Nuclear magnetic resonance is a technique requiring breath holding or staying still for long periods of time for image acquisition. For this reason, paediatric patients need to be given sedation or anaesthesia, creating additional risks to those found in other areas.
ObjectiveTo describe the incidence of adverse events in paediatric patients in the magnetic resonance service with the presence of an anaesthetist.
Materials and methodsDescriptive observational study to assess the incidence of adverse events in 4786 patients under 15 years of age taken to magnetic resonance imaging with an attending anaesthetist for sedation or anaesthesia at Instituto de Alta Tecnología Médica between 2010 and 2014.
ResultsThere were 12 adverse events with a rate of 2.5 for every 1000 paediatric patients. Of these, there were 6 serious, 4 moderate and 2 mild adverse events. The proportion of mortality was 0.04%.
ConclusionPerforming magnetic resonance imaging studies under sedation or anaesthesia given by an anaesthetist in patients under 15 years of age is safe. However a risk-benefit analysis is required in hospitalized or decompensated patients, in order to assess the best option.
La resonancia magnética es una técnica donde se requieren apneas o periodos de inmovilidad considerables para la adquisición de imágenes. Inmovilidad que por condiciones de los pacientes pediátricos son difíciles mantener, requiriendo administración de sedación o anestesia generando riesgos adicionales a los existentes en otras áreas.
ObjetivoDescribir la incidencia de eventos adversos en pacientes pediátricos en el servicio de resonancia magnética bajo asistencia por anestesiólogo.
Materiales y métodosEstudio observacional descriptivo, donde se valoró la incidencia de eventos adversos en 4786 pacientes menores de 15 años que fueron llevados a resonancia bajo asistencia por anestesiólogo para sedación o anestesia en el Instituto de Alta Tecnología Médica entre los años 2010-2014.
ResultadosSe presentaron 12 eventos adversos, con un índice de 2.5 por cada 1000 pacientes pediátricos, de los cuales 6 eventos adversos fueron graves, 4 moderados y 2 leves. La proporción de mortalidad fue del 0.04%.
ConclusiónRealizar estudios de resonancia magnética bajo sedación o anestesia por anestesiólogo en pacientes menores de 15 años es seguro, sin embargo en pacientes hospitalizados o descompensados debe hacerse un análisis riesgo - beneficio y valorar la mejor opción.
Magnetic resonance imaging (MRI) is an evolving diagnostic technique aimed at achieving better quality images to diagnose increasingly complex diseases in a shorter period of time.1,2 Despite new advances, image acquisition still requires relative long periods where the patient needs to remain still, and even short breath-holding periods.3 Immobility in patients under 15 years of age is seldom possible, hence the need for sedation and, occasionally, anaesthesia in order to ensure the right conditions.3
Sedation during magnetic resonance imaging entails additional risks to those existing in other settings.1,4 They include risks associated with the powerful electromagnetic field, high frequency electromagnetic waves, high noise levels, and low lighting.1 The most significant limitations come from the electromagnetic field which precludes the use of many of the devices used regularly in anaesthesia, given the risk that they may become projectiles inside the machine because of their ferromagnetic characteristics.1,5 This requires instituting safety measures to reduce the incidence of adverse events in the resonance imaging area.1
The following safety measures are described in the world literature1,5,6 for reducing the incidence of adverse events:
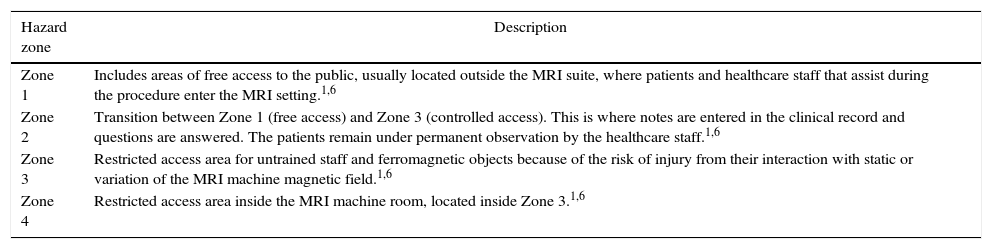
Hazard zone: The MRI area is access-restricted in accordance with hazard zones. See Table 1.
