Levobupivacaine and ropivacaine are relatively new local anaesthetics developed in order to address the issue of bupivacaine toxicity. Although certain differences do exist between their pharmacological profiles, its clinical relevance at equipotent doses is not evident so far.
ObjectiveTo compare the efficacy and characteristics of equipotent doses of intrathecal levobupivacaine with ropivacaine.
MethodologySixty ASA grade I/II patients of 18–60 years, either sex posted for lower limb orthopaedic surgery under spinal anaesthesia were randomly given either 15mg levobupivacaine or 22.5mg ropivacaine. Sensory and motor block, haemodynamic characteristics, as well as any side effects, were recorded.
ResultsOnset of sensory block to T10 was more rapid in group R than group L, p<0.0001. The median (range) height achieved in group R was T7 (T5–T10) while in group L was T7 (T4–T10). Time to reach maximum height and time to modified Bromage grade 3 was shorter in group R as compared to group L, p<0.0001. Levobupivacaine produced significantly longer (290.50±34.67) duration of motor block compared to ropivacaine (222.50±23.00). Duration of analgesia was significantly longer in group L (309.83±36.45) than group R (249.50±22.83). No serious adverse effects were recorded.
ConclusionLevobupivacaine produces significantly longer duration of analgesia than ropivacaine when used in a ratio of 0.6:1. Efficacy, toxicity and haemodynamic profile make ropivacaine suitable agent for surgeries with low threshold for hypotension.
La levobupivacaína y la ropivacaína son anestésicos locales relativamente nuevos, desarrollados con el fin de abordar la cuestión de la toxicidad de la bupivacaína. Aunque existen ciertas diferencias entre sus perfiles farmacológicos, su relevancia clínica en dosis equipotentes no es evidente hasta ahora.
ObjetivoComparar la eficacia y las características de las dosis equipotentes de levobupivacaína por vía intratecal con las de ropivacaína.
MetodologíaA Sesenta pacientes de grado ASA I/II de 18 a 60 años y de ambos sexos, programados para cirugía ortopédica del miembro inferior bajo anestesia espinal, se les dio al azar o bien 15mg de levobupivacaína o 22,5mg de ropivacaína. El bloqueo motor, el bloqueo sensorial, las características hemodinámicas y cualquier otro efecto secundario fueron registrados.
ResultadosEl inicio del bloqueo sensorial en T10 fue más rápido en el grupo R que en el grupo L, p<0,0001. El nivel mediano (rango) alcanzado en el grupo R fue T7 (T5-T10), mientras en el grupo L fue T7 (T4-T10). El tiempo para alcanzar el nivel máximo y para alcanzar un grado 3 en la escala de Bromage fue más breve en el grupo R en comparación con el grupo L, p<0,0001. La levobupivacaína produce una duración significativamente más larga (290.50±34.67) del bloqueo motor que la ropivacaína (222.50±23.00). La duración de la analgesia fue significativamente más larga en el grupo L (309.83±36.45) que en el grupo R. No se registraron efectos adversos graves.
ConclusiónLa levobupivacaína produce una duración de la analgesia significativamente más larga que la ropivacaína cuando se utiliza en una proporción de 0,6:1. La eficacia, toxicidad y perfil hemodinámico hacen de la ropivacaína un agente adecuado para cirugías con un umbral bajo de hipotensión.
Traditionally, bupivacaine has been the drug of choice for the subarachnoid block. However, significantly long duration of action delays recovery of motor function and prolongs post-anaesthesia care unit stay. In addition, several studies have shown that bupivacaine produces higher neurological and cardiac toxicity compared to other local anaesthetics.1 The problems associated with the toxicity of racemic bupivacaine triggered the development of alternative suitable ‘single enantiomeric’ local anaesthetic agents with low cardiac and CNS toxicity. Levobupivacaine and Ropivacaine are two relatively new amide local anaesthetic agents that have been produced in order to address the issues of bupivacaine toxicity.
Levobupivacaine is a high potency, long-acting local anaesthetic with a relatively slow onset of action.2 It has a lower propensity to block inactivated sodium and potassium channels along with faster rate of dissociation compared to its racemic form.3 The majority of in vitro, in vivo and human pharmacodynamic studies of nerve block indicate that levobupivacaine has similar potency, yet lower risk of cardiovascular and CNS toxicity than bupivacaine.4 So, having a higher threshold for cardiac and neurotoxicity compared to racemic bupivacaine, anaesthetists feel safer working with levobupivacaine5 and has the potential to replace bupivacaine as the standard drug.6
Ropivacaine is the ‘S’ isomer of the propyl analogue of bupivacaine with longer duration of action, low lipid solubility, low potency and low cardiovascular and CNS toxicity.7 Ropivacaine blocks nerve fibres involved in pain transmission (Aδ and C fibres) to a greater degree than those controlling motor function (Aβ fibres).8 Therefore, ropivacaine has been found to induce less intense motor blockade than bupivacaine. Hence, its comparatively shorter duration, faster recovery of motor function and lower toxicity profile have been identified as a potential benefit for surgery of intermediate duration as well as for ambulatory surgery in day care surgical units.
