This case describes the anesthetic technique used in two symptomatic neonates with a prenatal diagnosis of cystic adenomatoid malformation that underwent surgery under general anesthesia at the San Ignacio University Hospital. One was under one-lung ventilation and ultrasound-guided caudal catheter analgesia and the second one with general anesthesia and two-lung ventilation.
A cystic adenomatoid malformation is a rare pathology with improved outcomes with early diagnosis and management, and a survival rate of over 95%.
Se describe la técnica anestésica en dos neonatos con diagnóstico prenatal de malformación adenomatoide quística sintomáticos, llevados a cirugía de resección pulmonar en el Hospital Universitario San Ignacio bajo anestesia general uno de los dos con ventilación unipulmonar y analgesia con catéter caudal guiada por ecografía y el otro con anestesia general y ventilación bipulmonar.
La malformación adenomatoide quística es una patología poco frecuente que mejora su desenlace con el diagnóstico y manejo temprano con una sobrevida mayor al 95%.
Cystic adenomatoid malformations are a rare condition with an incidence of 1 in 10,000 to 1 in 35,000 pregnancies.1 It is a congenital lesion resulting from the proliferation of the terminal bronchioles preventing the normal development of the alveoli. There is no predilection for gender, race, or laterality and 100% of the cases are diagnosed with echography at 20 weeks. Based on the size