Cough at the time of extubation may trigger anaesthesia-related adverse events. A technique that has been found to limit cough during this stage of the anaesthesia procedure is the use of remifentanil.
ObjectiveTo compare cough frequency and intensity at the time of extubation with two different plasma concentrations of remifentanil, 3–4ng/ml and 2–3ng/ml, using target controlled anaesthesia.
Materials and methodsRandomised controlled clinical trial carried out at the Institute for Blind and Deaf Children in Valle del Cauca, in patients taken to elective ear surgery. Patients were randomly assigned to one of two groups. The first group (T) received an infusion of remifentanil at a plasma concentration of 3–4ng/ml (n=50). The second group (U) received an infusion of remifentanil at a plasma concentration of 2–3ng/ml (n=51). Data were analysed using the Student t test and the non-parametric Mann Whitney U test; the Chi square test was used for determining associations.
ResultsCough intensity and frequency were less in group T compared to group U (OR: 3.73; 95% CI: 1.3–10.7), and there was no difference between the two groups regarding emergence from anaesthesia.
ConclusionsThe presence of at least one cough episode during extubation is less with plasma concentrations of remifentanil of 3–4ng/ml than 2–3ng/ml.
La presencia de tos al momento de la extubación puede desencadenar eventos adversos asociados a la anestesia. Una técnica reportada para disminuir la tos durante esta parte del acto anestésico es la extubación con remifentanilo.
ObjetivoComparar la frecuencia y la intensidad de la tos en el momento de la extubación con dos concentraciones plasmáticas de remifentanilo de 3-4 y de 2-3ng/ml, a través de la técnica anestésica con objetivo controlado.
Materiales y métodosSe realizó un ensayo clínico controlado aleatorizado en el Instituto para Niños Ciegos y Sordos del Valle del Cauca, en pacientes sometidos a cirugía programada de oído. Los pacientes fueron divididos aleatoriamente en dos grupos. El primer grupo (T) recibió una infusión de remifentanilo con una concentración plasmática entre 3-4ng/ml (n=50). El segundo grupo (U) recibió una infusión de remifentanilo con una concentración plasmática entre 2-3ng/ml (n=51). Los datos se analizaron mediante la prueba estadística de la t de Student y la prueba no paramétrica de la U de Mann Whitney; para establecer asociaciones se realizó la prueba Chi-cuadrado.
ResultadosLa intensidad y la frecuencia de la tos fue menor en el grupo T que en el grupo U (OR: 3.73; IC 95%: 1.3–10.7); el tiempo de despertar no mostró diferencia entre ambos grupos.
ConclusionesLa presencia de al menos un episodio de tos durante la extubación es menor cuando se alcanzan concentraciones plasmáticas de remifentanilo entre 3-4ng/ml que entre 2-3ng/ml.
Extubation takes place at a time in which the plasma concentration (PC) of the anaesthetic agents is reaching zero and the patient is still subject to a mechanical painful stimuli such as tracheal intubation that elicit coughing and haemodynamic changes including hypertension, tachycardia, increased pressure (intra-abdominal, intraocular, intracranial pressure), myocardial ischaemia and arrhythmias. Opioid doses have been used to suppress the cough stimulus at the time of extubation (CTE); however, published studies report a high incidence of cough. With remifentanil at a PC of 1.5ng/ml, 31% of patients presented CTE when sevoflurane was administered. These studies are not clear regarding the concentration of the hypnotic agent at the time of extubation. This could explain why, in different studies, different remifentanil PC have the same results in terms of cough frequency. Unfortunately, prior designs have not considered the concept of no-response probability (NRP) based on target controlled anaesthesia (TACAN), which consists of keeping in mind the synergistic interactions between these drugs.
To avoid this bias, synergistic interactions must be considered, and this requires adjusting the opioid dose according to age and making sure that there is no effect of the hypnotic agent during extubation. In this study, infusions were adjusted according to age in order to achieve a PC of remifentanil, and care was taken to ensure a final concentration of the halogenated drug under 0.1 ET.
The objective of this study was to compare cough frequency and intensity at the time of extubation with two infusions of remifentail that predict a plasma concentration between 3–4 and 2–3ng/ml, using target controlled anaesthesia.
Materials and methodsRandomised double-blind clinical trial carried out in a Clinic in Cali in patients taken to ear surgery after obtaining the approval of the ethics committee and patient informed consents. Patients were randomly assigned to two groups: the intervention group received an infusion of remifentanil to predict a PC between 3 and 4ng/ml, and the control group received a remifentanil infusion to predict a PC between 2 and 3ng/ml at the time of extubation.
