Left ventricular pseudoaneurysms are usually associated with acute myocardial infarction; however, these conditions may emerge in the late postoperative period of valvar surgery, and this can also occur with aortic pseudoaneurysms. These pseudoaneurysms often affect patients with high surgical risk, and percutaneous treatment is usually performed in reference centers for treatment of congenital heart diseases, due to anatomical characteristics of these defects. We present two cases of left ventricular pseudoaneurysms treated by transapical approach without need for cardiopulmonary bypass, and a case of aortic pseudoaneurysm treated by femoral approach, in which a snare was introduced by contralateral access, to allow for adequate support and guidance of the long sheath for accessing the defect.
Pseudoaneurismas do ventrículo esquerdo são geralmente associados a infarto agudo do miocárdio, entretanto, podem surgir no pós-operatório tardio de cirurgias valvares, assim como os pseudoaneurismas aórticos. Acometem frequentemente pacientes com alto risco cirúrgico, e o tratamento percutâneo é habitualmente realizado em centros de referência para o tratamento de cardiopatias congênitas devido às características anatômicas dos defeitos. Apresentamos dois casos de pseudoaneurismas do ventrículo esquerdo tratados por via transapical, sem necessidade de circulação extracorpórea, e um caso de pseudoaneurisma aórtico tratado por via femoral, no qual foi utilizado laço por acesso contralateral para permitir suporte e direcionamento adequados da bainha longa para acessar o defeito.
Left ventricular pseudoaneurysms usually are the result of left ventricular wall rupture after acute myocardial infarction (AMI) or heart surgery, with or without valve replacement. The risk of rupture is between 30 to 45% in the first year, and surgery is associated with a mortality of about 20 to 35%.1–5 Percutaneous or transapical therapy seems to be a viable, effective and possibly safer option.6,7
In the same line, pseudoaneurysms of the ascending aorta are more often diagnosed, among other less common causes, in patients who has previously undergone aortic surgery.8 In addition to being technically difficult, patients usually show a not negligible surgical risk, making the percutaneous technique an attractive alternative, mainly in centers specialized in the treatment of congenital heart defects.
This study reports two cases of left ventricular pseudoaneurysm successfully treated by a transapical technique in a hybrid room, and a case of pseudoaneurysm of the aorta treated by femoral access.
Case reportCase 1This is a 79-year-old female patient with a history of AMI complicated by rupture of left ventricle and formation of pseudoaneurysm. During coronary artery bypass graft surgery, it was not possible to access the pseudoaneurysm location, due to the extensive presence of adhesions, caused by radiotherapy for breast cancer. Thus, we favored a conservative treatment; however, the patient developed heart failure and pseudoaneurysm growing, which reached 6 6cm in the last assessment, when the patient was referred to the invasive cardiology team to evaluate the possibility of a percutaneous closure.
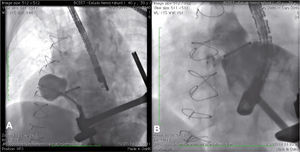
During cardiac catheterization, extensive peripheral vascular disease was evidenced, which ruled out the feasibility of a percutaneous treatment by retrograde route, possibly the best option due to the posterior-anterior position of the defect. Thus, our final choice was an apical puncture through mini-throracotomy, through which a 14 F introducer sheath was inserted in the left ventricular cavity. However, due to the unfavorable angle between the short sheath and the defect entry, we could not access the defect with a standard long sheath for implantation of the occluder device.
