The Orsiro is a hybrid stent which has passive (amorphous silicon carbide) and active (poly-L-lactic acid, PLLA) coatings. The first layer encapsulates the stent struts, promoting lower local inflammation, whereas the second layer releases sirolimus through a biodegradable matrix. This study's aim was to compare the results of percutaneous coronary interventions (PCI) with Orsiro and Xience™ V stents (everolimus-eluting stent) in daily clinical practice.
MethodsObservational study in which patients were divided into two groups: those who received only one or more Orsiro stents, and those who received only XienceTM V stents. Early and late outcomes were prospectively collected.
ResultsBetween September 2012 and March 2014, this study included 92 and 108 patients treated with Orsiro and Xience™ V stents, respectively. Clinical, angiographic, and procedure characteristics were mostly similar between groups. Rates of procedure success (98.9% vs. 95.4%; p=0.22), in-hospital mortality (1.1% vs. 0%; p=0.40) and stent thrombosis (0% vs. 0.9%, p=0.30) did not differ between groups. Time of follow-up was 434±111 and 477±66 days (p=0.23), respectively, and differences in mortality (0.9% vs. 0%, p=0.30), stent thrombosis (0% vs. 0.9%; p=0.30), or need for repeat revascularization of the target lesion (0% vs. 0.9%; p=0.30) were not observed.
ConclusionsOrsiro and Xience™ V stents showed similar performance, with low rates of early and late clinical and angiographic events.
O stent Orsiro é um stent híbrido que possui revestimentos passivo (carbeto de silício amorfo) e ativo (ácido poli-L-lático, PLLA). O primeiro encapsula as hastes do stent, promovendo menor inflamação local, e o segundo libera o sirolimus por meio de matriz biodegradável. Nosso objetivo foi comparar os resultados das intervenções coronárias percutâneas (ICP) dos stents Orsiro e Xience® V (eluidor de everolimus) na prática clínica diária.
MétodosEstudo observacional em que os pacientes foram alocados em dois grupos: os que receberam exclusivamente um ou mais stents Orsiro e os que receberam exclusivamente stents Xience® V. Desfechos iniciais e tardios foram prospectivamente coletados.
ResultadosEntre setembro de 2012 e março de 2014, incluímos 92 e 108 pacientes tratados com stent Orsiro e Xience® V, respectivamente. Características clínicas, angiográficas e do procedimento foram, em sua maioria, semelhantes entre os grupos. As taxas de sucesso do procedimento (98,9% vs. 95,4%; p=0,22), mortalidade (1,1% vs. 0%; p=0,40) e trombose do stent (0% vs. 0,9%; p=0,30) hospitalares não diferiram entre os grupos. O tempo de seguimento foi de 434±111 e 477±66 dias (p=0,23), respectivamente, não sendo observadas diferenças na mortalidade (0,9% vs. 0%; p=0,30), trombose do stent (0% vs. 0,9%; p=0,30) e nem na necessidade de revascularização da lesão alvo (0% vs. 0,9%; p=0,30).
ConclusõesOs stents Orsiro e Xience® V apresentaram desempenho semelhante, com baixas taxas de eventos clínicos e angiográficos iniciais e tardios.
The use of drug-eluting stents (DES) represented a major step forward in current interventional cardiology, as it has significantly reduced restenosis rates1 and the need for repeat revascularization.2 How-ever, some specific issues, such as late thrombosis3 and local inflammatory response,4,5 have promoted significant advances in the development of prostheses, to allow better stent performance associated with greater patient safety.
DES consist of a platform, a polymer, and an antiproliferative drug.6 Bioengineering of materials has improved stent components, resulting in changes in the polymers and the use of several antiproliferative drugs. The Orsiro stent (Biotronik AG, Bulach, Switzerland), a hybrid sirolimus-eluting stent, is currently available for clinical use, but its utilization in Brazil is scarcely known.
The aim of this study was to evaluate early and late outcomes of patients from daily clinical practice submitted to percutaneous coronary intervention (PCI) with Orsiro stents, and to compare them to those receiving the Xience™ V stent (Abbott Vascular, Santa Clara, USA).
MethodsDesignThis was an observational study with prospective data collection.
Sample selectionPatients undergoing PCI for the treatment of ischemic heart disease in the CINECORS service of the Centro de Cardiologia do Hospital Ernesto Dornelles, in Porto Alegre (RS), Brazil were assessed. Variables related to risk factors for cardiovascular disease, procedure indication, technical details of the intervention, complications, and in-hospital and late follow-up were prospectively recorded on a specific form and entered in a dedicated database. For comparison, patients were divided into two groups: patients who received only Orsiro stents and patients who received only Xience™ V stents. All patients signed the free and informed consent form.