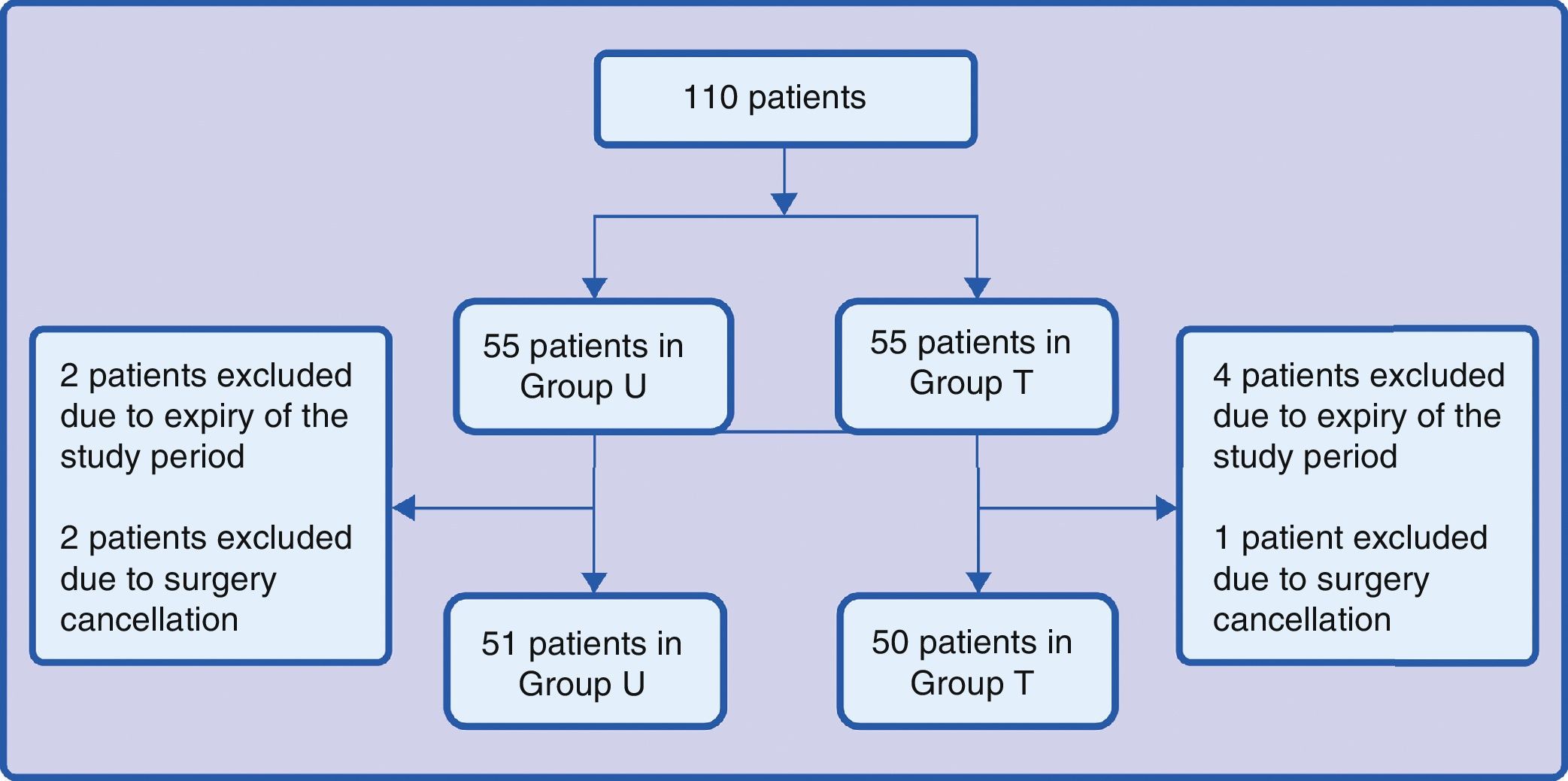
Based on preliminary studies in which the cough frequency with a PC of 1.5ng/ml of remifentanil was 30%, the one-tail sample size to reduce cough frequency from 30% to 10% with an alpha error of 0.05 and a power of 0.8 is 46 patients in each group.1,2 With an estimated loss of 20%, the total is 55 patients in each group.
Considering the need to manage two groups – remifentanil concentration of 2–3ng/ml (group U) and remifentanil concentration of 3–4ng/ml (group T), the epidemiology department of Hospital Departamental del Valle Evaristo García carried out a randomisation using balanced blocks of six in order to ensure 55 patients in each group. The randomisation was maintained in non-see-through envelopes together with the data collection forms that were numbered from 1 to 110, and the order was followed by the anaesthetist on call.
Management was blinded for the patient, the observers and the researchers in order to control for patient evaluation bias.