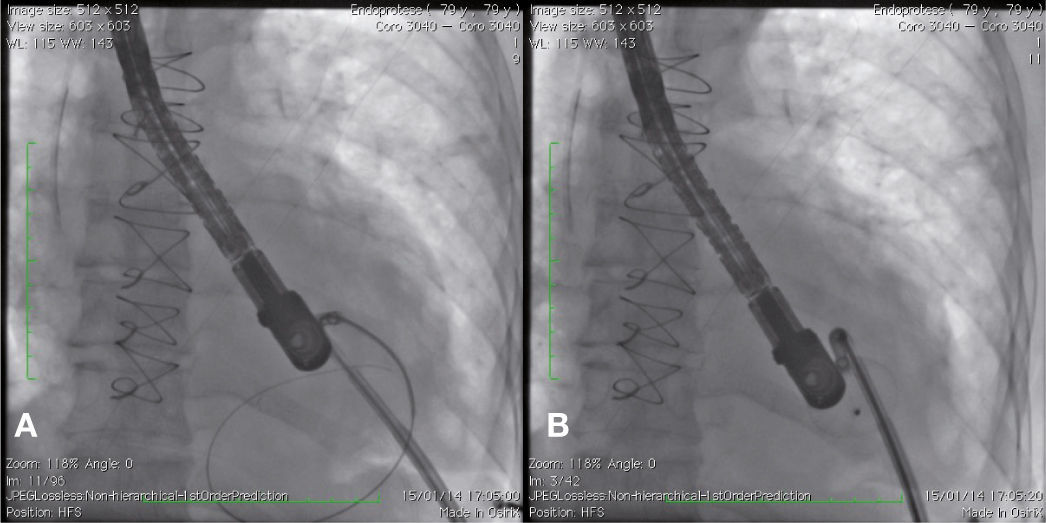
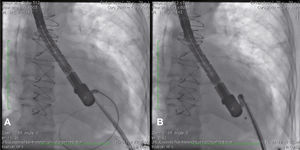
After angiography and appropriate location and measurement of the defect (15mm diameter), a long deflectable sheath (FustarTM, Lifetech Scientific Inc., Shenzhen, China) was introduced, making possible the access to the defect with a catheter, the positioning of a stiff guide and, following that, the release of a 20mm CERATM ASD (Lifetech Scientific Inc., Shenzhen, China) device (Fig. 1). This patient recovered uneventfully, being discharged after 48hours of admission and with a transthoracic echocardiogram without residual shunt through the defect. Three months after surgery, the patient was asymptomatic.
Case 2This is a 39-year-old male patient who underwent three previous heart surgeries. The first of these operations, carried out 14 years ago, was for a mitral-aortic aneurysm repair. After 1 year, the patient developed severe aortic insufficiency, requiring a metallic aortic prosthesis implant. Eight years after the first surgery, the patient showed signs of class II NYHA heart failure, and the echocardiogram showed moderate-to-severe perivalvar leak and a pseudoaneurysm of the ascending aorta. The patient was referred for surgical implant of an aortic valved tube, a 25mm prosthetic metallic aortic valve, and coronary reimplantation by Cabrol technique. Intraoperatively, the patient developed severe bleeding; it was necessary to maintain the chest opened with hemostatic pack use for 48hours. Then, the chest was closed, with hemothorax drainage. During the postoperative period, the patient showed right pleural effusion, sepsis and atrial flutter reverted with electrical cardioversion.
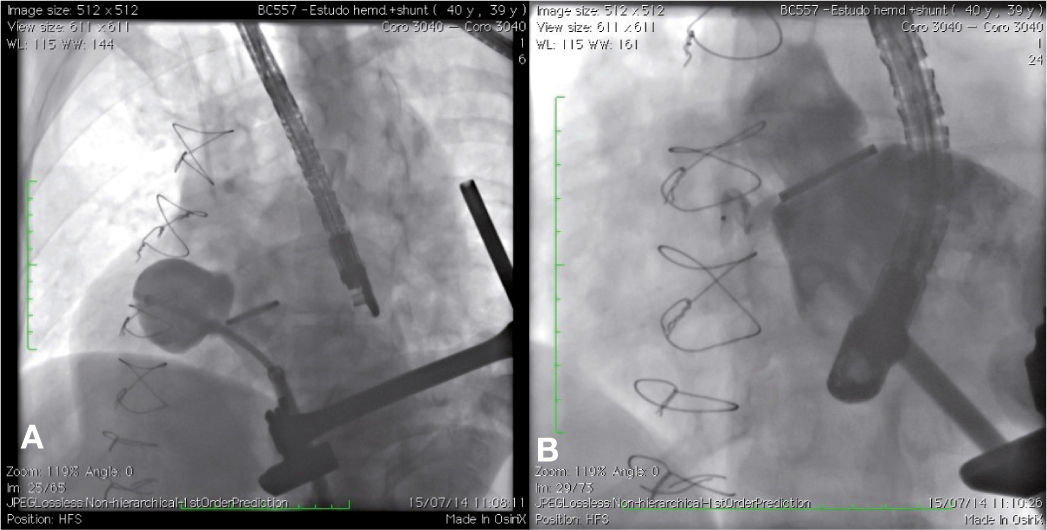
The patient had a good outcome; however, 6 years later, a transthoracic echocardiography was suggestive of residual pseudoaneurysm. A transesophageal echocardiogram showed pulsatile sacculation with an ostium located in the left ventricular outflow tract between the prosthetic valve ring and the anterior mitral leaflet, measuring 4.4 2.6cm. By transapical approach and with an 18 F introducer sheath, a catheter was placed inside the defect, and an angiography was carried out, revealing a pseudoaneurysm in the aortic subvalvar portion with a hole measuring about 4mm in diameter and a course of approximately 20mm (Fig. 2A). The defect was accessed by means of a hydrophilic guide, which was later replaced by a molded extra-rigid guide, from which the 9 F releasing system was introduced. Then, an 8-mm AmplatzerTM Muscular VSD device (St. Jude Medical Inc., St. Paul, US) was successfully implanted. A control ventriculography and an intraoperative transesophageal echocardiography showed good positioning of the device, without interfering with the prosthetic aortic heart valve dynamics, and with minimal residual shunt through the device (Fig. 2B). An echocardiogram carried out 24hours after the procedure showed complete occlusion of the defect.
