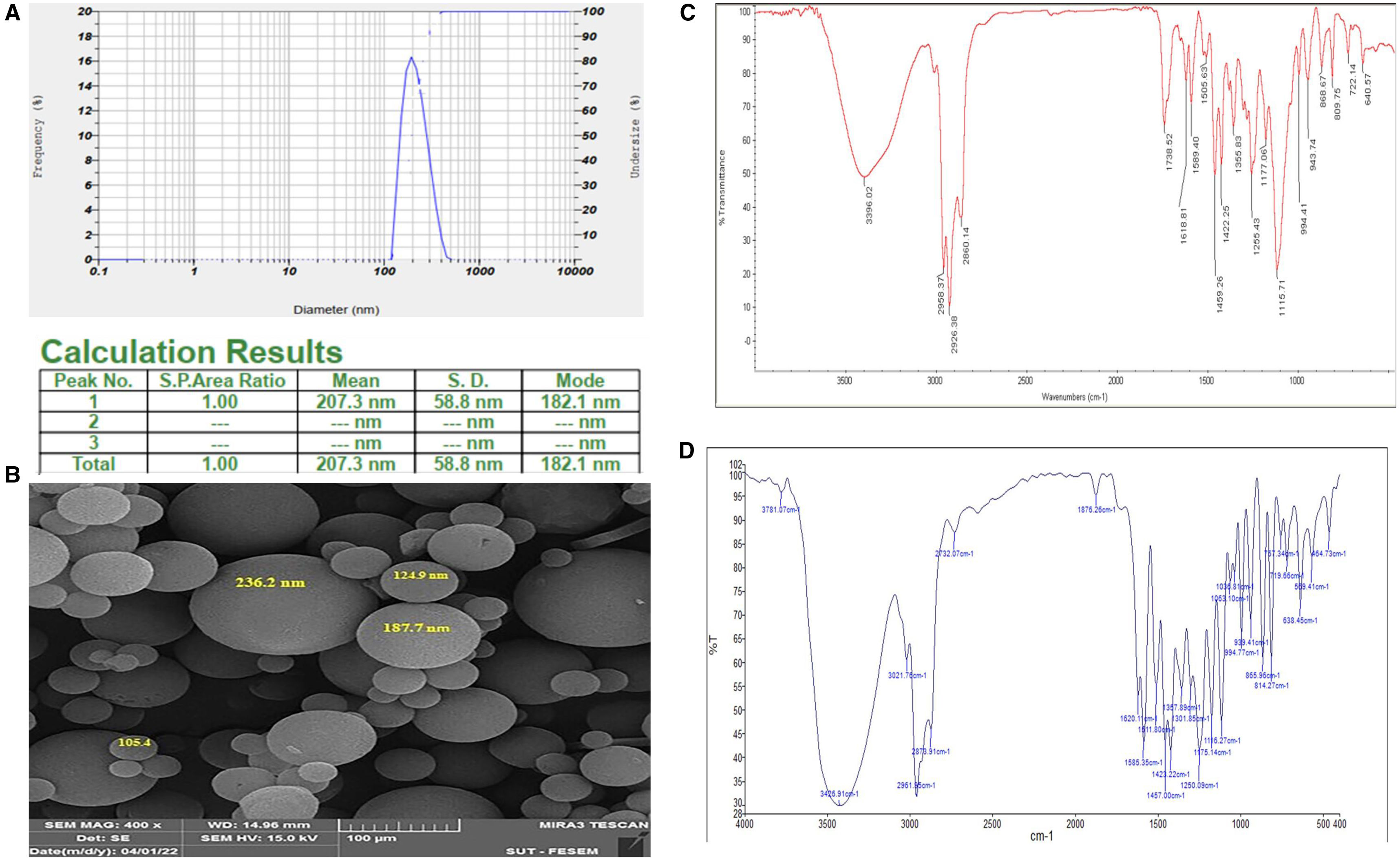
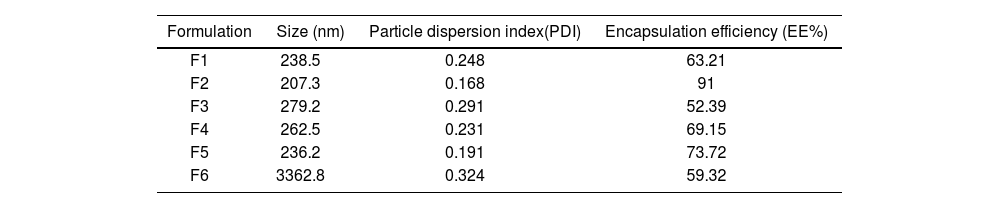
ResultsCharacterization of carvacrol–niosomesThe carvacrol–niosomes in six different formulations were successfully prepared. DLS analysis in Figure 1A reveals synthesized carvacrol–niosomes with a particle size of 207.3nm. Table 2 shows the size (nm), PDI, and EE (%) of carvacrol–niosomes. The SEM was utilized to examine the morphology and size of the niosome formulations (Fig. 1B). The findings indicated that the nanoparticles have a spherical structure.