To ensure blinding of the observer (treating anaesthetist), a nurse was trained in the adjustment of the infusion pump. Ten minutes before the end of the procedure and upon request by the anaesthetist, the nurse proceeded to place the pump in such a way that the anaesthetist was unable to see the infusion setting and then opened the envelope and adjusted the infusion in accordance with the (U) or (T) randomisation. Without knowing the emergence infusion, the anaesthetist had to assess cough, intensity and the Ramsay score.
Eligibility criteriaInclusion criteriaPatients that were entered in the study were those between 18 and 70 years of age, classified as ASA I–II.
Exclusion criteriaPatients with lung disease (asthma, COPD), with a body mass index higher than 35, smokers and patients with a respiratory picture within three weeks prior to the procedure were excluded.
Primary and secondary end pointsThe variables analysed included age, sex, body mass index, surgical procedure, length of the surgery, duration of the anaesthesia, mean arterial pressure, heart rate, and train of four (TOF) at the end of the surgery.
Primary end points measured were cough intensity and frequency at the time of extubation, defined as follows:
Grade 0=no cough
Grade 1=mild (a single cough episode)
Grade 2=moderate (more than one cough episode lasting less than 5s)
Grade 3=severe (more than one cough episode lasting more than 5s or unwanted limb movements)
Secondary end points measured were the Ramsay score and emergence time defined as the time the patient took to open the eyes after having stopped the infusion of the halogenated drug.
Description of the procedureOnce the informed consent was signed, patient weight and size were determined, a 20 gauge Jelco catheter was put in place, vital signs were taken before induction and the monitor was set for taking vital signs every three minutes. The induction technique was applied using TACAN, setting a PNR of 95% (remifentanil PC of 7–8ng/ml and propofol PC of 3–4mcg/ml) for patient intubation.
To avoid adverse events, the concentration of remifentanil in saline solution was 8 mcg/ml and the line for this medication was placed proximal in the vein, using a three-way lock. Rocuronium was used as a muscle relaxant at an ED95 two minutes before patient intubation. The volume control setting was used in the ventilator in order to maintain an end-tidal CO2 concentration (ETCO2) of 30mmHg. After intubation, maintenance anaesthesia was achieved with remifentanil to predict a PC of 4–5ng/ml (50% PNR for soft tissue surgery), reaching “steady state” in 10min. Rugloop simulations were made for all the infusions in order to ensure that the final concentration was the same as predicted by the Tafur nomogram. The sevofluorane vaporiser was opened and kept at a maximum flow of 0.8L/min of oxygen, maintaining the necessary end-tidal V% to reach a 0.5 MAC according to age, with the help of the Lerou nomogram.
Ten (10) minutes before the end of the surgery, a nurse who had not participated in the anaesthesia procedure adjusted the remifentanil infusion pump to reach a PC of 2–3ng/ml or 3–4ng/ml by age group, as follows:
20 years: group U 6.0mcg/kg/h–group T 9.0mcg/kg/h
30 years: group U 5.7mcg/kg/h–group T 8.5mcg/kg/h
40 years: group U 5.3mcg/kg/h–group T 8.0mcg/kg/h
50 years: group U 5.0mcg/kg/h–group T 7.5mcg/kg/h
60 years: group U 4.6mcg/kg/h–group T 7.0mcg/kg/h
70 years: group U 4.3mcg/kg/h–group T 6.5mcg/kg/h
80 years: group U 4.0mcg/kg/h–group T 6.0mcg/kg/h
The nurse placed the infusion pump in such a way that it could not be seen by the treating anaesthetist and proceeded to open the respective envelope according to the consecutive numbering. She then adjusted the dose of remifentanil in accordance with the randomisation: letter U, 2–3ng/ml, letter T, 3–4ng/ml.
Upon completion of closure and within less than 5min of the change in the remifentanil infusion, the sevoflurane vaporiser was closed and fresh gas flow was set at 4L/min. Neuromuscular relaxation was monitored using the train of four and the patient was reverted if the TOF was less than 90%.
After 10min of the change in the remifentanil infusion and when the gas analyser showed ET 0.1V%, the patient's name was called every minute asking him/her to open the eyes or the mouth, and this was defined as the emergence time. Once the patient reacted to the call and to avoid the tracheal stimulus caused by the sudden decompression of the endotracheal tube balloon (ETB), the balloon retention connection was closed, allowing for passive deflation. The patient was extubated once a response to the command was obtained. The blinded observer (the attending anaesthetist) recorded the time elapsed between the moment the flow of the halogenated drug was shut off and the moment the patient opened the eyes and mouth, as well as cough frequency and intensity, Ramsay sedation score at the time of extubation, and vital signs such as heart rate and mean arterial pressure.
