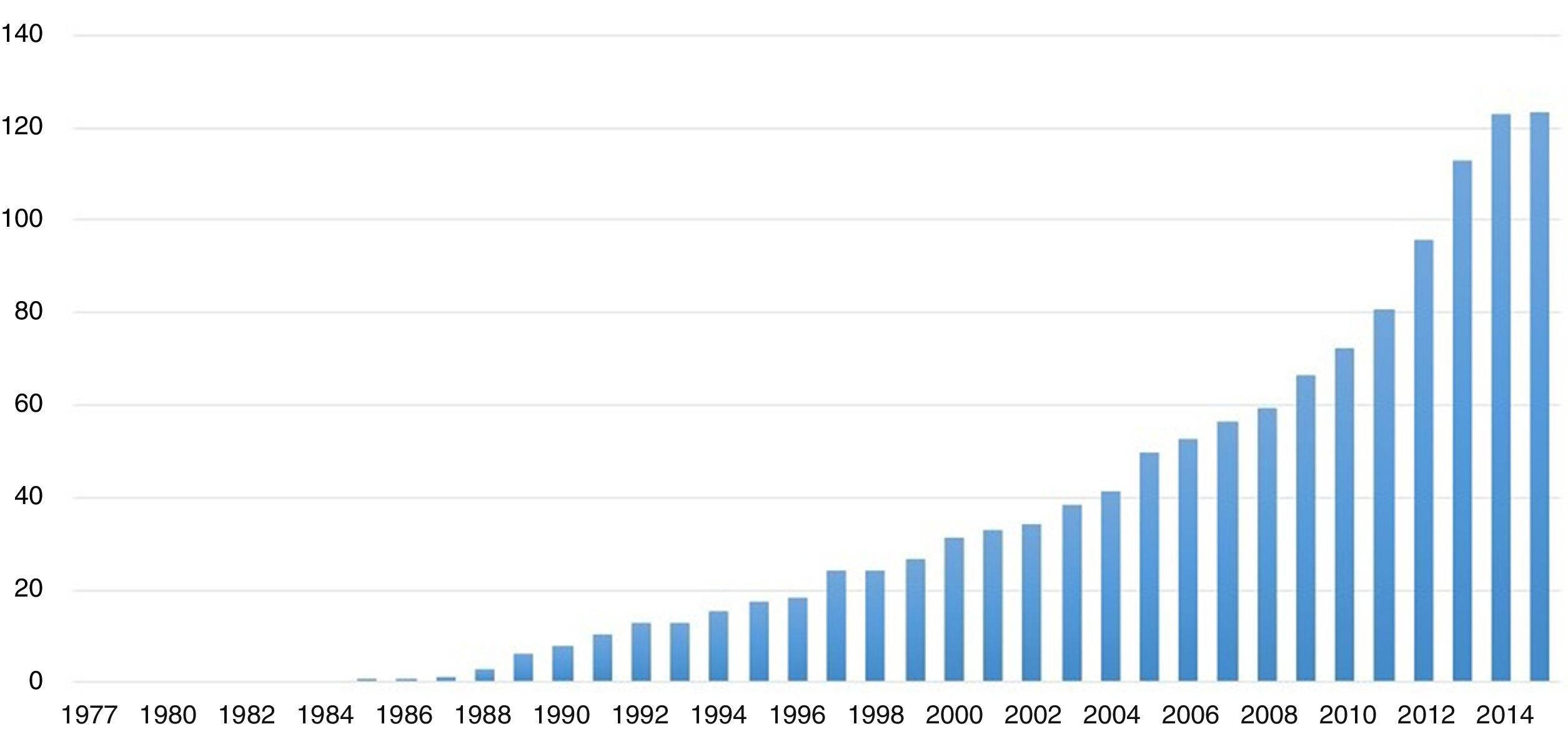
Systemic reviews and meta-analyses continue to enjoy exponential growth, as demonstrated by the number of publication indexed in MEDLINE, numbering 84,197 systematic reviews in 2014. Of these, 13,012 are meta-analyses, as we can see in Fig. 1. This summarizes the importance that this method of answering research questions continues to have in terms of contributions to knowledge and clarifications of evidence surrounding topics that may be controversial with regard to treatment, diagnosis, and even prognosis.