Physicochemical characterization of prepared carvacrol–niosomes. (A) Particle size distribution of carvacrol–niosomes by DLS analysis; (B) morphological structure and size of niosome preparations by SEM (magnification of 400×); (C) FTIR spectra of carvacrol–niosomes; (D) FTIR spectra of carvacrol alone.
Characterization of different carvacrol-loaded niosome formulation synthesis via thin layer hydration method.
| Formulation | Size (nm) | Particle dispersion index(PDI) | Encapsulation efficiency (EE%) |
|---|---|---|---|
| F1 | 238.5 | 0.248 | 63.21 |
| F2 | 207.3 | 0.168 | 91 |
| F3 | 279.2 | 0.291 | 52.39 |
| F4 | 262.5 | 0.231 | 69.15 |
| F5 | 236.2 | 0.191 | 73.72 |
| F6 | 3362.8 | 0.324 | 59.32 |
The FTIR spectra of carvacrol–niosomes and free carvacrol are presented in Figures 1C and D, respectively. FTIR spectroscopy revealed characteristic peaks corresponding to the functional groups of the constituent compounds of the niosomes. Notably, the peak at 1096cm−1 is associated with the C–O alcoholic bond stretching, a feature that is present in the molecular structures of both cholesterol and Span 60. Furthermore, the peaks at 1044cm−1 and 1278cm−1 were identified as indicative of the presence of carvacrol.
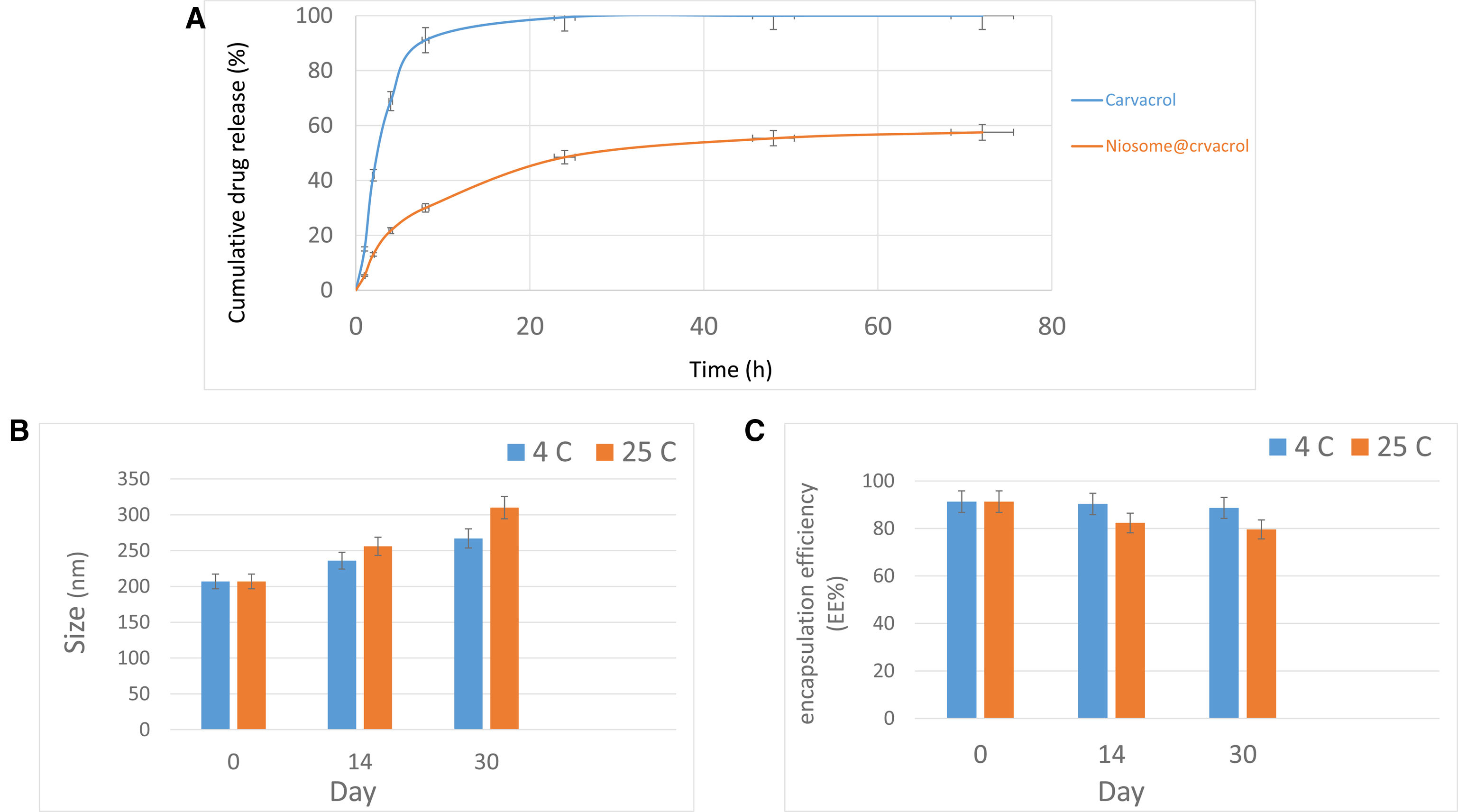
In vitro carvacrol releaseFigure 2A displays the 72-h cumulative release of carvacrol in its pure form and when encapsulated in a nanocarrier in a PBS-SDS medium. The nanocarrier form showed a slower release, reaching only 57% at 72h, compared to the 100% release of free carvacrol in the same period. Initially, 41.90% of free carvacrol was released in 8h, while just 13.06% was released from the niosomes. At 24h, these figures were 99% for free carvacrol and 48.46% for the nanocarrier. The release from the nanocarrier gradually increased to 55% at 48h and 57% at 72h.
Stability studies of carvacrol–niosomesFigures 2B and C illustrate that carvacrol–niosomes stored at 4°C maintained better stability in terms of size and encapsulation efficiency than those kept at room temperature (25°C) over 30 days. The niosomes at 4°C showed minimal changes in these parameters. In essence, 4°C maintained samples exhibited a reduced rate of size increase and a slower decline in encapsulation efficiency compared to those at room temperature. The stability analysis for both storage conditions indicated a gradual size enlargement and EE reduction over time. There is a marked distinction in the size of samples stored for 14 and 30 days at these different temperatures. Furthermore, a notable discrepancy in EE was observed on the 30th day between samples stored under the two conditions.