The infusion pump was turned off upon patient extubation. During transfer to the post-anaesthesia care unit (PACU), care was taken to ensure that the patient was ventilated. The patient was entrusted to the care of the PACU nurse only after spontaneous breathing, a saturation of more than 96% and a Ramsay score of 2 were achieved.
Statistical analysisQuantitative variables were summarised using central trend and scatter measurements. Absolute and relative frequencies were calculated for the qualitative variables. The Shapiro Wilk test was used to assess normality of variables in order to make the comparison between the groups. The Student t test was used for normality comparison, and the Mann Whitney non parametric U test was used for non-normality comparison. The Chi square test was used to study associations in the contingency tables. A level of statistical significance of α=0.05 was established in advance. The statistical analysis was performed using the Stata 11.1 software package. Overall, 101 patients were analysed: 51 in group U and 50 in group T.
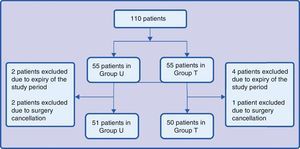
ResultsOverall, 101 patients were enrolled during the research period: 51 for group U and 50 for group T. Group distributions are illustrated in Fig. 1. Two patients in group U were excluded because of the end of the study period and 2 because of surgery cancellation when they were already in the operating room. Four patients in group T were excluded because of the end of the study period and 1 because of cancellation of the surgery in the operating room.
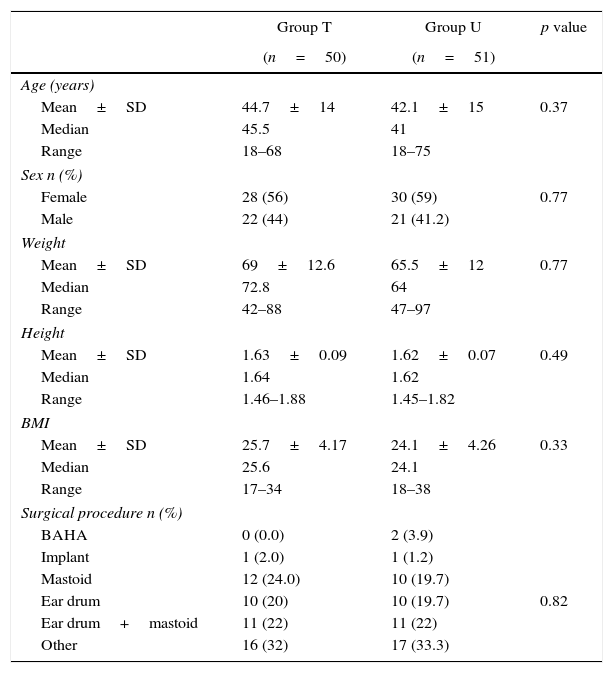
Table 1 describes the social and demographic characteristics of the individuals assigned to each group.
General characteristics.
| Group T | Group U | p value | |
|---|---|---|---|
| (n=50) | (n=51) | ||
| Age (years) | |||
| Mean±SD | 44.7±14 | 42.1±15 | 0.37 |
| Median | 45.5 | 41 | |
| Range | 18–68 | 18–75 | |
| Sex n (%) | |||
| Female | 28 (56) | 30 (59) | 0.77 |
| Male | 22 (44) | 21 (41.2) | |
| Weight | |||
| Mean±SD | 69±12.6 | 65.5±12 | 0.77 |
| Median | 72.8 | 64 | |
| Range | 42–88 | 47–97 | |
| Height | |||
| Mean±SD | 1.63±0.09 | 1.62±0.07 | 0.49 |
| Median | 1.64 | 1.62 | |
| Range | 1.46–1.88 | 1.45–1.82 | |
| BMI | |||
| Mean±SD | 25.7±4.17 | 24.1±4.26 | 0.33 |
| Median | 25.6 | 24.1 | |
| Range | 17–34 | 18–38 | |
| Surgical procedure n (%) | |||
| BAHA | 0 (0.0) | 2 (3.9) | |
| Implant | 1 (2.0) | 1 (1.2) | |
| Mastoid | 12 (24.0) | 10 (19.7) | |
| Ear drum | 10 (20) | 10 (19.7) | 0.82 |
| Ear drum+mastoid | 11 (22) | 11 (22) | |
| Other | 16 (32) | 17 (33.3) | |
Source: Authors.
TACAN required remifentanil infusions at the time of extubation to achieve an estimated PC of 2–3ng/ml and 3–4ng/ml and the halogenated agent to be at ET0.1. The Tafur nomogram was used for determining the remifentanil concentrations. In order to determine that the infusions set using the nomogram actually predicted the required concentrations, PC was estimated using the Rugloop simulation based on patient demographic data and the infusions recorded in the data collection form.