Percutaneous coronary interventionThe PCIs were carried out according to standard techniques and following the current guidelines,7 through femoral or radial access. Lesion assessment was performed by quantitative angiographic analysis, after intracoronary nitrate administration. Lesions were classified according to the definition of the American College of Cardiology/American Heart Association (A, B1, B2 and C).8 Pre- and post-dilation were used at the interventionist's discretion. All patients received unfractionated heparin at the begining of the procedure (70 to 100 U/kg), with additional doses administered if required, to maintain an activated clotting time (ACT) from 250 to 350seconds. Dual antiplatelet therapy was used in all patients with the administration of aspirin, 75 to 200mg a day, together with a P2Y12 inhibitor (clopidogrel, prasugrel or ticagrelor).
Orsiro stent descriptionThe Orsiro stent (Registered with the Brazilian National Health Surveillance Agency [Agência Nacional de Vigilância Sanitária - ANVISA] No. 80224390190) has a PRO-Kinetic Energy stent platform (L605 Chromium Cobalt alloy with 60μm thick struts). It is considered a hybrid stent, because it has two coatings: a passive (PROBIO - amorphous silicon carbide coating) and an active coating (BIOlute - poly-L-lactic acid, PLLA). The passive PROBIO coating encapsulates the stent struts and reduces the interaction between metal and the tissue. Consequently, it promotes less local inflammation. The BIOlute active coating contains a highly biocompatible polymer that releases sirolimus drug through a biodegradable matrix for 12 to 14 weeks. The BIOlute polymer is slowly degraded into carbon dioxide and water. After complete polymer degradation, only the PRO-Kinetic Energy stent remains (Fig. 1).
Clinical follow-up and outcomesPatients were clinically followed during the hospital stay and later through clinical consultation, chart review, and/or telephone contact. The following were defined as hospital outcomes for the analysis: procedure success rate (stent implantation with residual lesion<10% and Thrombolysis in Myocardial Infarction [TIMI] 3 flow after the procedure), hospital mortality, stent thrombosis, bleeding assessed by the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) study criteria, contrast-induced nephropathy (50% increase over baseline serum creatinine), or need for dialysis. As for the late follow-up, the following were evaluated as outcomes: mortality, stent thrombosis, and symptom-guided target-lesion revascularization.
Statistical analysisThe variables are shown in percentages, as mean±standard deviation (SD). For comparison between groups, the Chi-squared test or Student's t-test were used for categorical or continuous variables, respectively. The Mann-Whitney test was used for variables with normal distribution. A two-tailed p-value of<0.05 was considered statistically significant. All data were analyzed using Statistical Package for the Social Science (SPSS) for Windows, version 18.0 (SPSS, Chicago, USA).
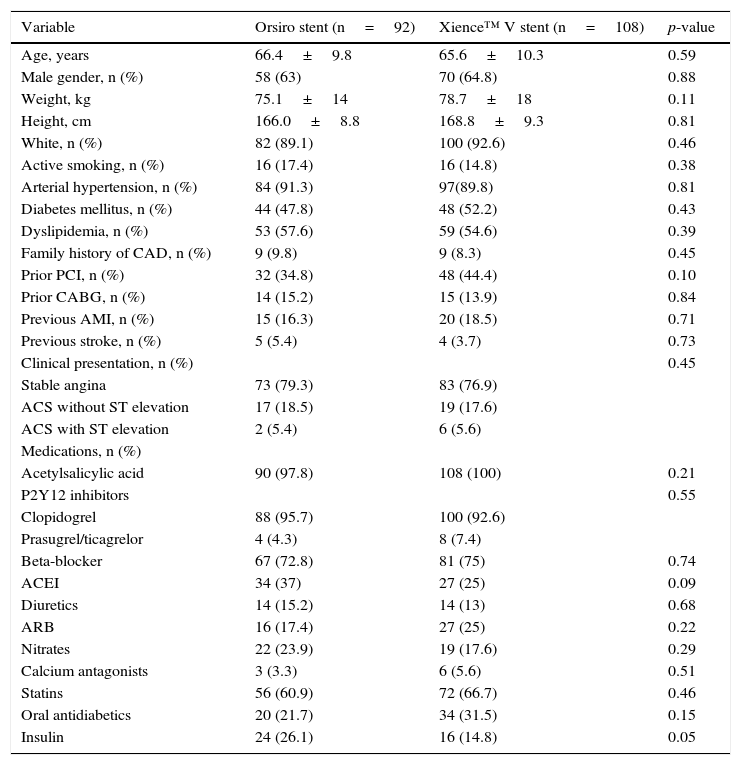
ResultsBetween September 2012 and March 2014, a total of 367 PCIs were performed in this service. After the inclusion criteria were applied, two groups of 92 and 108 patients participated in the study, treated exclusively with Orsiro and Xience™ V stents, respectively. As shown in Table 1, it was observed that both groups showed no differences in risk factors for cardiovascular disease, medications, and clinical indication for the procedure.