Case 3This is a 58-year-old female patient, with a history of fusiform aneurysm of ascending and transverse aorta measuring 6.3 6.0cm, operated 3 years ago. The patient was admitted because of syncope and chest pain. A catheterization was carried out, revealing a distal saccular aneurysmal region in the ascending aorta, probably corresponding to a pseudoaneurysm at the suture area. Due to the presence of a previous surgery and considering the high surgical risk, we opted for a technique of percutaneous occlusion of the pseudoaneurysm.
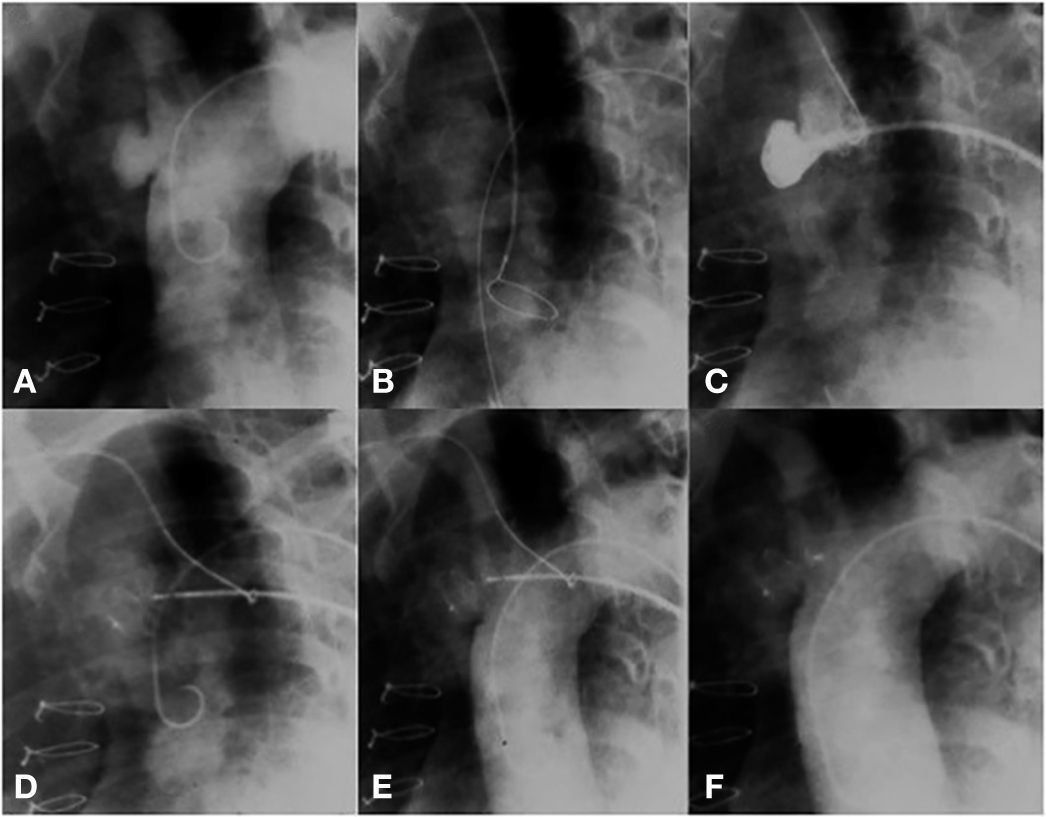
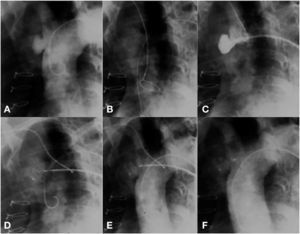
The technical challenge to be overcome in this case was the location of the pseudoaneurysm, just below the insertion of the innominate artery. The location prevented access through the right subclavian artery; thus, direct positioning of the long sheath into the aneurysm became a risky task, due to system tension. Our solution was to control the sheath positioning through a support snare. A 20-mm snare was introduced from the radial artery; this device was left open into the ascending aorta, near the aortic valve. Then, a long sheath, compatible with an appropriately sized prosthesis for defect occlusion, was introduced up to the ascending aorta. The distal part of the sheath was captured by the snare and brought to the pseudoaneurysm orifice. Using the support provided with the use of this snare, the sheath was gently introduced into the cavity, without putting pressure on its walls. The snare was maintained until the introduction of the Amplatzer ASO (15mm St. Jude Medical Inc., St. Paul, USA) device, thus allowing that there was no retrocession or loss of position when the distal disk of the device was open. The prosthesis was released in the usual way, after confirmation by an angiogram of an adequate position of the device; with this, the snare was opened, releasing the long sheath (Fig. 3).
(A) Aortography showing a pseudoaneurysm of the ascending aorta. (B) A snare in the ascending aorta through contralateral radial access. (C) Snare facilitating the access of a long sheath to the defect. (D) Beginning of device release. (E) A well-positioned device, still attached to the delivery cable. (F) Aortography without residual shunt.
The evolution of the patient had no further complications, but 3 years after the procedure, she was hospitalized with hypotension and chest pain. An angiotomography revealed the presence of a dissecting aneurysm of the ascending aorta, which was surgically corrected. During the intraoperative period, the patient developed massive airway bleeding and died probably due to an aortopulmonary fistula.