of the cysts, the most widely used classification in neonates is Adzick's with type 1 or the macrocystic classification being the most prevalent (58%), versus type 2 or microcystic.2 The pre-surgical diagnosis shall be done with CT with a sensitivity of 100%.3
The purpose of this report is to educate about the anesthetic management at the San Ignacio Hospital for patients with a rare condition.
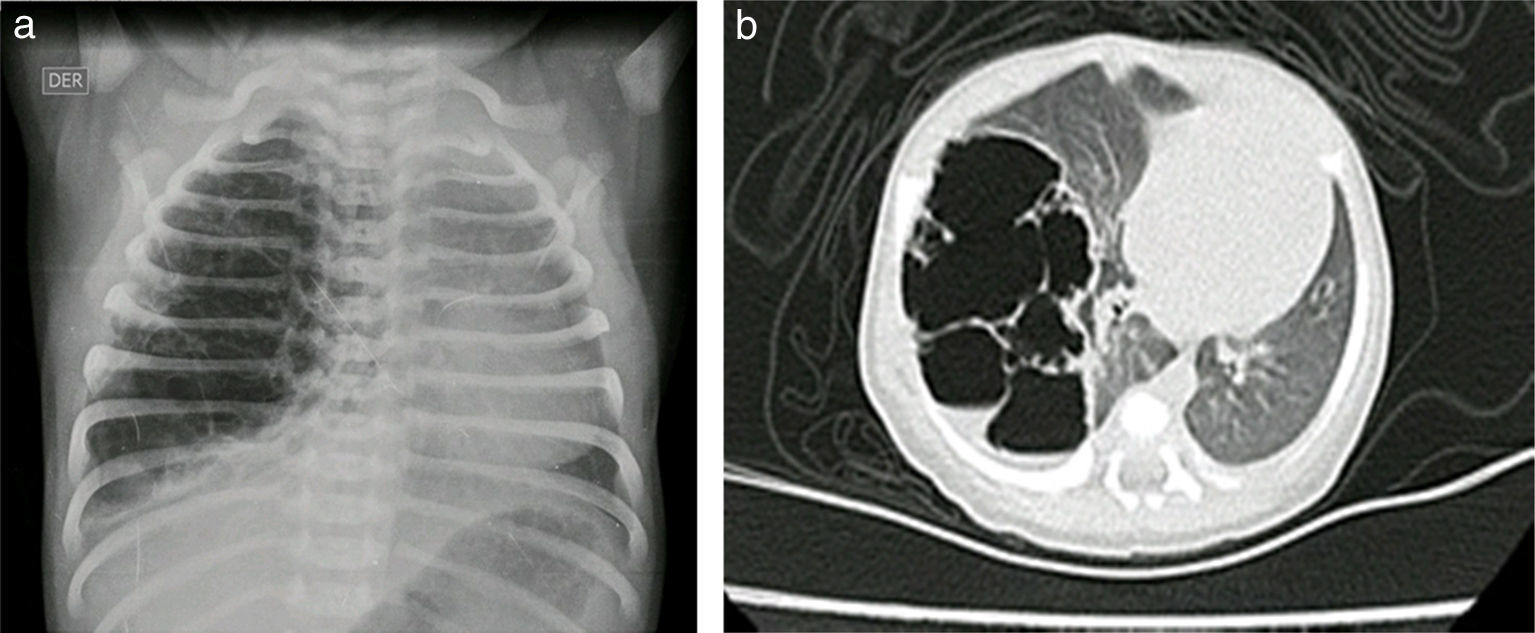
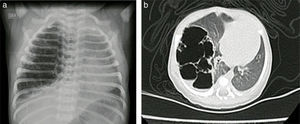
Case reportsCase no. 143-week post-conception age male patient with prenatal diagnosis of a right cystic adenomatoid malformation. At birth, the oxygen saturation ranged between 75 and 85%, requiring neonatal ICU admission. The birth weight was 3.2kg. The pre-surgical paraclinical tests are described in Table 1. The ultrasound scan showed a patent foramen ovale, good biventricular function, mild pulmonary hypertension PSAP 35mmHg. A CT scan is requested prior to the surgical procedure in order to characterize the lesion (Fig. 1a and b).
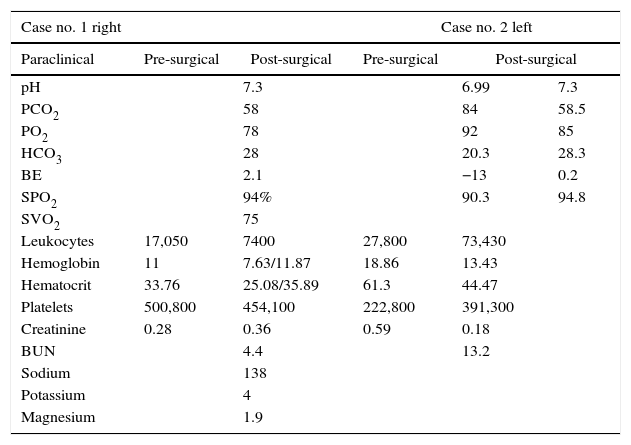
Paraclinical and post-surgical tests.
| Case no. 1 right | Case no. 2 left | ||||
|---|---|---|---|---|---|
| Paraclinical | Pre-surgical | Post-surgical | Pre-surgical | Post-surgical | |
| pH | 7.3 | 6.99 | 7.3 | ||
| PCO2 | 58 | 84 | 58.5 | ||
| PO2 | 78 | 92 | 85 | ||
| HCO3 | 28 | 20.3 | 28.3 | ||
| BE | 2.1 | −13 | 0.2 | ||
| SPO2 | 94% | 90.3 | 94.8 | ||
| SVO2 | 75 | ||||