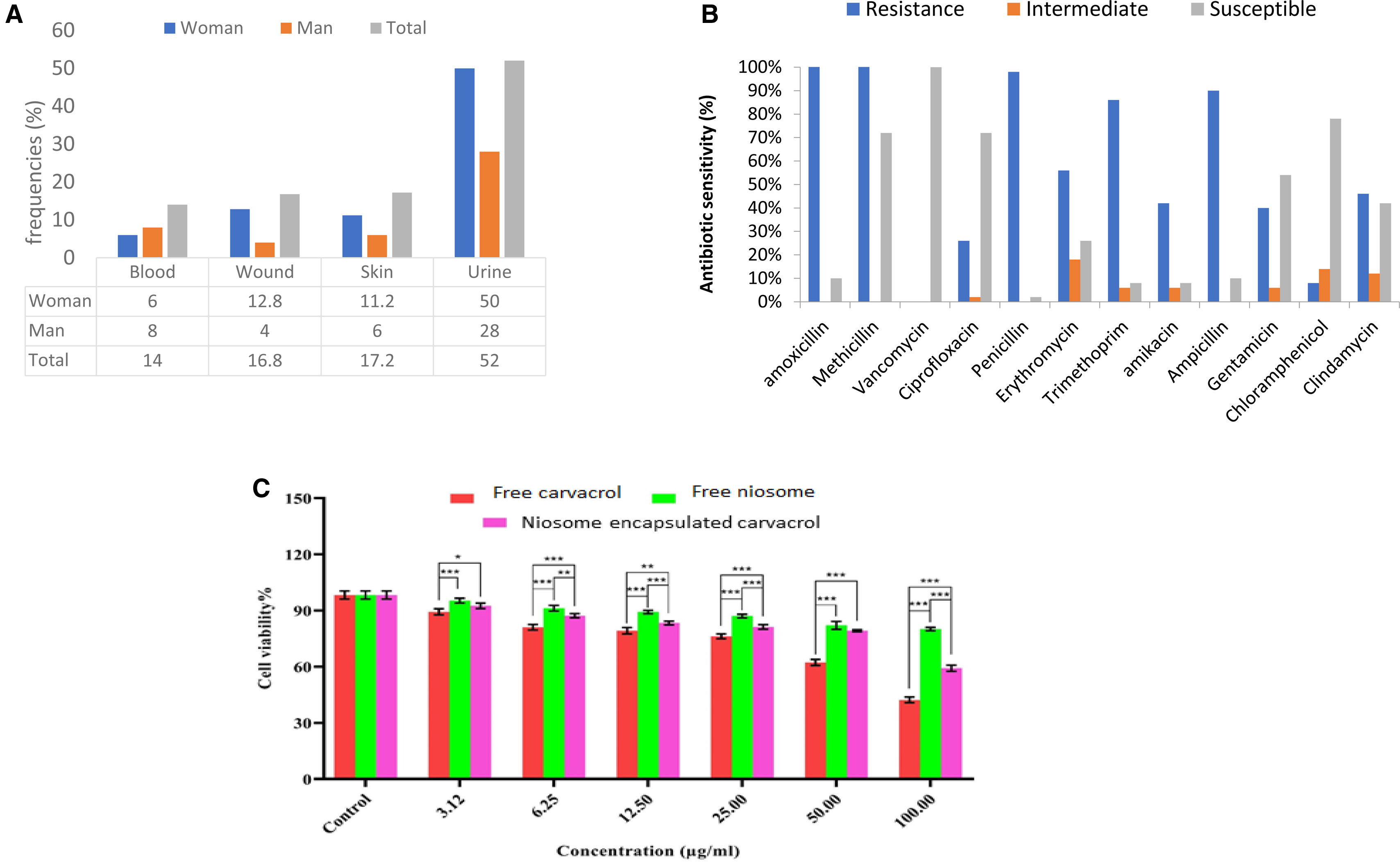
Demographic characteristics of the clinical specimenFifty samples of S. aureus were isolated from a pool of 250 clinical samples obtained from patients, as outlined in the demographic characteristics provided in Figure 3A. Furthermore, the analysis of antibiotic resistance patterns in S. aureus revealed that the highest resistances were to penicillin (98%), ampicillin (90%), amoxicillin and trimethoprim (86%), and cefoxitin (68%). Conversely, the lowest resistance rates were observed with vancomycin (100% sensitivity) and ciprofloxacin (56% sensitivity). Overall, strains isolated from urine and wound samples exhibited higher antibiotic resistance than those from other sources. Notably, no resistance to vancomycin was detected in any of the strains. The detailed resistance levels of the strains to various antibiotics are shown in Figure 3B.
