Point-of-care thromboelastography is used for guiding peri-operative haemostatic therapy.
ObjectiveTo identify a thromboelastographic pattern in children with prolonged cardiopulmonary bypass exposure.
Material and methodsA cohort study of 62 patients undergoing prolonged cardiopulmonary bypass was performed. Patients with preexisting coagulopathy, use of drugs known to interfere with clotting, hematocrit>60%, weight<3kg, or hepatic disease were excluded. The thromboelastography study was conducted at the point of care.
ResultsBaseline and rewarming reaction time values were 8.24±6.35 and 7.66±2.15min, respectively (p=0.102). Baseline and rewarming angle values were 64.88±13.08 and 54.67±8.98 degrees, respectively (p<0.001). Baseline and rewarming maximum amplitude values were 64.54±12.31 and 43.14±12.47mm, respectively (p=0.001). The same trend was observed when the cohort was divided into patients under and over 3 years of age, and patients under and over 10kg of body weight.
DiscussionThis study suggests the existence of a thromboelastographic pattern independent of age or weight in patients undergoing paediatric cardiac surgery with prolonged cardiopulmonary bypass exposure, characterised by a reduction of angle and maximum amplitude values, with no change in reaction time.
La tromboelastografía se emplea para la orientación en el manejo de la coagulación perioperatoria en el sitio de atención.
ObjetivoIdentificar un patrón de coagulación en niños sometidos a tiempos prolongados en circulación extracorpórea así como su asociación con edad y peso.
Material y MétodosRealizamos un estudio de cohorte en 62 pacientes sometidos a circulación extracorpórea prolongada. Excluimos pacientes con coagulopatía pre - existente, empleo de medicamentos interfiriendo con la coagulación, hematocrito>60%, peso<3 Kg o con enfermedad hepática. El estudio de tromboelastografía fue realizado en el sitio de atención.
ResultadosLos valores para el tiempo de Reacción basales y durante recalentamiento fueron: 8,24 +/- 6,35 y 7,66 +/- 2,15 minutos respectivamente (p= 0,102). Los valores para el Ángulo basales y durante recalentamiento fueron: 64,89 +/- 13,08 y 54,67 +/- 8,98 grados (p<0,001). Los valores para Amplitud Máxima basales y durante recalentamiento fueron: 64,54 +/- 12,31 y 43,14 +/- 12,47mm respectivamente (p=0,001). Dividiendo la cohorte en pacientes menores o mayores a 3 años o bien en menores o mayores a 10 Kg se observó el mismo comportamiento.
DiscusiónEste estudio sugiere la existencia de un patrón tromboelastográfico independiente de la edad o peso en pacientes sometidos cirugía cardiaca pediátrica con permanencia prolongada en circulación extracorpórea caracterizado por reducción en los valores de ángulo y amplitud máxima, sin modificación en el tiempo de reacción.
Peri-operative bleeding is perhaps the most common complication in paediatric cardiac surgery (PCS). This event is often associated with an increase in hospital length of stay or the need for surgical reintervention during the postoperative period.
One of the main factors associated with increased bleeding is prolonged exposure to cardiopulmonary by-pass circulation (CPB), which is also related to the complexity of the surgery required to address the cardiac condition. Likewise, other factors associated with a higher risk of bleeding include age under 1 year, level and duration of the hypothermia, and a low preoperative platelet count.1
Several strategies have been proposed for reducing peri-operative bleeding in different situations, including CPB, but no consensus has been reached so far2; however, a group of experts from the European Society of Anaesthesia under the coordination of Sibylle A. Kozek-Langeneker have published management guidelines for the control of massive bleeding.3
These guidelines include the following recommendations:
We recommend the use of transfusion algorithms incorporating pre-defined intervention triggers to guide interventions designed to achieve control of intra-operative bleeding (Level 1B Recommendation).
We recommend the application of transfusion algorithms incorporating pre-defined triggers based on point-of-care (POC) coagulation monitoring studies to guide corrective manoeuvres during cardiovascular surgery (Level 1C Recommendation).