MRI hazard zones.
| Hazard zone | Description |
|---|---|
| Zone 1 | Includes areas of free access to the public, usually located outside the MRI suite, where patients and healthcare staff that assist during the procedure enter the MRI setting.1,6 |
| Zone 2 | Transition between Zone 1 (free access) and Zone 3 (controlled access). This is where notes are entered in the clinical record and questions are answered. The patients remain under permanent observation by the healthcare staff.1,6 |
| Zone 3 | Restricted access area for untrained staff and ferromagnetic objects because of the risk of injury from their interaction with static or variation of the MRI machine magnetic field.1,6 |
| Zone 4 | Restricted access area inside the MRI machine room, located inside Zone 3.1,6 |
In patients under 15 years of age or high risk patients, sedation (moderate or profound) and anaesthesia are given by the anaesthesia specialist.1
Equipment brought into Zone 4 must be approved by the manufacturer before use, including the anaesthesia machine and all airway devices.1
A fully equipped basic and advanced cardiovascular resuscitation area must be available in Zone 2.1
In the search for continuous improvement of the safety and efficiency measures implemented at Fundación Instituto de Alta Tecnología Médica (IATM) in Medellín-Colombia, a study was conducted to describe the incidence of adverse events over the past 5 years in patients under 15 years of age taken to magnetic resonance imaging under sedation or anaesthesia given by the attending anaesthetist.
Materials and methodsPatient selection for sedation or anaesthesiaRetrospective observational descriptive study in patients under 15 years of age taken to magnetic resonance imaging (1.5 and 3.0T machines) under sedation or anaesthesia given by a specialist in anaesthesia during the time period between 2010 and 2014 at IATM.
Patients were classified according to mutually exclusive age groups7; neonates (1–30 days), infants (>1 month to <1 year), toddlers (1–2 years), pre-schoolers (3–4 years), school children (5–10 years) and adolescents (11–14 years).
Data recording and verificationData were taken from the clinical record completed by the nurse and from the anaesthesia record. The former includes identification, informed consent for entering the MRI machine area, patient interview (background information, clinical record summary and order from the treating physician), and anthropometric data. The information was verified with the radiologist.
The anaesthesia record completed by the anaesthetist includes patient identification, date, classification of the anaesthetic risk (ASA classification),8 personal history and physical examination. Basic ASA monitoring (or advanced, depending on the physical condition), was performed during anaesthesia and the variables (blood pressure, oximetry, heart rate and capnography) were documented in a paper form that also included timing and dosing of medications, which were administered at the therapeutic doses recommended in the literature.9
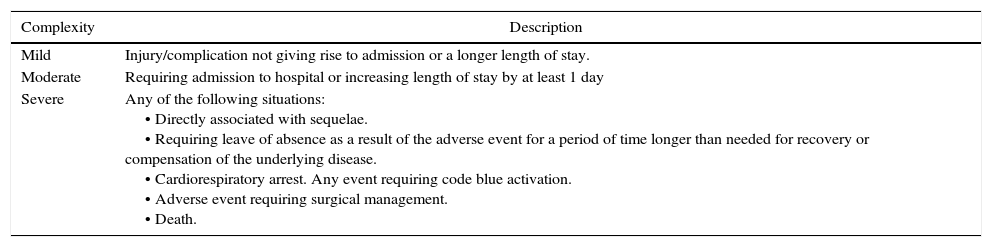
After collecting the information, the study population was described and adverse events were identified and classified according to the complexity of the outcome (mild, moderate or severe). They were also characterised by sex, age, type of study performed, length of the procedure and ASA level. This study included all adverse outcomes of allergic origin as well as moderate to severe outcomes of non-allergic origin. See Table 2.
Classification of adverse events.
| Complexity | Description |
|---|---|
| Mild | Injury/complication not giving rise to admission or a longer length of stay. |
| Moderate | Requiring admission to hospital or increasing length of stay by at least 1 day |
| Severe | Any of the following situations: • Directly associated with sequelae. • Requiring leave of absence as a result of the adverse event for a period of time longer than needed for recovery or compensation of the underlying disease. • Cardiorespiratory arrest. Any event requiring code blue activation. • Adverse event requiring surgical management. • Death. |
Source: Adapted from the Ministerio de la Protección Social.10
After the adverse event, all patients were followed-up by phone at 48h through their relatives (parents or companions) or by the nursing staff in hospitalised patients.