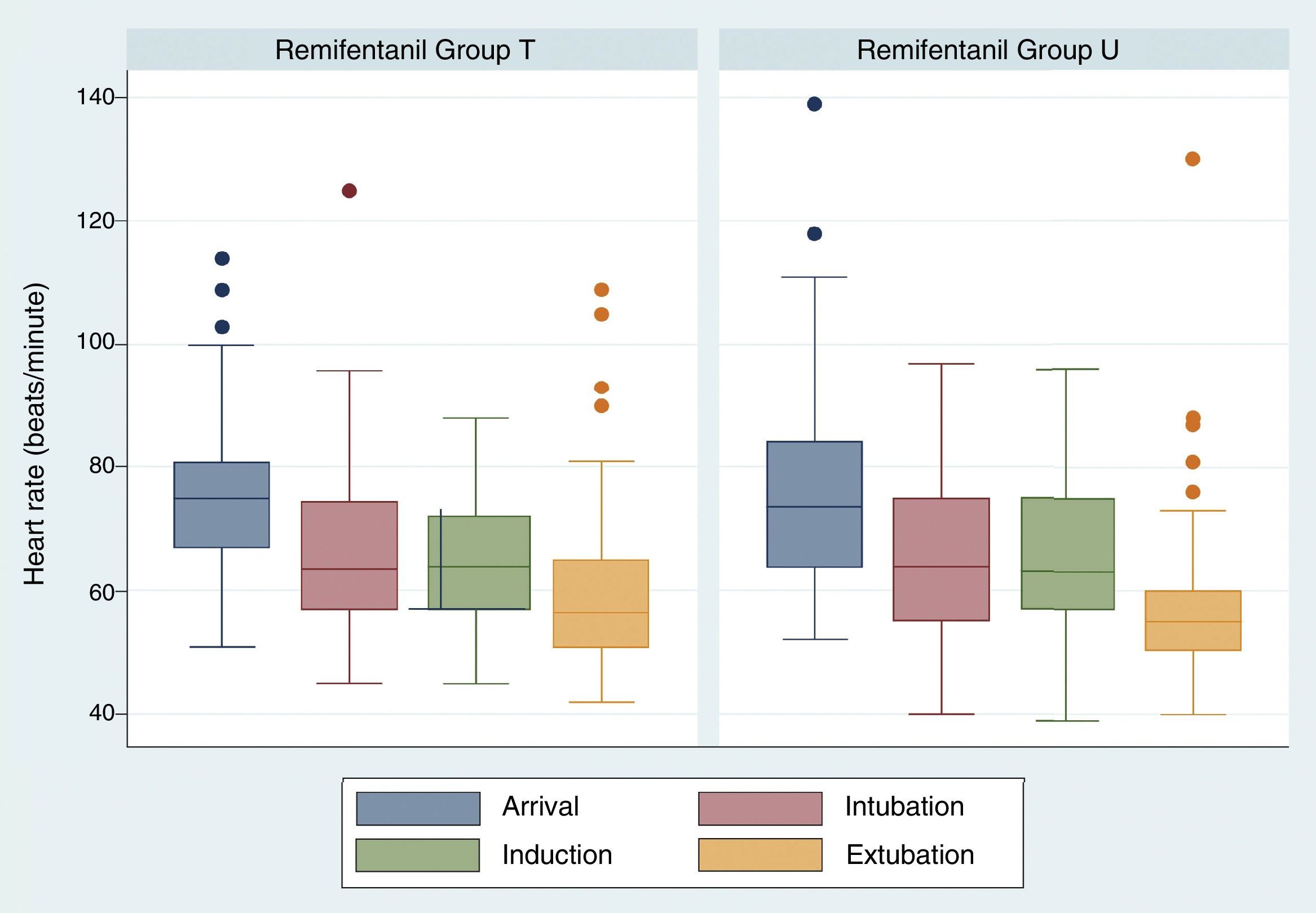
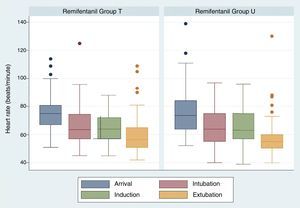
There were no significant differences regarding heart rate values over time. Heart rate at the time of extubation was 60.26+/−14.47 in group U and 58.25+/−14.70 for group T, p=0.3514 (Fig. 2).