More recently, the American Society of Anaesthesiologists (ASA)4 has published guidelines for the peri-operative management of blood products, reaching the same conclusion as those published by the European Society in the sense that the use of viscoelastic tests to build algorithms or protocols for peri-operative management reduces the need for using blood products during the peri-operative period.
At the Instituto Nacional de Pediatría (National Paediatrics Institute) in Mexico we use the concept of point-of-care (POC) diagnosis, and as far a clotting is concerned, we use thromboelastography (TEG) for monitoring haemostasis abnormalities. This study was designed to determine whether there is a coagulation pattern in children undergoing prolonged exposure to CPB, and to analyse the effects of weight and age on the outcomes for these variables.1,5
Materials and methodsBoth the Ethics as well as the Research Committee of Instituto Nacional de Pediatría granted registration number 060/2012 on June 17 and July 20, 2012, respectively. The informed consent for anaesthetic and surgical procedures, and for transfusion if needed, was obtained from the parents or guardians.
This prospective, descriptive and observational cohort study included all patients undergoing PCS with the potential of remaining under CPB for more than 90min between February 2013 and February 2014. Prolonged exposure to CBP was defined as more than 90min, based on previously reported experiences.1,5
Exclusion criteria were pre-existing coagulopathy (primary or secondary), use of medications that interfere with coagulation, hematocrit>60%, term neonates<3kg of body weight or premature babies, and known liver disease.
Surgical risk was determined using the RACHS-1 score (Risk Adjusted Classification for Congenital Heart Surgery).6 Conventional clotting tests were made in all cases, including prothrombin time, partial thromboplastin time, INR and platelet count. No baseline fibrinogen levels were obtained.
A thromboelastography machine (TEG Hemostasis Analyzer, Model 5000, Haemonetics Corporation, 400 Wood Road, Braintree, MA, USA) was used for POC testing. The machine is located in an office next door to the cardiovascular surgery room at Instituto Nacional de Pediatría and it is calibrated every day just before every surgical event. Individual patient data are stored in the computer where the analysis software is installed.
A baseline 1mL blood sample was obtained upon patient arrival at the operating room, before the incision and after anaesthesia induction; the sample was processed in a 360μL tray with kaolin added as response accelerator.
A second sample was obtained towards the end of surgery, with the patient still on BPC and upon start of rewarming at 32°; this sample was processed in a different 360μL tray with heparinase, again with kaolin added.
In all cases, tranexamic acid as anti-fibrinolytic was used after induction, both for the patient and for priming the by-pass circulation pump circuit. The doses used were 10mg/kg as an intravenous bolus given in 5min plus an additional 10mg/kg for priming, followed by a continuous infusion of the drug at a rate of 5mg/kg/h during the entire surgical procedure. The infusion was discontinued after protamine administration.
Concentrated red blood cells were used for priming the cardiopulmonary by-pass pump in patients <10kg. Fresh plasma was used in all cases.
During rewarming at 32°C, 125mL of fresh plasma were added to the circuit, plus an additional 125mL when 36°C were reached, in all cases. Continuous ultrafiltration was used during the procedure.
The samples were processed by the perfusion team which had been previously trained in the performance of the test, and TEG curve readings and interpretations were made by the researchers.
Statistical analysisResults were expressed in percentages, central trend measurements and scatter.
The Student T test for paired data was used to determine differences between TEG variables (baseline and rewarming).
Correlation between age and body weight and CPB time was analysed using the Pearson correlation test with a 95% confidence interval. All “p” values were considered significant.
ResultsOverall, 183 patients were taken to surgery during the study period and, of them, 63 met the inclusion criteria. The data for one patient were removed because it was not possible to obtain the complete information. Consequently, a total of 62 patients were analysed, 28 of them females (45%) and 34 males (55%). Mean age was 3.71 years (0.004–16) and mean weight was 13.53kg (3–51.5).