Analysis planAbsolute and per cent distributions were used for the descriptive analysis. Likewise, the rate of adverse events indicator was also used, where the numerator is the number of adverse events during the time period and the denominator is the number of paediatric patients, multiplied by a constant of 1000.
Ethical considerationsAfter review, approval and authorisation by the Research Ethics Committee of IATM, the data from the clinical record were collected from the Radiology Information System (RIS). In accordance with the Declaration of Helsinky, the Belmont Report and Colombian Resolution 8430 of 1993, this research was classified as no-risk and, for this, reason, no informed consent was obtained before patient inclusion.
ResultsGeneral considerationsOverall, 4786 patients under 15 years of age taken to MRI and requiring care from an anaesthetist for deep sedation or general anaesthesia were identified during the time period between 2010 and 2014.
Of the total number of patients 57.2% were males. Age groups according to sex are shown in Fig. 1.
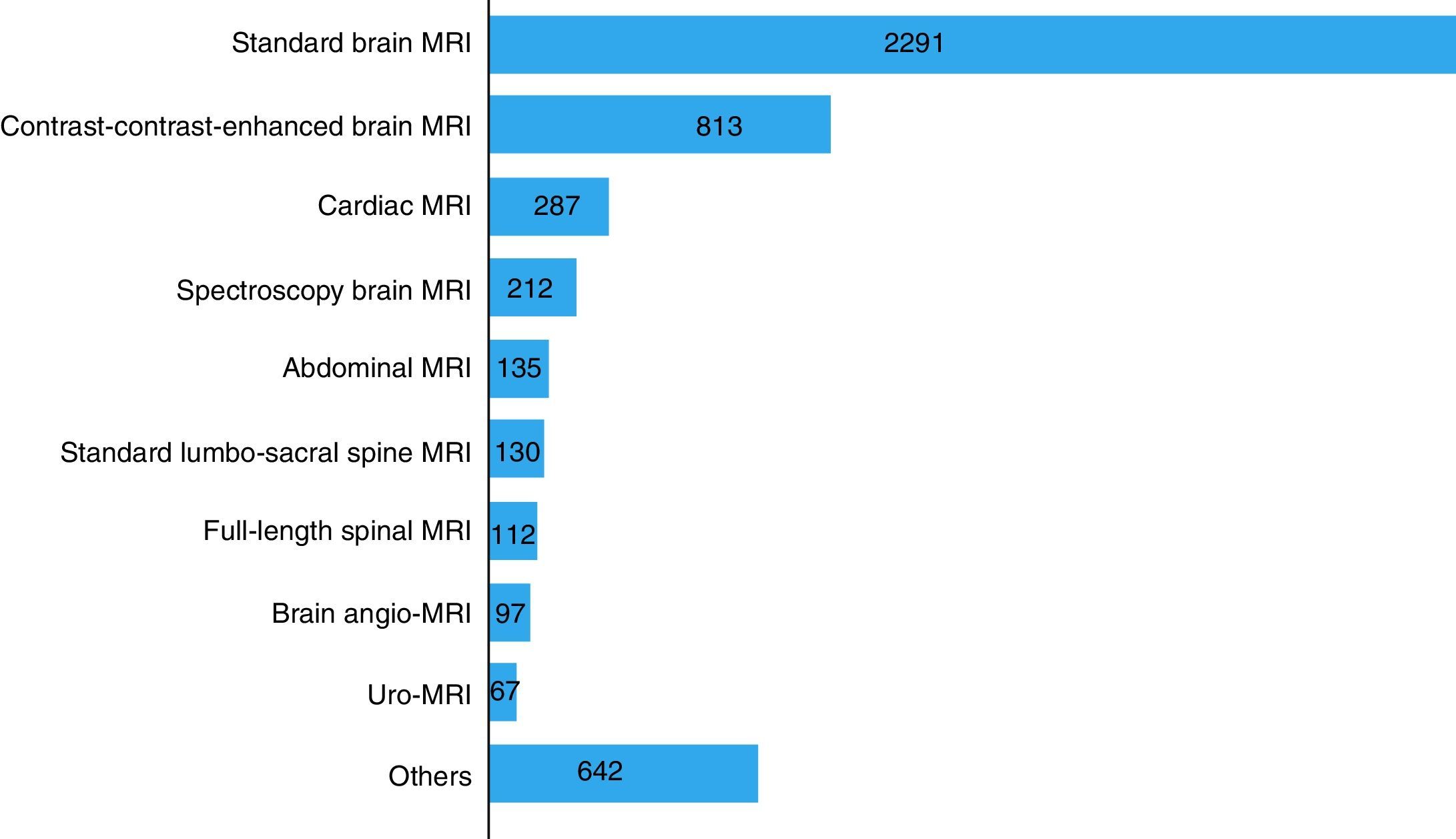
The most frequent MRI studies performed were: standard brain MRI, 47% (n=2291); contrast-contrast-enhanced brain MRI, 16% (n=813) and cardiac MRI, 6% (n=287). See Fig. 2.