Clinical characteristics.
| Variable | Orsiro stent (n=92) | Xience™ V stent (n=108) | p-value |
|---|---|---|---|
| Age, years | 66.4±9.8 | 65.6±10.3 | 0.59 |
| Male gender, n (%) | 58 (63) | 70 (64.8) | 0.88 |
| Weight, kg | 75.1±14 | 78.7±18 | 0.11 |
| Height, cm | 166.0±8.8 | 168.8±9.3 | 0.81 |
| White, n (%) | 82 (89.1) | 100 (92.6) | 0.46 |
| Active smoking, n (%) | 16 (17.4) | 16 (14.8) | 0.38 |
| Arterial hypertension, n (%) | 84 (91.3) | 97(89.8) | 0.81 |
| Diabetes mellitus, n (%) | 44 (47.8) | 48 (52.2) | 0.43 |
| Dyslipidemia, n (%) | 53 (57.6) | 59 (54.6) | 0.39 |
| Family history of CAD, n (%) | 9 (9.8) | 9 (8.3) | 0.45 |
| Prior PCI, n (%) | 32 (34.8) | 48 (44.4) | 0.10 |
| Prior CABG, n (%) | 14 (15.2) | 15 (13.9) | 0.84 |
| Previous AMI, n (%) | 15 (16.3) | 20 (18.5) | 0.71 |
| Previous stroke, n (%) | 5 (5.4) | 4 (3.7) | 0.73 |
| Clinical presentation, n (%) | 0.45 | ||
| Stable angina | 73 (79.3) | 83 (76.9) | |
| ACS without ST elevation | 17 (18.5) | 19 (17.6) | |
| ACS with ST elevation | 2 (5.4) | 6 (5.6) | |
| Medications, n (%) | |||
| Acetylsalicylic acid | 90 (97.8) | 108 (100) | 0.21 |
| P2Y12 inhibitors | 0.55 | ||
| Clopidogrel | 88 (95.7) | 100 (92.6) | |
| Prasugrel/ticagrelor | 4 (4.3) | 8 (7.4) | |
| Beta-blocker | 67 (72.8) | 81 (75) | 0.74 |
| ACEI | 34 (37) | 27 (25) | 0.09 |
| Diuretics | 14 (15.2) | 14 (13) | 0.68 |
| ARB | 16 (17.4) | 27 (25) | 0.22 |
| Nitrates | 22 (23.9) | 19 (17.6) | 0.29 |
| Calcium antagonists | 3 (3.3) | 6 (5.6) | 0.51 |
| Statins | 56 (60.9) | 72 (66.7) | 0.46 |
| Oral antidiabetics | 20 (21.7) | 34 (31.5) | 0.15 |
| Insulin | 24 (26.1) | 16 (14.8) | 0.05 |
CAD: coronary artery disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass surgery; AMI: acute myocardial infarction; ACS: acute coronary syndrome; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin II receptors blockers.