Although it is true that meta-analyses allow researchers to improve statistical power by increasing the probability of finding the effect of an intervention by combining the individual results of several studies—especially when the studies lack an appropriate sample size, it is also true that meta-analyses offer us the opportunity to clarify why results may differ in the direction of effect depending on the characteristics of the studies.
In this issue, Dr. Trujillo and collaborators, in their publication “Lactato Ringer versus Solución Salina Normal para Trasplante Renal. Revisión sistemática y Meta-análisis,1 perform, with a valid and relevant question, a systematic review and meta-analysis in order to determine the best crystalloid solution option for post-operatively managing patients that underwent kidney transplants, specifically when there are outcomes of hyperchloremic acidosis metabolic acidosis, hyperkalemia, volume of fluids infused, and renal graft failure.
The authors rigorously follow the recommendations of the Cochrane Collaboration2 and finally select four studies to combine the outcomes presented3–6. The first of these outcomes refers to potassium levels, which are compared through the difference of the potassium averages, without finding sufficient precisions in the summary measure obtained, with a wide confidence interval containing zero. In addition, from the point of view of the assessment of heterogeneity, an I2 of 75% was found. The lack of precision was repeated with the creatinine levels, this time with a smaller number of subjects (81). In terms of the outcomes for the levels of chlorine, again the point estimate is imprecise and a high level of heterogeneity (99%) was evident. For this reason, an adequate interpretation of the results was not possible, and an adequate precision for the outcome of volume of fluids infused was not found either. Only with the outcome of the bicarbonate levels was it possible to be satisfied with the summary measure in which the greatest acidosis is found after the infusion of normal saline solution.
The results mentioned above are the product of some of the difficulties that researchers embarking on systematic reviews and meta-analyses, in which an insufficient number of studies are found, have to face. The lack of precision and the heterogeneity are two limitations that researchers deal with when combining the results of individual studies. The former determines the statistical power, while the second could be our greatest ally.
Among the main reasons for performing an analysis is to increase the statistical power, something that depends on the sample size that will be increased after the integration of several studies. In this way, the significance of the estimates is improved. However, in a contrasting position are the differences that can be found between the studies, corresponding to the heterogeneity expressed by the degree of variation of the effect between the studies, something that is not up to chance. The sources of heterogeneity are diverse; as such, the first thing to do is to identify the different types of heterogeneity, including: (1) Clinical heterogeneity, due to the differences in the types of patients, treatments, or outcomes; (2) Methodological heterogeneity, due to variability of designs and the control o bias; and (3) Statistical heterogeneity, those differences that can only be made evident through statistical tests like the Cochran Q test and the I2. These are differences that can not be explained by chance and are derived from true differences7,8.
Heterogeneity is common in meta-analyses, and it should be our ally when we explore its sources9,10; researchers must assess which studies could combine their results in a quantitative was. This depends on the presence and magnitude of the heterogeneity. In cases in which the value is greater than 50%, the recommendation is to not combine the individual results to obtain a summary measure since there is a risk of acquiring an aggregate of bias from different sources11.
Finally, heterogeneity is the opportunity to be able to perform pertinent stratifications that, based on the researchers’ judgment, lead to finding where the sources of this heterogeneity lie. As mentioned above, these may be in the characteristics of the population, due to differences in comorbidities, in the magnitude of the treatment, the presentation of outcomes, or in the time of the monitoring of the subjects. Once the results of the individual studies are combined again by performing the pertinent stratifications, we can evaluate whether or not the heterogeneity disappears and, in this way, contribute valuable information about the identification of sources of heterogeneity. This should be taken into account when it comes to interpreting results and making recommendations for future studies.
To conclude, we can say the following: before undertaking a meta-analysis, researchers must decide whether there is a sufficient number of studies, which ensures the performance of the assessment of the heterogeneity through different strategies like stratification, analysis of sensitivity, or meta-regression.
FundingThe authors did not receive sponsorship to undertake this article.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Oliveros H. La heterogeneidad en los meta-análisis, ¿es nuestra mejor aliada? Rev Colomb Anestesiol. 2015;43:176–178.