| Leukocytes | 17,050 | 7400 | 27,800 | 73,430 | |
| Hemoglobin | 11 | 7.63/11.87 | 18.86 | 13.43 | |
| Hematocrit | 33.76 | 25.08/35.89 | 61.3 | 44.47 | |
| Platelets | 500,800 | 454,100 | 222,800 | 391,300 | |
| Creatinine | 0.28 | 0.36 | 0.59 | 0.18 | |
| BUN | 4.4 | 13.2 | |||
| Sodium | 138 | ||||
| Potassium | 4 | ||||
| Magnesium | 1.9 | ||||
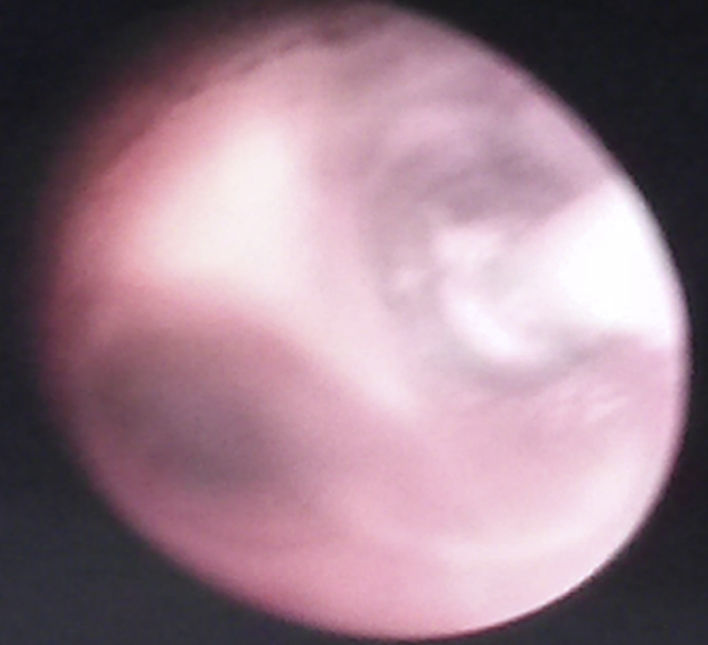
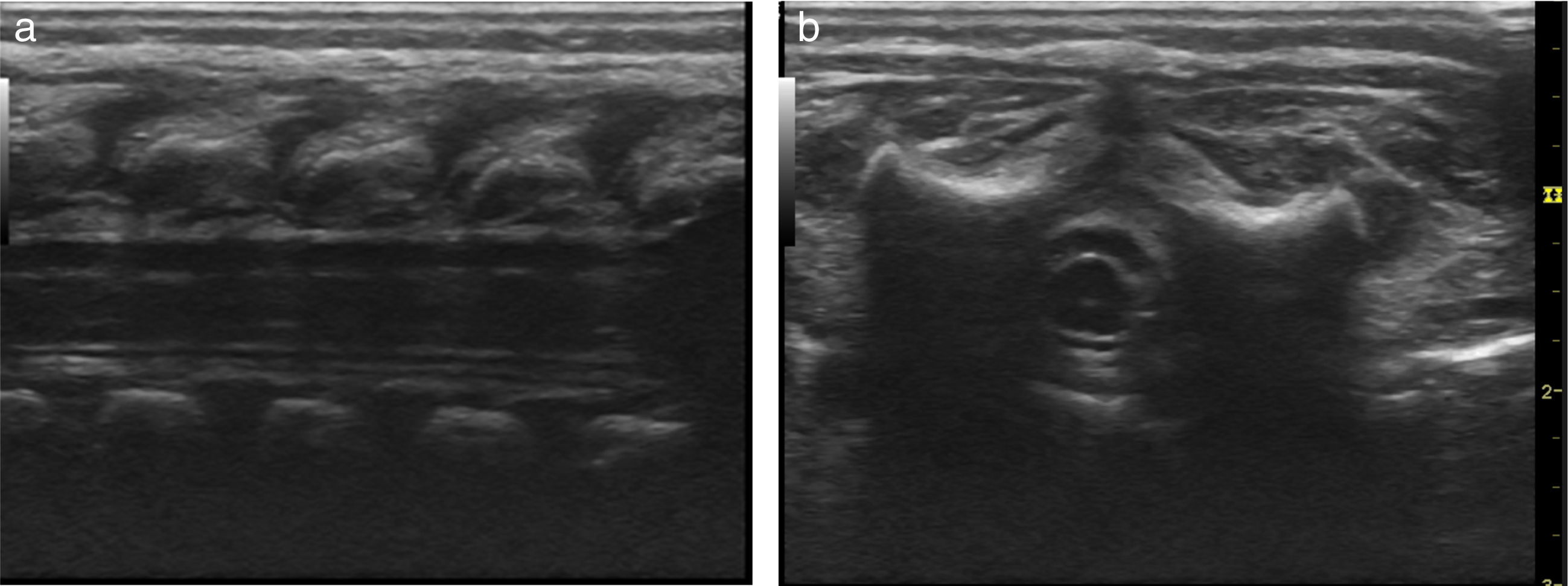
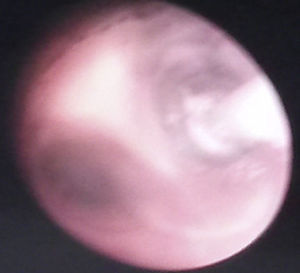
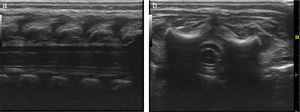
Basic monitoring was performed, pre oxygenation with facemask and intravenous induction with fentanyl 3mcg/kg, lidocaine 1mg/kg, propofol 1mg/kg, and for maintaining spontaneous ventilation a rigid bronchospy was performed for inserting a Fogarty catheter 3 French in the right source bronchus for one-lung ventilation (Fig. 2); then orotracheal intubation with a 3.5 fixed tube at 9cm from the labial commissure was performed uneventfully, invasive monitoring with right radial arterial line and right internal jugular central venous catheter monitoring. For the management of analgesia, a caudal puncture with a pediatric Touhy N 20 needle was performed, and an epidural catheter was advanced up to T6. This space had been previously marked under ultrasound and high frequency transducer. Bupibucaine 0.25% was injected through the catheter in a volume of 1.5cc3 visualizing the spread of the local anesthetic through the epidural space at this level (Fig. 3a and b).