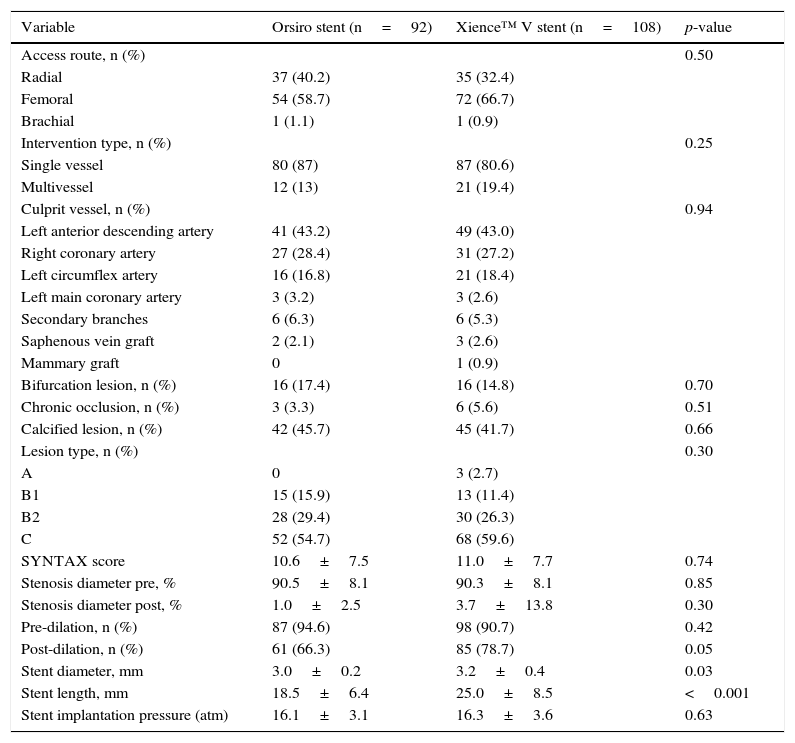
A total of 95 and 114 lesions were treated, respectively, in patients using Orsiro and Xience™ V stents. Vessel diameter (3.0mm±0.2 vs. 3.0±0.3mm; p=0.51) and mean lesion length (18.0±6mm vs. 20.0±7mm; p=0.08) were equivalent in both groups. The numbers of Orsiro and Xience™ V stents per patient were 1.3±0.56 and 1.4±0.55 (p=0.60). A total of 91 and 103 patients from the Orsiro and Xience™ V groups (98.9% vs. 95.4%; p=0.22) were successfully treated, respectively. Table 2 describes the main angiographic and procedure characteristics.
Angiographic and procedure characteristics.
| Variable | Orsiro stent (n=92) | Xience™ V stent (n=108) | p-value |
|---|---|---|---|
| Access route, n (%) | 0.50 | ||
| Radial | 37 (40.2) | 35 (32.4) | |
| Femoral | 54 (58.7) | 72 (66.7) | |
| Brachial | 1 (1.1) | 1 (0.9) | |
| Intervention type, n (%) | 0.25 | ||
| Single vessel | 80 (87) | 87 (80.6) | |
| Multivessel | 12 (13) | 21 (19.4) | |
| Culprit vessel, n (%) | 0.94 | ||
| Left anterior descending artery | 41 (43.2) | 49 (43.0) | |
| Right coronary artery | 27 (28.4) | 31 (27.2) | |
| Left circumflex artery | 16 (16.8) | 21 (18.4) | |
| Left main coronary artery | 3 (3.2) | 3 (2.6) | |
| Secondary branches | 6 (6.3) | 6 (5.3) | |
| Saphenous vein graft | 2 (2.1) | 3 (2.6) | |
| Mammary graft | 0 | 1 (0.9) | |
| Bifurcation lesion, n (%) | 16 (17.4) | 16 (14.8) | 0.70 |
| Chronic occlusion, n (%) | 3 (3.3) | 6 (5.6) | 0.51 |
| Calcified lesion, n (%) | 42 (45.7) | 45 (41.7) | 0.66 |
| Lesion type, n (%) | 0.30 | ||
| A | 0 | 3 (2.7) | |
| B1 | 15 (15.9) | 13 (11.4) | |
| B2 | 28 (29.4) | 30 (26.3) | |
| C | 52 (54.7) | 68 (59.6) | |
| SYNTAX score | 10.6±7.5 | 11.0±7.7 | 0.74 |
| Stenosis diameter pre, % | 90.5±8.1 | 90.3±8.1 | 0.85 |
| Stenosis diameter post, % | 1.0±2.5 | 3.7±13.8 | 0.30 |
| Pre-dilation, n (%) | 87 (94.6) | 98 (90.7) | 0.42 |
| Post-dilation, n (%) | 61 (66.3) | 85 (78.7) | 0.05 |
| Stent diameter, mm | 3.0±0.2 | 3.2±0.4 | 0.03 |
| Stent length, mm | 18.5±6.4 | 25.0±8.5 | <0.001 |
| Stent implantation pressure (atm) | 16.1±3.1 | 16.3±3.6 | 0.63 |
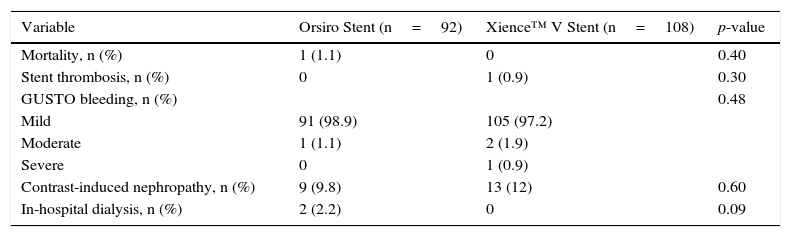
During the in-hospital follow-up, no differences were observed between the groups regarding mortality, development of stent thrombosis, bleeding or contrast nephropathy (Table 3). One patient from the Orsiro group died due to cardiogenic shock during acute myocardial infarction. The only case of acute thrombosis occurred with a Xience™ V stent, caused by prosthesis underexpansion. There was a slight tendency to increased need for dialysis in the Orsiro group, although this finding has no direct link to the type of stent used.