DiscussionHistorically, both left ventricular pseudoaneurysm as aortic pseudoaneurysm are treated surgically. However, these two problems often occur in previously operated patients with surgical adhesions that hinder the intraoperative management, and with comorbidities leading to a high procedural mortality. In this scenario, it is not uncommon that some patients have surgical contraindications due to the severity of their condition. From this context, percutaneous treatment by femoral or transapical approach has been gaining space and has become a viable alternative, with satisfactory results.6,7
In most cases, the diagnosis can be established by transthoracic echocardiography. A ratio < 0.5 between neck diameter and inner diameter of the aneurysmal sac suggests a diagnosis of pseudoaneurysm. Chest CT angiography and three-dimensional transesophageal echocardiography help in planning the intervention and in the choice of the device, by determining the anatomical characteristics of the aneurysmal sac and also of the neck of the defect.2 Furthermore, it is critical to determine the relations of the defect with adjacent structures.8–10Left ventricular pseudoaneurysms usually result from rupture of the left ventricular wall, which is temporarily restrained by scar tissue, thereby avoiding pericardial tamponade or free bleeding to the pleural cavity. However, the risk of rupture is around 30 to 45% in the first year. It usually occurs after AMI or a surgical valve replacement. Less frequently, the rupture may occur after a pacemaker implant, resection of a left ventricular aneurysm, Ross surgery or percutaneous aortic valve implant.2–7,11,12 Clinically, left ventricular pseudoaneurysm may occur in an asymptomatic patient, in a patient with chest pain, or even causing symptoms of congestive heart failure. Surgical repair is the most widely described treatment; however, its high risk and the presence of previous surgery in most patients have made percutaneous therapy a viable, effective and possibly safer option.6,7
As described in the literature, in this study two cases were successfully treated by direct left ventricular puncture, without cardiopulmonary bypass, using percutaneous occlusion techniques for congenital heart disease. Considering that this is an acquired cardiopathy with several etiologies, there is a number of measures and neck size and location that corroborates an individualized approach with respect to access and to the device.7
Initially described by Clift et al.,4 approximately 19 cases have been published in the literature, with many devices of common use in the treatment of congenital heart disease being used off-label, such as septal occluders of interatrial or muscular interventricular communications, and vascular plugs. In general, these defects are occluded by a percutaneous retrograde access through the femoral artery, or else, by a transapical access through a mini-thoracotomy.3–7,11–16 In the two cases presented in this study, the transapical approach was used. In the first case, thanks to the extensive peripheral vascular disease; and in the second case, because of the proximity and the unfavorable angle close to the aortic valve.