Anesthetic management is continued with sevorane at 2 vol % and with the patient in left lateral decubitus with right lung isolation, a 10cm diameter resection of the right upper lobe malformation was performed. The patient remained hemodynamically stable throughout the procedure, with saturations between 94% and 96%, total bleeding was 10cc3, and adequate acid base balance in arterial gases monitoring (Table 1) enabling for the patient's extubation at the end of the procedure and post-operative pain management with thoracic caudal catheter infusion for the next 48h. This provided adequate respiratory mechanics and early postoperative recovery with hemodynamic stability and no oxygen therapy requirements.
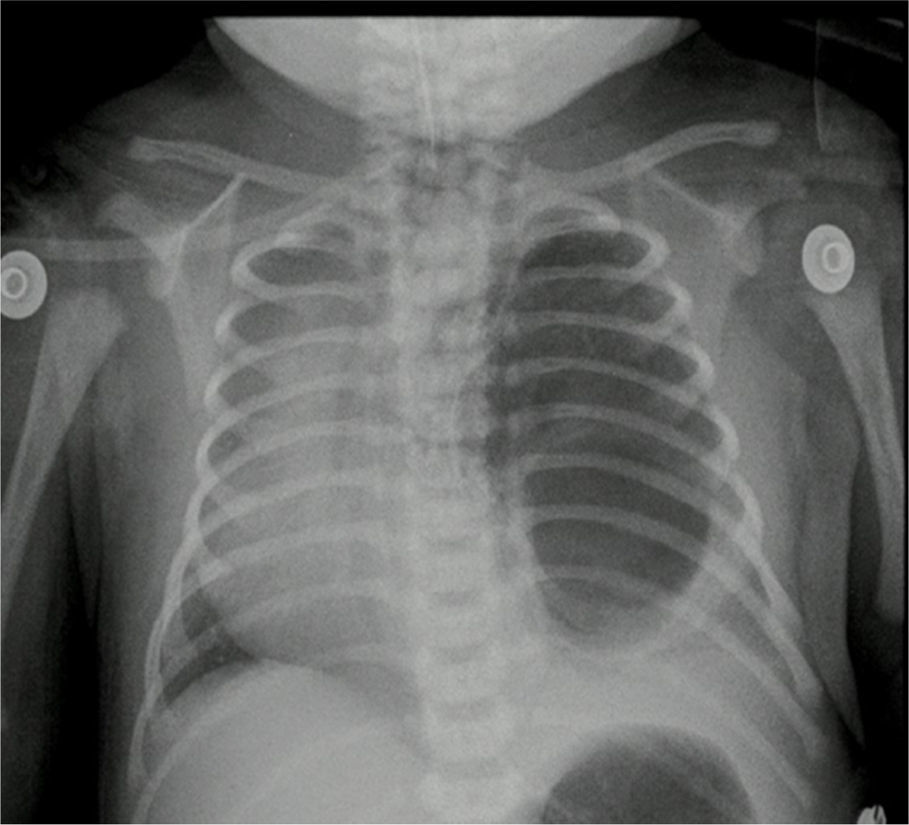
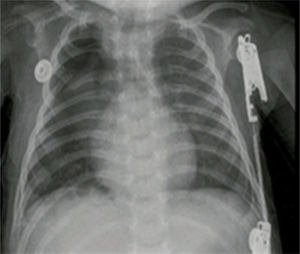
The pathology report of the right upper lobe indicated a Type II cystic adenomatoid malformation and the X-ray control showed adequate pulmonary expansion (Fig. 4).