(A) Absolute and relative frequencies of clinical S. aureus isolates are categorized by sample type and gender distribution of the isolated clinical strains; (B) antibiotic sensitivity (%) pattern of S. aureus; (C) impact of various concentrations of carvacrol-loaded niosomes, carvacrol alone, and free niosomes on the cell viability of the HFF cell line, over 24h. In this graph, data are presented as the mean percentage of cell viability±standard deviation (p-value<0.05: *, p-value<0.01: **, p-value<0.001: ***, n=3).
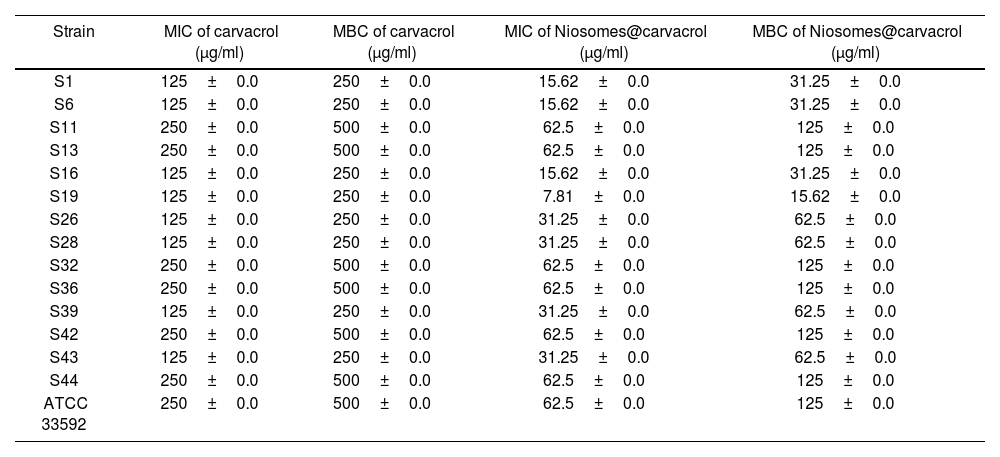
The results from the MIC test demonstrated that the carvacrol–niosomes showed enhanced antimicrobial effects compared to free carvacrol, leading to a 4-fold reduction in the MIC level (Table 3). The MIC of carvacrol–niosomes against S. aureus (ATCC 33592) was 62.5±0.0μg/ml, with an MBC of 125±0.0μg/ml. In comparison, free carvacrol exhibited a MIC of 250±0.0μg/ml, with an MBC of 500±0.0μg/ml.
MIC and MBC of carvacrol–niosomes and free carvacrol against tested S. aureus strains.
| Strain | MIC of carvacrol (μg/ml) | MBC of carvacrol (μg/ml) | MIC of Niosomes@carvacrol (μg/ml) | MBC of Niosomes@carvacrol (μg/ml) |
|---|---|---|---|---|
| S1 | 125±0.0 | 250±0.0 | 15.62±0.0 | 31.25±0.0 |
| S6 | 125±0.0 | 250±0.0 | 15.62±0.0 | 31.25±0.0 |
| S11 | 250±0.0 | 500±0.0 | 62.5±0.0 | 125±0.0 |
| S13 | 250±0.0 | 500±0.0 | 62.5±0.0 | 125±0.0 |
| S16 | 125±0.0 | 250±0.0 | 15.62±0.0 | 31.25±0.0 |
| S19 | 125±0.0 | 250±0.0 | 7.81±0.0 | 15.62±0.0 |
| S26 | 125±0.0 | 250±0.0 | 31.25±0.0 | 62.5±0.0 |
| S28 | 125±0.0 | 250±0.0 | 31.25±0.0 | 62.5±0.0 |
| S32 | 250±0.0 | 500±0.0 | 62.5±0.0 | 125±0.0 |
| S36 | 250±0.0 | 500±0.0 | 62.5±0.0 | 125±0.0 |
| S39 | 125±0.0 | 250±0.0 | 31.25±0.0 | 62.5±0.0 |
| S42 | 250±0.0 | 500±0.0 | 62.5±0.0 | 125±0.0 |
| S43 | 125±0.0 | 250±0.0 | 31.25±0.0 | 62.5±0.0 |
| S44 | 250±0.0 | 500±0.0 | 62.5±0.0 | 125±0.0 |
| ATCC 33592 | 250±0.0 | 500±0.0 | 62.5±0.0 | 125±0.0 |
Figure 3C shows the results of treating HFF cells at concentrations spanning from 3.125 to 100μg/ml of empty niosomes, free carvacrol, and niosomes containing carvacrol. We found that empty niosomes exhibited minimal cytotoxic effects. At a concentration of 100μg/ml, cell viability was 88.32±1.32%, demonstrating the biocompatibility of the niosomes. Conversely, treatment with free carvacrol at the same concentration resulted in only 39.46±1.26% cell viability, indicating significant cytotoxicity compared to the control. Nevertheless, when carvacrol was encapsulated in niosomal form, the cell survival rate at the highest concentration (100μg/ml) was notably higher, at 59.67±1.62%. This represents an almost 1.5-fold increase in cell survival compared to treatment with carvacrol alone.