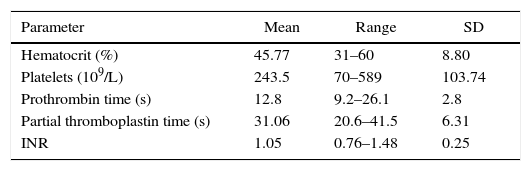
The conventional coagulation profile (prothrombin time, partial thromboplastin time, INR and platelet count) is shown in Table 1. The average preoperative values for the conventional coagulation tests were found within normal ranges. Likewise, the baseline hematocrit level was 45.77±8.80% and 42.03±8.52% after CPB (p=0.07).
Pre-operative coagulation parameters.
| Parameter | Mean | Range | SD |
|---|---|---|---|
| Hematocrit (%) | 45.77 | 31–60 | 8.80 |
| Platelets (109/L) | 243.5 | 70–589 | 103.74 |
| Prothrombin time (s) | 12.8 | 9.2–26.1 | 2.8 |
| Partial thromboplastin time (s) | 31.06 | 20.6–41.5 | 6.31 |
| INR | 1.05 | 0.76–1.48 | 0.25 |
SD, standard deviation; INR, International Normalised Ratio.
Source: Authors.
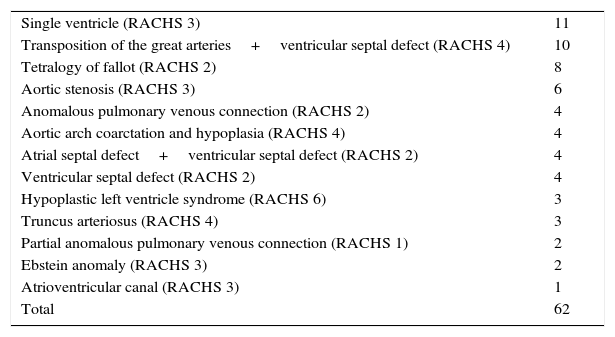
Table 2 shows the pre-operative diagnoses. The mean value on the RACHS-1 scale for the cohort, according to the various diagnoses, was 2.6 (range 1–6).
Pre-operative diagnoses.
| Single ventricle (RACHS 3) | 11 |
| Transposition of the great arteries+ventricular septal defect (RACHS 4) | 10 |
| Tetralogy of fallot (RACHS 2) | 8 |
| Aortic stenosis (RACHS 3) | 6 |
| Anomalous pulmonary venous connection (RACHS 2) | 4 |
| Aortic arch coarctation and hypoplasia (RACHS 4) | 4 |
| Atrial septal defect+ventricular septal defect (RACHS 2) | 4 |
| Ventricular septal defect (RACHS 2) | 4 |
| Hypoplastic left ventricle syndrome (RACHS 6) | 3 |
| Truncus arteriosus (RACHS 4) | 3 |
| Partial anomalous pulmonary venous connection (RACHS 1) | 2 |
| Ebstein anomaly (RACHS 3) | 2 |
| Atrioventricular canal (RACHS 3) | 1 |
| Total | 62 |
Abbreviation: RACHS, Risk Adjusted Congenital Heart Surgery Score.6
Source: Authors.
Regarding the characteristics of the surgery, mean CPB time was 217±103.35min, aortic clamping 128.03±76.80min, and total surgical time 402.95±157.93min; average temperature during CPB was 24.02°C.
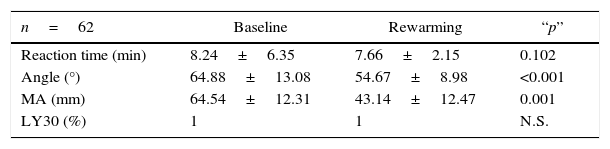
Table 3 shows the behaviour of the thromboelastographic parameters for the cohort. No significant differences were found for reaction time (R) or for clot lysis at the 30min time point (LY30) either at baseline (Bs) or rewarming (Rw). However, significant reductions were observed in angle and maximum amplitude (MA) values, specifically during the rewarming period.
Baseline and rewarming TEG characteristics.
| n=62 | Baseline | Rewarming | “p” |
|---|---|---|---|
| Reaction time (min) | 8.24±6.35 | 7.66±2.15 | 0.102 |
| Angle (°) | 64.88±13.08 | 54.67±8.98 | <0.001 |
| MA (mm) | 64.54±12.31 | 43.14±12.47 | 0.001 |
| LY30 (%) | 1 | 1 | N.S. |
Significance levels of baseline and rewarming TEG values in the general population. No significant differences were observed for reaction and lysis values at the 30-min time point (LY30).