In the first case, even using direct left ventricular cavity puncture, the angle between the inserted sheath and the neck of the defect was not favorable for introduction of a long and free-of-tension large-bore sheath. After multiple unsuccessful attempts, we opted for the use of a deflectable sheath, which was easily introduced until reaching the defect entry and allowed for a successful implant of the device. Since this was a large defect with a shallow neck, it was decided to use a device for atrial septal defect occlusion, which was adequate for this type of defect.
In the second case, the apical puncture allowed coaxial access to the defect close to the aortic valve, permitting the implantation of the device to be carried out safely, effectively and without major difficulties with a 6 F catheter. In this case, we chose an interventricular defect closure device, because this defect had an extensive neck, and also because type II vascular plugs were temporarily unavailable. Both cases were performed at the same institution, in a hybrid room and with a team of professionals accustomed with team work (Heart Team).
Pseudoaneurysms of the ascending aorta, although well-known entities, are uncommon, with potentially fatal complications. These defects occur most often after reconstructive surgery of the aorta and valve repair, and less often after chest trauma or infection.10
The regions potentially amenable to the emergence of pseudo-aneurysms of the ascending aorta are those sites of cannulation and aortic clamping, and graft suture zones.9 Some complications related to surgical technique, graft or tissue degeneration, as well as deterioration of suture material are among the causes commonly associated with the development of pseudoaneurysms of the aorta.
The behavior of this defect is similar to that of pseudoaneurysms of the left ventricle, i.e., there is risk of rupture, thrombosis and distal embolization, and of fistula formation.8 The standard treatment is surgical, but the risk can reach 30% and sometimes this option is not technically or clinically feasible.
In patients with clinical manifestations, the most common presentations are myocardial ischemia due to coronary compression, a chest pulsatile mass, skin ulceration and subsequent bleeding, embolic events and complications related to infection and/or pseudoaneurysm rupture.
Percutaneous occlusion of pseudoaneurysms of the aorta was first described in 2005 in a patient with a prohibitive surgical risk.8 Based on the experience of septal occluders in congenital heart defect patients; we chose to use this technique in our patient, with an excellent and immediate result. One of the setbacks often encountered in using this technique is the tension generated by the long sheath during the access towards the defect, besides the intrinsic difficulty of access. The literature describes some alternatives to facilitate implantation and minimize risks. Bashir et al.,8 after numerous unsuccessful attempts, described the use of a Hausdorff sheath (Cook Medical Inc., Bloomington, USA), which enabled a successful implantation. According to Stark et al.,17 the customization of the 8F introducer sheath with a “hockey stick” guide-catheter is required, and this also permitted the access to the defect and percutaneous occlusion of the pseudoaneurysm, with adequate result. However, depending on the location and size of the isthmus of entry for the defect, the occlusion with an AmplatzerTM device may be beyond question, for instance, in the case of an isthmus larger than the prosthesis, or when there would be risk of occlusion of supra-aortic vessels.
As described in the literature, in this case the main difficulty was the access to the defect with the long sheath. To overcome this limitation, the authors describe a technique that uses an contralateral access snare, which lends adequate support and direction for the long sheath, without causing significant tension in the aortic wall.
This case clearly elucidates that percutaneous aortic pseudo-aneurysm occlusion in selected cases is a safe and viable alternative to surgical procedure, with fewer risks related to the procedure and less short-term complications. However, it is critical that the operator has experience in percutaneous occlusion, which may result in higher success rates.
In addition, the Heart Team cooperation and the provision of a hybrid room at our institution were extremely important in our initial experience in the treatment of left ventricular pseudoaneurysms; thus, it was shown that teamwork results in a wide range of patients with the possibility of a safer and more effective treatment.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.