DiscussionMRSA infection, a key contributor to deaths from antibiotic resistance, is known for hospital-acquired infections which infects both patients and healthy individuals38. In our study, 28% of the total 50 S. aureus isolates were identified as MRSA strains. Furthermore, our results highlight a higher occurrence of MRSA strains in the infectious, surgical, and neurology hospital departments. However, a study that analyzed 104 nosocomial MRSA outbreaks from 18 countries, with the majority reported in the US, UK, France, Canada, and Japan, reported that neonatology, surgery, internal medicine, burn units, and gynecology departments had the highest prevalence of MRSA, with 40% of outbreaks occurring in ICUs23. The therapeutic approach to these infections is complicated due to evolving resistance and S. aureus biofilm formation, making treatments less effective36. Studies indicated that herbal extracts could boost antibiotic efficacy as adjuvants, offering a promising strategy against MRSA. This combination not only restores antibiotic sensitivity, lowers their dosage, and minimizes toxic effects but also reverses MRSA resistance5,30. Carvacrol, an herbal bioactive substance found in essential oils, is the main monoterpenic phenol in oregano and thyme and possesses antioxidant and antimicrobial properties10,22. In this study, carvacrol loaded in niosomes was employed to explore its antimicrobial effects. Six distinct carvacrol–niosome formulations were synthesized, with formulation F2 (Span 60/Tween 60 ratio: 50:50, cholesterol 1:1) emerging as the optimal choice due to its favorable size (207.3nm) and high encapsulation efficiency (91%). In the course of our investigation, the encapsulation efficiency of carvacrol surpassed that of a niosomal gel study with a 180nm size (EE: 90.61%)13, yet fell below the range observed in a phytosome study (92–100%)32. The lower EE% in our study, as compared to the preceding study, can be attributed to the distinct encapsulation techniques employed. Unlike the previous study where carvacrol was chemically bound to phospholipids within the phytosome, our niosome study relied on the physical entrapment of carvacrol32. Studies indicated that drug delivery systems such as niosomes exhibit a biphasic release pattern with a primary fast release followed by a steady or gradual level secretion1, a pattern that was similarly observed in our study. In vitro drug release studies of optimal carvacrol–niosome formulation in a PBS-SDS buffer indicated a slower release rate for this formulation compared to its free drug. This demonstrates the potential of niosomes for the sustainable and controlled delivery of carvacrol, highlighting their effectiveness as a promising herbal drug delivery system6. We observed that carvacrol was released from the niosome at 48.46% after 24h, compared to a 99% release when unencapsulated. This was lower than the previous study13. The stability of carvacrol–niosomes was assessed through their size and encapsulation efficiency, over 30 days at 4°C and 25°C. The comparative analysis revealed that the samples kept at 4°C demonstrated greater stability than those at room temperature. The stability of niosomal myrtle essential oil at 4°C, without any change in size over three months, confirms our result25. Additionally, the stability results of our study under two storage conditions indicate that over time, there was an increase in size and a corresponding decrease in EE%. Moreover, the elevation in size of the samples maintained at 4°C was more gradual compared to those kept at 25°C, likely owing to the reduced mobility of niosome bilayers at the lower temperature of 4°C. The findings of EE% indicate significant drug leakage at 25°C, attributed to the enhanced fluidity of lipid vesicles at high temperatures26. Antimicrobial activity of carvacrol–niosomes against a MRSA strain by determining the MIC and MBC revealed that carvacrol–niosome possesses notably stronger antimicrobial properties compared to free carvacrol, achieving a 4-fold reduction in MIC concentrations. The MIC of