Case no. 2Female newborn of 38.5 weeks of post-conception age, with antenatal diagnosis of Type I left cystic adenomatoid malformation and a birth weight of 2.875g. The pre-surgical echocardiography findings indicated a 3.8mm patent ductus without hemodynamic disruptions, patent foramen ovale, and good biventricular function. The pre-surgical paraclinical tests are shown in Table 1. A chest X-ray and CT scan were performed prior to surgery (Fig. 5).
Basic monitoring was performed with prior oxygenation, intravenous induction with fentanyl 3mcg/kg, lidocaine 1mg/kg, propofol 3mg/kg and cisatracurium 0.1mg/kg; then an orotracheal intubation with a 3 tube was performed without pneumoplug, fixed at 10cm from the labial commissure, selective for the right lung and verified through pulmonary auscultation. The left radial artery was catheterized and the patient had an epicutaneous catheter in position. During the intraoperative of the lower lobectomy and the lingula, the patient experienced ventilation difficulties and required manual ventilation for pressure control of the airway, with episodes of desaturation of up to 84% during the resection and the right lateral decubitus position; Arterial gases control with important respiratory acidosis with CO2 in 55 that prevented the extubation. The intraoperative bleeding was 15ml.
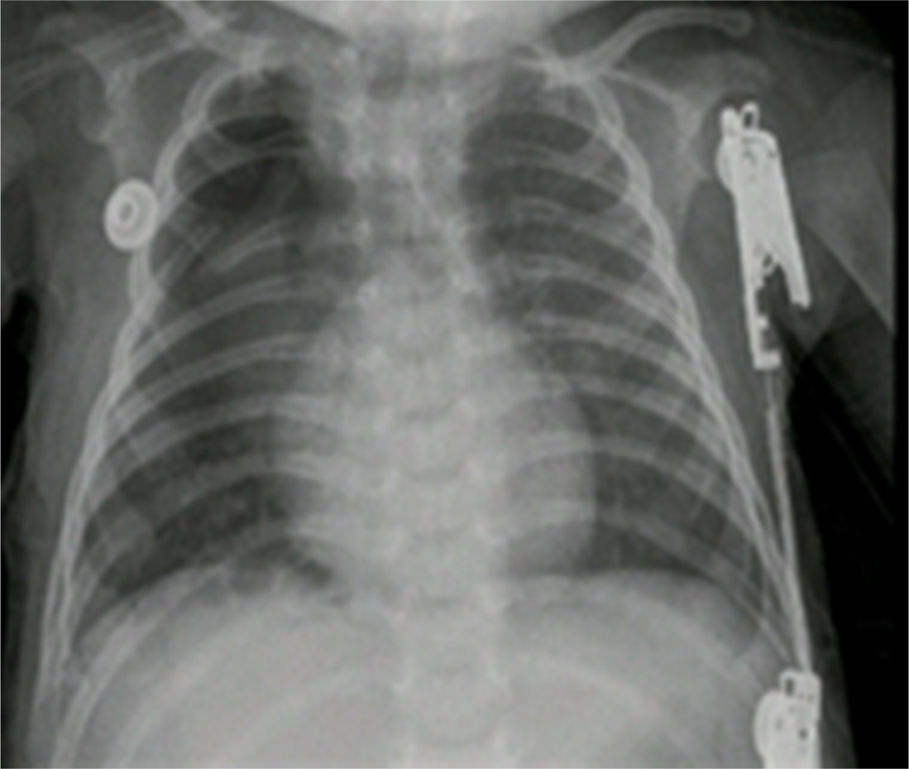
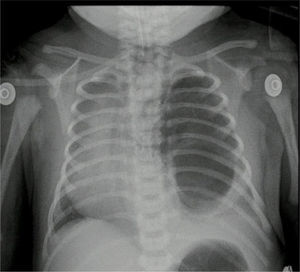
The pathology report indicated a left lung lower lobule cystic lesion and intralobal pulmonary sequestration. The post-operative control X-ray showed adequate thoracic expansion.