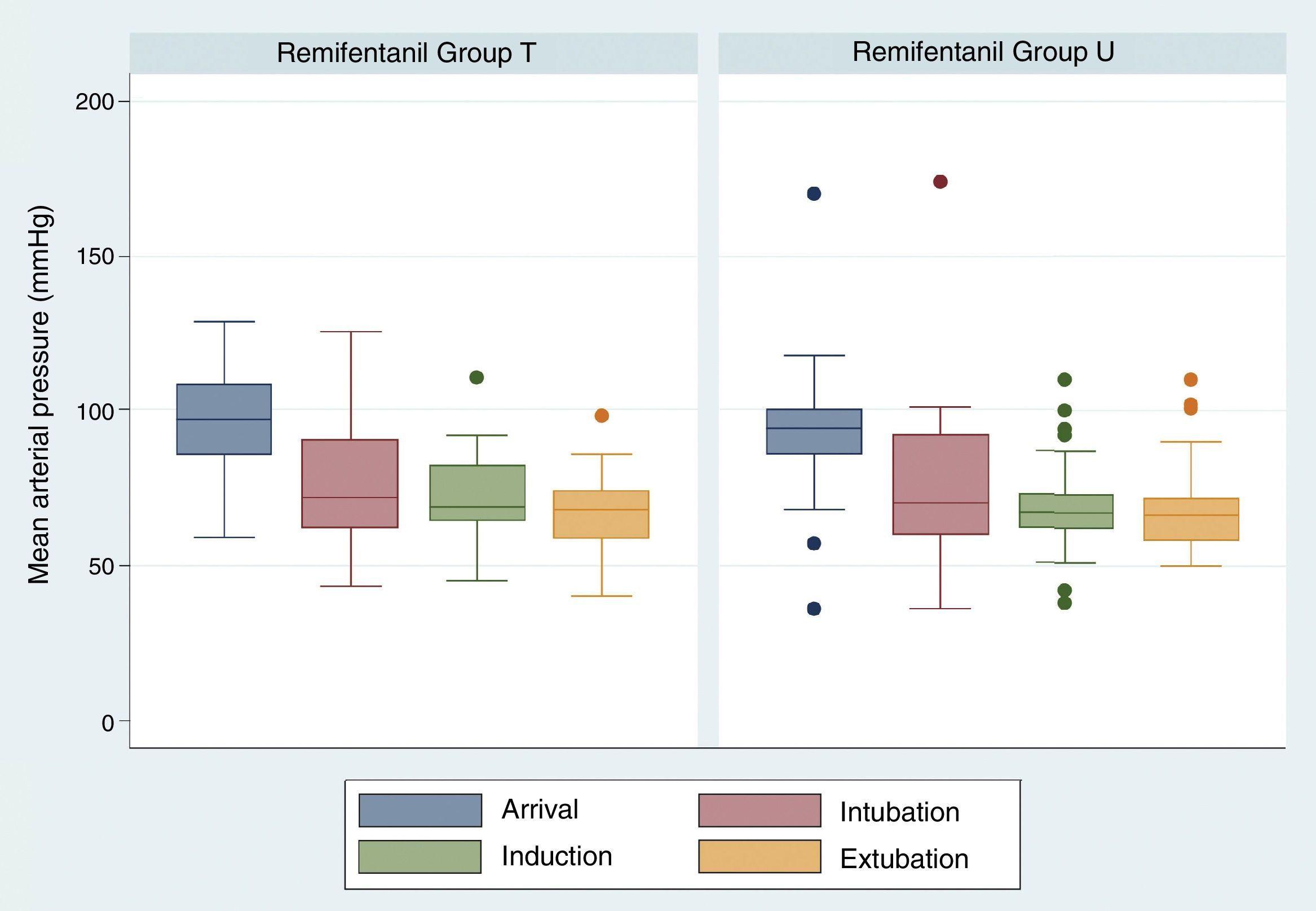
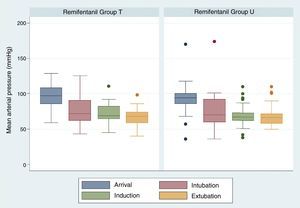
The comparison of mean arterial pressure in the two study groups did not show differences on arrival, after induction and during extubation. Mean arterial pressure at the time of extubation was 67.47±11.29 in group T and 67.67±13.61 in group U, p=0.3811 (Fig. 3). There were no respiratory complications associated with the intervention.