carvacrol–niosomes against S. aureus (ATCC 33592) was 62.5±0.0μg/ml, with MBC of 125±0.0μg/ml. In comparison, free carvacrol exhibited a MIC of 250±0.0μg/ml, with an MBC of 500±0.0μg/ml. However, in a study evaluating the antibacterial effects of pure thymol/carvacrol and liposome-encapsulated form against S. aureus, the reported MIC was 0.662mg/ml. Interestingly, the liposome-encapsulated form did not exhibit an increased antimicrobial activity compared to carvacrol alone12. This may be due to the different chemical composition between niosomes and liposomes. In another study that used modified cyclodextrin types and dehydration methods to encapsulate carvacrol, the MIC and MBC for S. aureus (USA300) were found to be higher than those in our study, ranging from 250 to 1000mg/ml and 450 to 1000mg/ml, respectively17. Carvacrol and thymol may target pathogen membranes and could interact with membrane proteins, enzymes, and intracellular targets12. Furthermore, carvacrol inhibits MRSA biofilm formation by downregulating the biofilm-associated sarA gene expression and reducing the expression of the crtM gene related to staphyloxanthin. Likewise, carvacrol at 75μg/ml demonstrated a 93% reduction in the formation of MRSA biofilms28. Our cell viability analysis of niosomes demonstrated that treating HFF cells with empty niosomes showed high biocompatibility (88.32% cell viability at 100μg/ml), while carvacrol alone showed significant cytotoxicity (39.46% cell survival). However, carvacrol in the niosomal form exhibited improved cell survival (59.67%) at the same concentration, showing nearly 1.5-fold efficacy compared to the standalone form. Another study found that the IC50 of carvacrol in MCF-7 cells was 305μM. This research indicated that carvacrol exhibited inhibitory effects on the growth of MCF-7 cells, inducing p53-dependent apoptosis, potentially associated with the Bax/Bcl-2 pathway20.
A limitation of our study is that we assessed the antimicrobial activity of carvacrol–niosomes only against MRSA strains. However, considering its high antimicrobial efficacy, carvacrol–niosomes could be explored for its effectiveness against other resistant pathogens, including ESBL, MBL, and others.
ConclusionIn summary, in the course of our investigation, we examined the antibacterial impact of carvacrol, an active plant compound, when encapsulated in a niosome drug delivery system. The physiochemical attributes of the niosomes were analyzed using FTIR, SEM, and DLS methods, followed by an assessment of their antimicrobial effects on MRSA strains. The findings indicated that the synthesized niosomes with spherical structure can enhance the antimicrobial effects by 2–4 times. Future investigations could concentrate on exploring the anticancer properties of niosomes containing carvacrol, with an emphasis on conducting in vivo studies. Additionally, optimizing drug delivery to specific tissues may enhance the antimicrobial and anticancer capabilities of this nanocarrier.
CRediT authorship contribution statementRonak Bakhtiari: She made a substantial contribution to the concept or design of the article; revised article critically for important intellectual content; approved the version to be published; agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Maliheh Shiri: She made a substantial contribution to the concept or design of the article; search database; designed figures, table and writing of the manuscript.
Mohammad Reza Mohammadi: Search database; designed figures and writing of the manuscript.
Mohammad Reza Pourmand: Search database; designed figures and writing of the manuscript.
Amir Mirzaie: Search database; designed figures and writing of the manuscript.
Zahra Taghiabadi: Search database; designed figures and writing of the manuscript.
Consent to participationN/A.
Ethical statementEthical code IR.TUMS.SPH.REC.1401.040 has been obtained from Tehran University of Medical Sciences, Tehran, Iran.
FundingThis study was supported by Tehran University of Medical Sciences, Tehran, Iran. (Grant code: 1401-2-296-57521)
Conflict of interestNone declared.