MA, maximum amplitude; N.S., not significant.
Source: Authors.
The correlation between angle and MA values and CPB time was analysed as well. The correlation index (r) regarding time on CPB was −0.468 for the angle and −0.667 for MA (p=0.001 and <0.001, respectively)
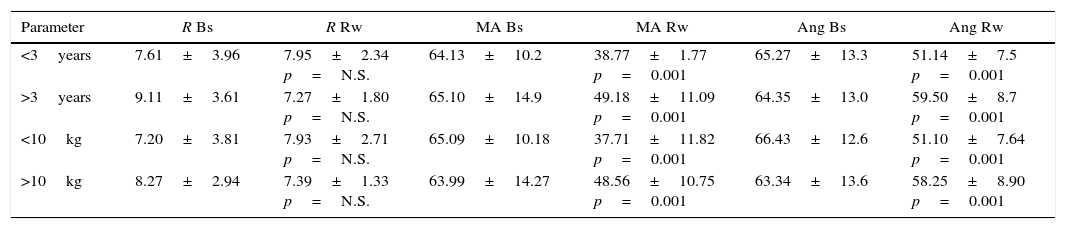
The cohort was divided on the basis of age and body weight into <3 years or >3 years and <10kg or >10kg (n=31 and 31, respectively). TEG values according to these groupings are shown in Table 4.
Behaviour of TEG values by age and weight.
| Parameter | R Bs | R Rw | MA Bs | MA Rw | Ang Bs | Ang Rw |
|---|---|---|---|---|---|---|
| <3years | 7.61±3.96 | 7.95±2.34 p=N.S. | 64.13±10.2 | 38.77±1.77 p=0.001 | 65.27±13.3 | 51.14±7.5 p=0.001 |
| >3years | 9.11±3.61 | 7.27±1.80 p=N.S. | 65.10±14.9 | 49.18±11.09 p=0.001 | 64.35±13.0 | 59.50±8.7 p=0.001 |
| <10kg | 7.20±3.81 | 7.93±2.71 p=N.S. | 65.09±10.18 | 37.71±11.82 p=0.001 | 66.43±12.6 | 51.10±7.64 p=0.001 |
| >10kg | 8.27±2.94 | 7.39±1.33 p=N.S. | 63.99±14.27 | 48.56±10.75 p=0.001 | 63.34±13.6 | 58.25±8.90 p=0.001 |
Bs, baseline; Rw, rewarming; Ang, angle; MA, maximum amplitude; N.S., not significant.
Source: Authors.
The table reveals that baseline R for children <3 years was 7.61±3.96min and 7.95±2.34min during rewarming (p=0.36). The baseline R value in patients >3 years was 9.11±3.61min and 8.27±1.80 during rewarming (p=0.08).
Likewise, a significant reduction of mean angle and MA values was observed after rewarming in both age and weight subgroups. Again, when the correlation between these parameters and time on CPB was analysed, the same trend as that described for the general population was observed.
DiscussionThe objective of this study was to identify a thromboelastographic pattern at the point of care in a paediatric population with prolonged exposure to CPB considering that, in our opinion, there is no clear definition of the intra-operative behaviour of thromboelastographic parameters in this group of patients.1,5
Most of the studies that analyse thromboelastography behaviour during paediatric cardiac surgery focus primarily on post-operative bleeding (in the intensive care unit) and fail to focus on the behaviour during CPB or immediately after coming off CPB.7,8
Moreover, although the assumption is that normal TEG values are no different in the paediatric population than those described for adults (in healthy patients), intra-population age differences (e.g. neonates vs. infants) are factors that need to be analysed; there are conditions that determine differences in haemostasis among groups, including concentration of the components of the haemostatic system, rate of synthesis of those components, total capacity to generate and regulate thrombin and plasma.9 Consequently, we believe that an analysis broken down by age may help clarify thromboelastographic behaviour.