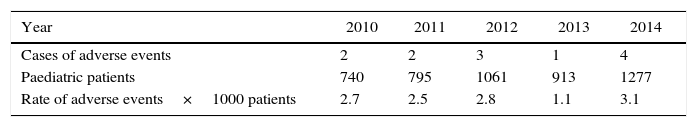
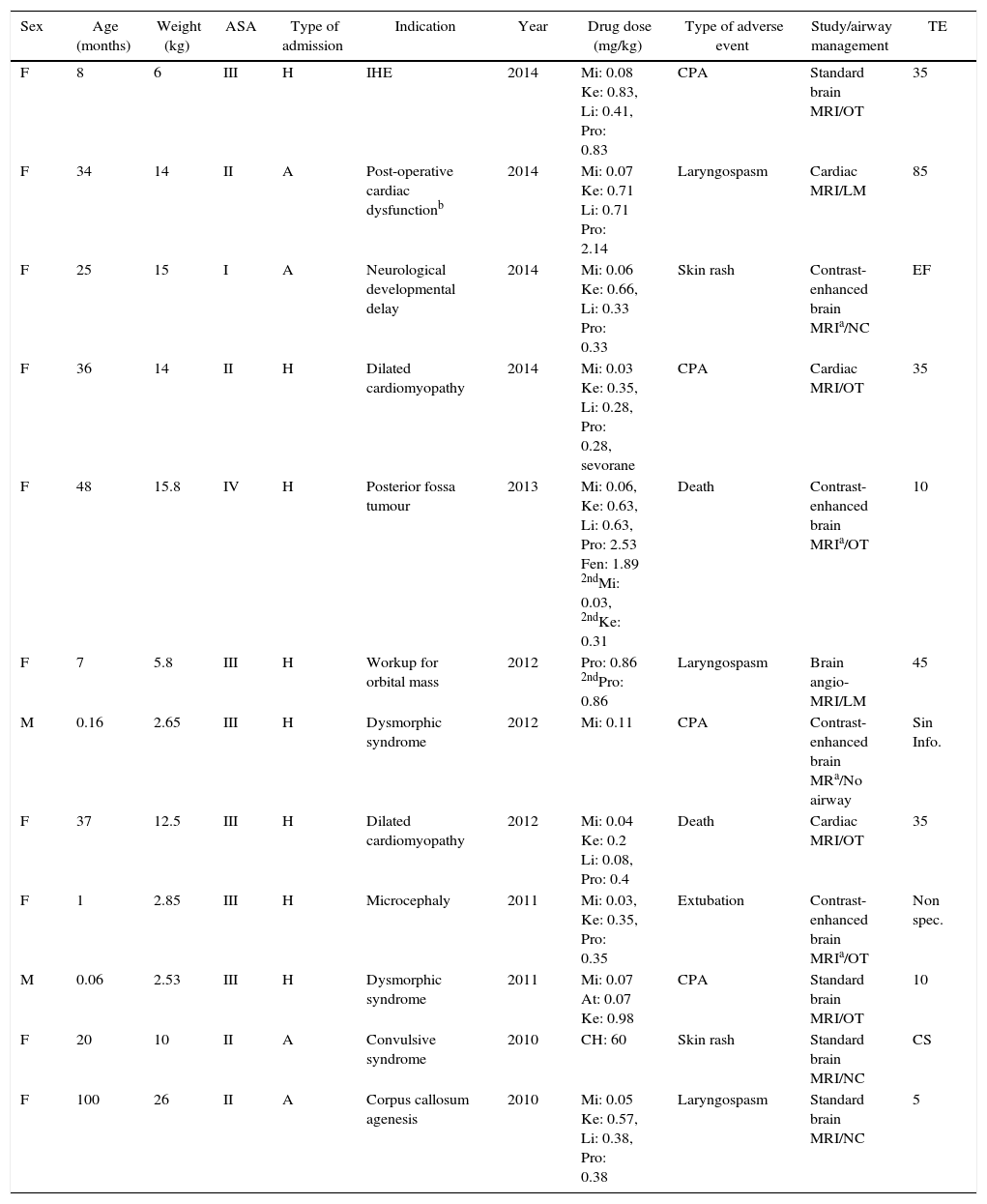
Adverse eventsThis study identified 12 adverse events, including 6 serious (0.12%), 4 moderate (0.08%) and 2 mild (0.04%) adverse events over a five-year period (Table 3).