DiscussionThe adenomatoid malformation was described for the first time in 1949 by Chi¿n Tang and is a low prevalence pathology of unknown etiology. This is a hamartoma-like lesion containing tissue from different lung sources with abnormal development of the alveoli, generating cysts of various sizes mostly unilateral: 80–95%. These cysts develop in the embryonic phase during the pseudoglandular and saccular period from week 7 through 17 of gestation. From the pathophysiological point of view, adenomatoid malformations are thought to be secondary to anomalous tissue proliferation, airway obstruction and dysplasia and metaplasia of the normal tissues.4
There are two classifications: Stocker separates malformations into five types, based on the lesion's histology and Adzick, based on the size of the cysts found in the lung. The prenatal ultrasound diagnoses over 80% of the lesions, so that in accordance with the head circumference it is possible to calculate the volume of the lesion that is directly correlated to the survival and risk of complications, such as marked mediastinal deviation, fetal hydrops, ascitis and heart failure. In some cases, antenatal surgery is performed without significant evidence supporting this management.5 Late diagnosis is occasionally done based on repeated pulmonary infections, bronchiectasis, pulmonary abscesses, hemoptysis, pioneumothorax, management resistant asthma or malignant transformation.5
The surgical procedure shall be performed prior to the occurrence of symptoms including respiratory distress, tachypnea, retractions and cyanosis and at an early stage for improved prognosis; this may result in compensatory growth of the lung, reduced risk of spontaneous pneumothorax, infection and malignant transformation.6,7 Additionally, it may help in sparing healthy adjacent parenchyma and compensates for the potential secondary mediastinal deviation.
The risk of surgical complications ranges from 6 to 9%, mainly secondary to air leaks, atelectasis, pleural effusion, pneumothorax, and bronchopleural fistulae. Mortality is practically zero and after the procedure the extubation is facilitated with regional/epidural analgesia.8
Some series describe the use of video-thoracoscopy resulting in a longer surgical time, with shorter hospital stay, better cosmetic outcomes and less postoperative pain.9–11
The anesthetic challenge in these patients is to maintain hemodynamic stability and adequate saturation during neonatal ventilation in lateral decubitus with open chest. In the previously described cases we discussed two anesthetic techniques with clearly different postoperative results. The use of one-lung ventilation in the first patient reduced bleeding, facilitated the resection of the malformation and maintained the ventilation/perfusion ratio reducing the Shunt in that lung, achieving adequate saturation and CO2 management. In contrast with the second patient in whom the isolation with selective orotracheal tube clinically verified trough auscultation failed to ensure adequate resection of the malformation and resulted in major intraoperative bleeding, permanence of the Shunt and difficult ventilation due to increased airway pressures with considerable CO2 increase, making extubation impossible at the end of the procedure. On the other hand, the combined anesthetic technique in the first case managed to reduce the use of the inhaled anesthetic to a small dose of local anesthetic, without causing any hemodynamic changes and facilitating the extubation and the postoperative management of analgesia through a continuous infusion of local anesthetic via the thoracic epidural catheter.
Ethics committeeThe report of cases was submitted to the medical ethics committee of the San Ignacio University Hospital and endorsed for publication.
Ethical disclosuresProtection of human and animal subjectsThe authors state that for this investigation, no experiments have been performed on humans or animals.
Confidentiality of dataThe authors state that they have followed the protocols of its work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document works in the power of the correspondence author.
Source of financingNone.
Conflict of interestThe authors have no conflicts of interest to disclose.
Please cite this article as: Fajardo-Escolar AP, Díaz-Bohada L. Manejo anestésico en dos neonatos con malformación adenomatoide quística. Reporte de caso. Rev Colomb Anestesiol. 2017;45:76–80.