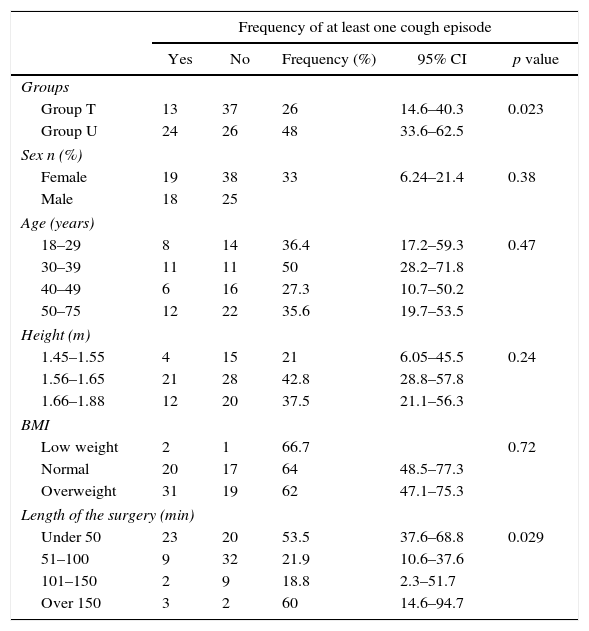
Table 2 shows significant differences between the study groups in terms of the frequency of at least one cough episode. In group U, frequency was 48% versus 26% in group T, RR 1.84, 95% CI (1.08–3.13). Despite a high frequency, the percentage of patients with moderate-to-severe cough was very low and, consequently, there is no statistically significant difference between the study subgroups. Another risk factor associated with the frequency of cough episodes is the length of the surgery, p=0.029. Cough frequency is higher in surgeries lasting less than 50min than in those lasting 150min. It is worth noting that the number of the latter surgeries was low. There are no significant differences for gender, age, height and BMI in relation to the frequency of at least one cough episode.
Frequency of at least one cough episode according to risk factors.
| Frequency of at least one cough episode | |||||
|---|---|---|---|---|---|
| Yes | No | Frequency (%) | 95% CI | p value | |
| Groups | |||||
| Group T | 13 | 37 | 26 | 14.6–40.3 | 0.023 |
| Group U | 24 | 26 | 48 | 33.6–62.5 | |
| Sex n (%) | |||||
| Female | 19 | 38 | 33 | 6.24–21.4 | 0.38 |
| Male | 18 | 25 | |||
| Age (years) | |||||
| 18–29 | 8 | 14 | 36.4 | 17.2–59.3 | 0.47 |
| 30–39 | 11 | 11 | 50 | 28.2–71.8 | |
| 40–49 | 6 | 16 | 27.3 | 10.7–50.2 | |
| 50–75 | 12 | 22 | 35.6 | 19.7–53.5 | |
| Height (m) | |||||
| 1.45–1.55 | 4 | 15 | 21 | 6.05–45.5 | 0.24 |
| 1.56–1.65 | 21 | 28 | 42.8 | 28.8–57.8 | |
| 1.66–1.88 | 12 | 20 | 37.5 | 21.1–56.3 | |
| BMI | |||||
| Low weight | 2 | 1 | 66.7 | 0.72 | |
| Normal | 20 | 17 | 64 | 48.5–77.3 | |
| Overweight | 31 | 19 | 62 | 47.1–75.3 | |
| Length of the surgery (min) | |||||
| Under 50 | 23 | 20 | 53.5 | 37.6–68.8 | 0.029 |
| 51–100 | 9 | 32 | 21.9 | 10.6–37.6 | |
| 101–150 | 2 | 9 | 18.8 | 2.3–51.7 | |
| Over 150 | 3 | 2 | 60 | 14.6–94.7 | |
Source: Authors.
The total number of surgeries lasting less than 50min was 43; of these, 39.53% (17) corresponded to group T, and 60.47% (26) to group U. Of the patients in group U, 35.29% (6) presented at least one cough episode, while in group T, 65.38% (17) had a cough episode. The RR of having at least one cough episode for group T compared to group U was 1.82, 95%CI (0.91–3.73), p=0.0531.
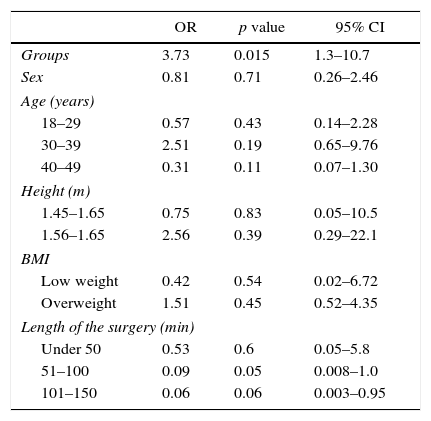
Logistic modelTable 3 shows the results obtained using the logistic model to determine the association of cough frequency based on study group, sex, age, height, BMI and length of the surgery. Variables were entered in a dichotomic way in order to ensure the validity of the logistic model. The p value for the fit test for this model was 0.002, leading to the conclusion that the model fits the data correctly at a significance level of 0.05.