Distribution of cases, patients and rates of adverse events in the paediatric population; IATM, 2010–2014.
| Year | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|
| Cases of adverse events | 2 | 2 | 3 | 1 | 4 |
| Paediatric patients | 740 | 795 | 1061 | 913 | 1277 |
| Rate of adverse events×1000 patients | 2.7 | 2.5 | 2.8 | 1.1 | 3.1 |
Source: Instituto de Alta Tecnología Médica – IATM, authors.
There were 6 serious cardiorespiratory arrest adverse events. The patients were given all basic cardiopulmonary resuscitation measures, with a successful outcome in 4 patients who recovered and two unsuccessful outcomes in patients who died. Serious adverse events were classified as ASA II (n=1), ASA III (n=4) and ASA IV (n=1).
The other four patients suffering from serious adverse events who recovered spontaneous circulation and breathing as a result of cardiopulmonary resuscitation had been previously classified as ASA II (n=1) and ASA III (n=3). Two of these cases were neonates with a diagnosis of dysmorphic syndrome, one of which occurred during brain MRI (8 months of age) due to hypoxic encephalopathy, and the last case was a 36-month old child during a study for dilated cardiomyopathy.
Regarding moderate adverse events, there were three cases of laryngospasm. The first event occurred during brain MRI in an 8-year old female patient classified as ASA II; the second case occurred during brain angio-MRI in a 7-month old patient classified as ASA III; and the third event occurred during cardiac MRI in a 2-year old patient. This latter moderate event was due to accidental extubation with no repercussions on the patient's basal status (it was the only event classified as preventable). See Table 4.
Adverse events of allergic origin and moderate-to-serious outcomes of non allergic origin.
| Sex | Age (months) | Weight (kg) | ASA | Type of admission | Indication | Year | Drug dose (mg/kg) | Type of adverse event | Study/airway management | TE |
|---|---|---|---|---|---|---|---|---|---|---|
| F | 8 | 6 | III | H | IHE | 2014 | Mi: 0.08 Ke: 0.83, Li: 0.41, Pro: 0.83 | CPA | Standard brain MRI/OT | 35 |
| F | 34 | 14 | II | A | Post-operative cardiac dysfunctionb | 2014 | Mi: 0.07 Ke: 0.71 Li: 0.71 Pro: 2.14 | Laryngospasm | Cardiac MRI/LM | 85 |
| F | 25 | 15 | I | A | Neurological developmental delay | 2014 | Mi: 0.06 Ke: 0.66, Li: 0.33 Pro: 0.33 | Skin rash | Contrast-enhanced brain MRIa/NC | EF |
| F | 36 | 14 | II | H | Dilated cardiomyopathy | 2014 | Mi: 0.03 Ke: 0.35, Li: 0.28, Pro: 0.28, sevorane | CPA | Cardiac MRI/OT | 35 |
| F | 48 | 15.8 | IV | H | Posterior fossa tumour | 2013 | Mi: 0.06, Ke: 0.63, Li: 0.63, Pro: 2.53 Fen: 1.89 2ndMi: 0.03, 2ndKe: 0.31 | Death | Contrast-enhanced brain MRIa/OT | 10 |
| F | 7 | 5.8 | III | H | Workup for orbital mass | 2012 | Pro: 0.86 2ndPro: 0.86 | Laryngospasm | Brain angio-MRI/LM | 45 |
| M | 0.16 | 2.65 | III | H | Dysmorphic syndrome | 2012 | Mi: 0.11 | CPA | Contrast-enhanced brain MRa/No airway | Sin Info. |
| F | 37 | 12.5 | III | H | Dilated cardiomyopathy | 2012 | Mi: 0.04 Ke: 0.2 Li: 0.08, Pro: 0.4 | Death | Cardiac MRI/OT | 35 |
| F | 1 | 2.85 | III | H | Microcephaly | 2011 | Mi: 0.03, Ke: 0.35, Pro: 0.35 | Extubation | Contrast-enhanced brain MRIa/OT | Non spec. |
| M | 0.06 | 2.53 | III | H | Dysmorphic syndrome | 2011 | Mi: 0.07 At: 0.07 Ke: 0.98 | CPA | Standard brain MRI/OT | 10 |
| F | 20 | 10 | II | A | Convulsive syndrome | 2010 | CH: 60 | Skin rash | Standard brain MRI/NC | CS |
| F | 100 | 26 | II | A | Corpus callosum agenesis | 2010 | Mi: 0.05 Ke: 0.57, Li: 0.38, Pro: 0.38 | Laryngospasm | Standard brain MRI/NC | 5 |