In-hospital complications.
| Variable | Orsiro Stent (n=92) | Xience™ V Stent (n=108) | p-value |
|---|---|---|---|
| Mortality, n (%) | 1 (1.1) | 0 | 0.40 |
| Stent thrombosis, n (%) | 0 | 1 (0.9) | 0.30 |
| GUSTO bleeding, n (%) | 0.48 | ||
| Mild | 91 (98.9) | 105 (97.2) | |
| Moderate | 1 (1.1) | 2 (1.9) | |
| Severe | 0 | 1 (0.9) | |
| Contrast-induced nephropathy, n (%) | 9 (9.8) | 13 (12) | 0.60 |
| In-hospital dialysis, n (%) | 2 (2.2) | 0 | 0.09 |
GUSTO: Global Use of Strategies to Open Occluded Coronary Arteries study.
Late follow-up was available for all 92 (100%) patients from the Orsiro group and the 104 (96,2%) patients of the Xience™ group. Mean follow-up was 434±111 and 477±66 days, respectively (p=0.23). There was one death (0.9%) in the Orsiro group from complications related to cirrhosis at 177 days of follow-up. In the Xience™ group, on the 90th day post-stenting, there was a case of late stent thrombosis (0.9%) due to dual antiplatelet therapy interruption. A case of target-lesion revascularization (0.9%, corresponding to one patient submitted to the implantation of 2 stents with the mini-crush technique) was also observed at 232 days of follow-up.
DiscussionThis study showed that the new hybrid sirolimus-eluting Orsiro stent showed excellent immediate results and sustained benefit at the late follow-up when compared to the everolimus-eluting Xience™ V stent.
The BIOFLOW-I study9 evaluated the first patients treated with the Orsiro stent in clinical practice. In that study, 30 patients were treated with the sirolimus-eluting biodegradable polymer stent. Similarly to the present study, there were no cases of late thrombosis. In the BIOFLOW-I study, however, angiographic follow-up with intravascular ultrasound was part of the protocol. Due to this characteristic, a better evaluation of the device was performed. There were no cases of binary restenosis. However, the procedures’ low complexity favored the excellent early and late outcomes in both studies.
The in-hospital clinical evolution was equivalent in the two groups of the present study. The only case of thrombosis in the Xience™ V group was due to an underexpanded stent. The consensus is that cases of thrombosis within the first days are usually due to the implantation technique.10 In the Orsiro group, the event of death was explained by the evolution of cardiogenic shock. There was a higher rate of contrast-induced nephropathy with the need for dialysis in the sirolimus-eluting stent group. Although this finding is not associated with the stent type, the higher prevalence of insulin-dependent diabetes in this group can justify what occurred.
Late follow-up was favorable for both the Orsiro and the Xience™ stents. Mortality in the Orsiro group was not due to cardiovascular causes. The two events related to the target-lesion in the Xience™ group (one late thrombosis and one restenosis) were justified by poor adherence to dual antiplatelet therapy and complex intervention with two stents. The low rate of clinical events is noteworthy in the present study. In the BIOFLOW-I and -II studies,11 the target-lesion revascularization rates were around 6.7% and 2.7%, respectively. However, the included patients had a low rate of complex interventions (87% single-vessel PCI), 3-mm vessel caliber, and lesion length of approximately 20mm. Additionally, the mean SYNTAX score of 10 is consistent with low complexity interventions. Considering these characteristics, the absence of clinical restenosis in the present study can be explained.
This study has the following limitations: it was a single-center study with a limited number of patients, and patients were followed only through clinical follow-up. Therefore, no comments regarding the angiographic performance of stents can be made. However, these data reflect the real-world experience, in which patients are referred to angiography when symptoms are present.
ConclusionsIn this clinical practice study, both the Orsiro (sirolimus-eluting) and Xience™ V (everolimus-eluting) stents showed similar performance, with low clinical event rates at the early and late follow-up.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.