As far as weight is concerned, it is worth mentioning that, in our population, 48.2% of the patients are malnourished and 1.8% show wasting (Source: institutional database, 2015. Database processed and audited by the IQIC [International Quality Improvement Collaborative] project in Boston Children's Hospital, USA). There are publications that have examined thromboelastographic changes in malnourished patients (including marasmus or kwashiorkor) and reported the following changes: thrombocytopenia, poor clot retraction, poor thrombin production (V curve) and a general tendency towards having poor coagulation.10 For this reason, we decided to conduct a subgroup analysis based on weight.
In a groundbreaking study in 1997, Bruce Miller et al. suggested that conventional clotting tests might no be useful for predicting post-operative bleeding. This study suggests that point-of-care thromboelastography (TEG) could be a method for analysing coagulation disorders in this group of patients.11 With this consideration in mind, we present normal thromboelastography values for the elements of the TEG curve analysed in this study: reaction time (R), 4–8min; angle (Ang), 47–64°; maximum amplitude (MA), 54–72mm; and lysis at 30min (LY30), 0–8%.12
Taking these parameters into account and based on our analysis of the general sample, the result was a consistent pattern: unaltered LY30 and R values during rewarming time as well as significant reductions of the angle and MA values in patients remaining on CPB for more than 90min. The same pattern as that observed for the general population was found in the breakdown by age and weight. Three factors may influence this finding: the use of fresh frozen plasma for priming the bypass pump circuit, the use of continuous ultrafiltration (CUF) during surgery, and the use of tranexamic acid throughout the procedure.
The use of plasma for priming the circuit has been challenged because it is associated with haemodilution and eventually leads to increased bleeding and mortality; however, the use of circuit priming with plasma is common practice for us and we believe that our results support its potential beneficial effect, as reflected by the R values in the cohort following cardiopulmonary bypass. Furthermore, we also administer a dose of plasma at 33° and 36° while still on by-pass and try to reduce haemodilution using CUF as part of the perfusion technique in all cases; we believe that this could have influenced the observed results. Some researchers have suggested that modified ultrafiltration (MUF) might lead to lower haemodilution and also reduce the consequences associated with the use of plasma during cardiopulmonary bypass. However, the objective of this study does not focus on filtration strategies and, therefore, we believe that studies designed to detect differences between the two procedures (CUF and MUF) should be performed.13–17
We looked for correlations between time of exposure to CPB and the behaviours of MA and Angle values, and found them to be consistent with what has been suggested by other authors.11 It is worth highlighting that reductions found in the MA values (platelets) during rewarming are significant and lower than the reference values; however, reductions in angle (fibrinogen) levels during that same period, although significant, are within normal parameters. New studies are needed in order to determine the clinical relevance of this finding.
Other studies have suggested similar behaviour patterns as those reported here but using other viscoelastic tests such as ROTEM in surgical procedures with CPB times longer than 90min, and describe alterations in clot formation and strength as a result of platelet changes like those found in our study.18
In conclusion, we describe a clotting pattern obtained through point-of-care thromboelastography in paediatric patients undergoing cardiac surgery with prolonged cardiopulmonary by-pass times. This pattern is characterised by a significant reduction of angle and maximum amplitude values, with no changes in reaction time or lysis at 30min during rewarming.
We believe that the effect of prolonged exposure to CPB, regardless of age or weight, acts on platelets (quantity and/or adhesiveness) and to a lesser degree on fibrinogen levels (represented by the angle). We suggest that the absence of changes in R values may be due to the use of plasma for priming the CPB circuit.
New research is needed in order to assess the clinical correlation of these findings.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study was funded with resources from the department.
Conflict of interestNone.
Please cite this article as: Tamariz-Cruz OJ, Cruz-Sánchez S, Pérez-Pradilla C, Motta-Amézquita LG, Díliz-Nava H, Palacios-Macedo-Quenot A. Identificación de un patrón tromboelastográfico en niños sometidos a cirugía cardiaca con exposición prolongada a circulación extracorpórea. Rev Colomb Anestesiol. 2017;45:108–113.