F: female; M: male; H: in-hospital; A: outpatient; IHE: ischaemic hypoxic encephalopathy; CS: completed scan. TE: time to event in minutes (time between the start of the anaesthetic intervention and the occurrence of the event). Non spec: non-specified; CPA: cardiopulmonary arrest; OT: orotracheal tube; LM: laryngeal mask; NC: nasal cannula (these patients were in moderate and deep sedation).22nd: second dose. Mi: midazolam. Ke: ketamine. Li: lidocaine. Pro: propofol. Fen: fentanyl. CH: chloral hydrate. At: atropine. No airway (the patient had a decompensation at the time of induction for intubation, and went on to recover spontaneous breathing).
There was evidence of two mild allergic events in the form of skin rash which did not require admission to hospital or scaling up of therapy. One of these occurred during a standard brain MRI with the use of chloral hydrate in a patient classified as ASA II; the second event occurred during contrast-enhanced brain MRI in an ASA I patient who received lidocaine, midazolam, ketamine and propofol. Both episodes took place after the patients were discharged from the institution and were reported by phone when the parents called to ask for instructions. On follow-up after 48h, the patients were reported to be asymptomatic and did not require in-hospital management.
Commonly used anaesthetics were give to the patients who experienced the adverse events, including lidocaine, midazolam, ketamine, propofol, fentanyl and chloral hydrate.
DiscussionMRI diagnostic yield depends to a large extent on the quality of the image obtained. For this reason, the patient is required to remain still for a long period of time, a condition that is not possible to achieve in the majority of paediatric patients. Hence the need to use medications at sedative or even anaesthetic doses, administered by an attending anaesthetist, given the risk entailed.
In Colombia, there are few studies describing the incidence of adverse events associated with high-complexity diagnostic tests as is the case of nuclear magnetic resonance. Delgado et al.2 conducted a review on the use of deep sedation by an anaesthetist in paediatric patients (<15 years) during 2009. The review included 113 patients and found an incidence of non serious adverse events of 4.4%. Internationally, the reported frequency of adverse events in the paediatric population ranges between 0.3 and 20.1% for diagnostic imaging services.11–18
Rangamani et al.15 reported an 8% incidence of adverse events in MRI. Of these, 0.69% were serious adverse events occurring in association with angio-MRI or cardiac MRI in patients under 120 days of age over a 10-year period. Dorfman et al.17 found 22 adverse events in 1334 cardiovascular MRI scans (1.6%), including 14 (63.5%) minor, 7 (32%) moderate and 1 (4.5%) serious. In turn, Kannikeswaran et al.14 reported an 11.9% incidence of adverse events in paediatric patients with impaired neurologic development, and of 7.9% in patients with no impaired neurological development. However, they excluded patients classified as ASA>III or who required general anaesthesia. In this study, the incidence of adverse events was 0.25%, without including mild, non-allergic events; most of the serious adverse events occurred in patients with a higher risk (ASA III and IV), consistent with what has been described in the literature by Metzner et al.18 and other authors15,19,20 in the sense that the frequency of serious complications is higher in patients with a higher risk level. In terms of adverse events in hospitalised paediatric patients, this study showed a proportion of 66%, a figure that is higher than the 32% reported by Dorfman et al.17 in hospitalised patients.