Completed logistic model.
| OR | p value | 95% CI | |
|---|---|---|---|
| Groups | 3.73 | 0.015 | 1.3–10.7 |
| Sex | 0.81 | 0.71 | 0.26–2.46 |
| Age (years) | |||
| 18–29 | 0.57 | 0.43 | 0.14–2.28 |
| 30–39 | 2.51 | 0.19 | 0.65–9.76 |
| 40–49 | 0.31 | 0.11 | 0.07–1.30 |
| Height (m) | |||
| 1.45–1.65 | 0.75 | 0.83 | 0.05–10.5 |
| 1.56–1.65 | 2.56 | 0.39 | 0.29–22.1 |
| BMI | |||
| Low weight | 0.42 | 0.54 | 0.02–6.72 |
| Overweight | 1.51 | 0.45 | 0.52–4.35 |
| Length of the surgery (min) | |||
| Under 50 | 0.53 | 0.6 | 0.05–5.8 |
| 51–100 | 0.09 | 0.05 | 0.008–1.0 |
| 101–150 | 0.06 | 0.06 | 0.003–0.95 |
Source: Authors.
The completed logistic model shows that the only variable that influences cough frequency is the type of treatment received, OR 3.73, 95% CI (0.77–1.29).
DiscussionFrequency of cough at the time of extubation is lower when the plasma concentration of remifentanil is maintained at 3–4ng/ml (group T=26%), than at 2–3ng/ml (group U=48%) (p<0.023). Although frequency was high, the percentage of patients with moderate-to-severe cough was very low. Therefore, a dose–response curve will show extremely high doses used for suppressing episodes of very mild cough that will give rise to adverse events. TME is just a sign in a cascade of events with the afferent pathway initiating in the airway epithelium and mucosa down to the alveoli3–6 and triggering responses in the ventilation system and the autonomous nervous system. This gives rise to clinical manifestations such as increased blood pressure, bronchospasm, arrhythmias, and increased intra-ocular, intracranial and gastric pressures.7–9
One strategy consists of extubating the patient under deep anaesthesia,10 which entails the risk of laryngospasm and airway complications. Dexmedetomidine11 may be time consuming and results do not appear to be conclusive. Topic lidocaine application12,13 may be of some benefit but it is dependent on the length of the surgery.
Opioids have been used to reduce the cough stimulus at the time of extubation.14–16 Lee et al. reported that effective PC50 of remifentanil for cough suppression was 1.5ng/ml17–20 with variable efficacy. Jun et al. reported that up to 31% of patients extubated under remifentanil concentrations of 1.5ng/ml had cough episodes.21 An analysis of these two papers showed that pharmacodynamic interactions between the levels of opioids and hypnotics at the time of extubation were not considered, and this may interfere with the interpretation of the results. This could explain why a similar frequency was found in our group with a remifentanil PC between 3 and 4ng/ml (26%), as was also the case in other studies which used 1.5 and 2ng/ml (31% incidence). Drug pharmacokinetics was taken into consideration in this study in order to make sure that the patient would only be under the effect of remifentanil at the time of the evaluation. To ensure that the desired concentration was reached, the Rugloop simulation was used for all infusions. Consequently, differences in cough frequency between the groups are only attributable to the different plasma concentrations of remifentanil. There were no significant differences in terms of age, sex, and body mass index between the two groups.
Considering that extubation takes place once the effect of the halogenated agent has waned out (ET 0.1), it may be concluded that, at the remifentanil concentration ranges studied, emergence time does not depend on the opioid PC but the PC of the hypnotic agent.
The pharmacokinetic behaviour of anaesthetic agents is not new.22,23 There are now sufficient studies supporting the safe and cost-effective use of drugs used in anaesthesia, aimed at determining the probability of attaining the desired response, planned and controlled by the anaesthetist.
The use of technology in the form of computerised infusion systems could provide information to allow the use of smaller ranges of remifentanil concentrations. However, the use of graphic representations of mathematical models has already been validated in the literature for remifentanil.24,25 This highlights the usefulness of nomograms, not only for this study, but also for planning infusions in any operating room, regardless of the availability of technological resources.
ConclusionThe presence of at least one cough episode during extubation is lower with a remifentanil PC of 3–4ng/ml than with a PC of 2–3ng/ml, the difference being statistically significant. Cough at the time of extubation is not only an unpleasant and dramatic event, but results also in higher pain levels, hoarseness and systemic events that may be prevented with the adequate use of this opioid.
Ethical disclosuresProtection of people and animalsThe authors state that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in agreement with the World Medical Association and the Declaration of Helsinki.
Confidentiality of the dataThe authors state that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the correspondence author.
FundingOwn resources.
Conflicts of interestAuthors declare having no conflict of interest.
Please cite this article as: Tafur-Betancourt LA, Arévalo-Sánchez M, Lema-Flórez E. Efecto de dos concentraciones plasmáticas de remifentanilo a través de TACAN sobre la frecuencia e intensidad de la tos durante la extubación: Ensayo Clínico Controlado Aleatorizado. Rev Colomb Anestesiol. 2017;45:92–99.
Clinical Trials: NCT02711904.