In terms of mortality, there were two deaths in this study. The first occurred in 2012 in a 37-month old female patient, ASA III, with underlying heart failure who went into cardiac arrest 35min into de anaesthetic procedure for cardiac MRI. The second case occurred in 2013 in a 48-month old female patient classified as ASA IV, with a posterior fossa tumour who was taken to contrast-enhanced brain MRI and went into cardiopulmonary arrest 10min into the start of anaesthesia. The two patients received all basic and advanced cardiopulmonary resuscitation measures under the direction of the anaesthesia specialist. It is difficult to find mortality information in studies on adverse events in the paediatric population seen in imaging services. One of the few studies reporting this fact is the one by Vitiello et al.20 which assessed 4952 patients and reported 7 deaths associated with cardiac catheterisation in critically ill paediatric patients.
Although some MRI studies require greater depth of anaesthesia and even short periods of apnea in order to minimise movement artefacts from diaphragmatic displacement, this research did not show a relationship between the complexity of the study and the occurrence of serious adverse events. These events occurred in 4 cases of brain MRI (requiring less depth of anaesthesia) and in 2 cases of cardiac MRI (more demanding in terms of anaesthesia). The two fatal cases occurred during different MRI studies (brain MRI and cardiac MRI).
Moderate adverse events in this study included 3 cases of laryngospasm (0.06%), in two of which a supraglottic device was used; the events happened during emergence from anaesthesia. In the third case, there was no airway device associated and the event occurred in a patient under light sedation. All the cases were solved with positive pressure ventilation given through the facial mask, and two of them required additional administration of propofol. No sequelae secondary to these outcomes were found in the study. Malviya et al.,16 in a study with 922 patients, found a 0.1% incidence of laryngospasm.
Unlike the report by Malviya et al.,21 with a reaction before discharge, this research found two allergic reactions after the end of care. On the other hand, in the study by Delgado et al.22 there are no reports of these types of reactions. These events were classified as mild, in the form of only a skin rash in both cases, and did not require hospital admission. The fist case was associated with chloral hydrate, an unpredictable medication with a long half life which is no longer widely used in our setting. The second event was associated with concomitant use of gadolinium, making it difficult to identify the culprit agent.
Schulte-Uentrop et al.23 reported general anaesthesia as the preferred technique for performing MRI in patients under 3 years of age or with major comorbidities who require some form of sedation. These factors were associated with a higher risk at the time of the diagnostic test. In our practice, a larger number of adverse events have been found only in patients with major comorbidities at the time of the scan, and no association was found with age (patients under 3 years of age); however, an adequate assessment requires a causality study which is outside the scope of this study.
In this study, the whole group of adverse events included 12 patients (10 females and 2 males) and the majority of adverse events occurred in females. As for serious adverse events, there were four in females and two in males. The study design does not allow to determine causal relationship but it is worth noting that the majority of adverse events occurred in females.
LimitationsThe data for this study came from databases. Considering that in mild non-allergic events there is evidence or underecording, these outcomes were not considered. Such is the case of occasional venous line leak identified at the time of the saline solution test but without drug administration, desaturation for short periods of time (<1m) or bradycardia that improved rapidly depending on the cause (<3min), without drug administration.
ConclusionMagnetic resonance imaging studies performed under sedation or anaesthesia given by the anaesthetist in paediatric patients under 15 years of age is a safe procedure with a risk as low as 0.25% of adverse events. However, as reported in the literature, hospitalised or decompensated patients require an in-depth risk-benefit assessment and consideration of the best option for each individual patient.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingWith their own resources the Instituto de Alta Tecnología Médica (IATM) financed the creation of the database and the time dedicated by researchers. No external resources were received.
Conflicts of interestThe authors declare not having any conflicts of interest.
Please cite this article as: Largo-Pineda CE, Arenas-Correa ID, Ángel-González GJ, Vélez-Arango JM, Calvo-Betancur VD, Arango-Zapata AN. Eventos adversos en pacientes pediátricos sometidos a resonancia magnética bajo sedación o anestesia. Rev Colomb Anestesiol. 2017;45:8–14.