In the present era of evidence-based medicine, each step of our management is thoroughly evaluated by properly controlled, peer-reviewed medical research, and subarachnoid block is not an exception. The concept of a single shot with bupivacaine can do all is now questioned and necessitate the judicious use of safer substitutes. As of Casati et al.9 theoretical as well as experimental differences do exist in toxicology and clinical profiles due to different anaesthetic potencies of these isomeric forms of bupivacaine, but reflections of these characteristics into clinical practice have not been evident so far. So, we have to explore the typical characteristics and potential uses of these newer drugs. Many studies have been done to compare various forms of bupivacaine, ropivacaine and levobupivacaine. However, most of them have used low doses which may be inadequate for hip surgeries.10 Furthermore, they have generally used hyperbaric forms11,12 and potency ratio between levobupivacaine and ropivacaine was not taken into consideration.13
Therefore, this study was conducted to compare the efficacy and characteristics of isobaric forms of intrathecal levobupivacaine 0.5% with ropivacaine 0.75% in equipotent doses for lower limb orthopaedic surgery.
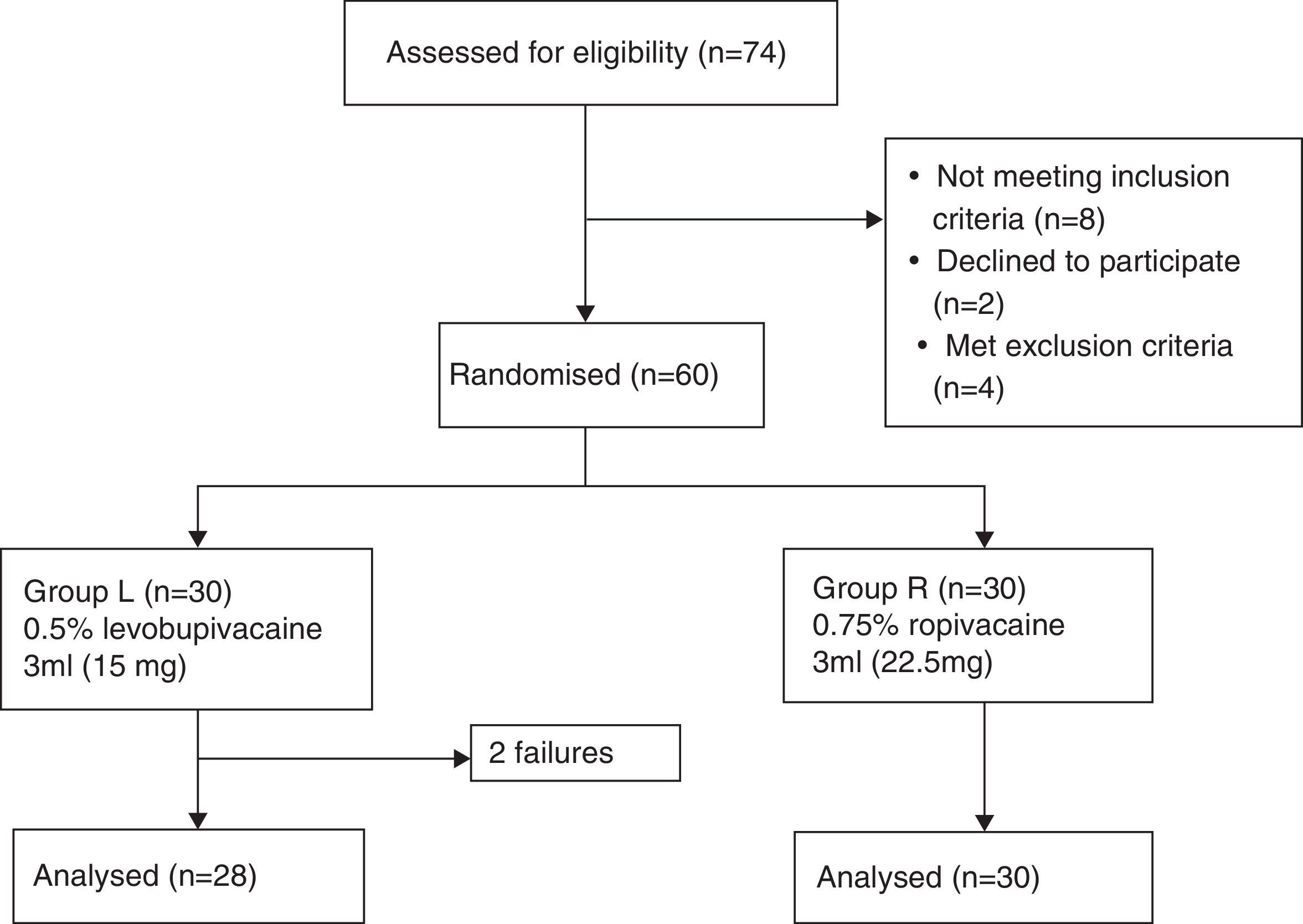
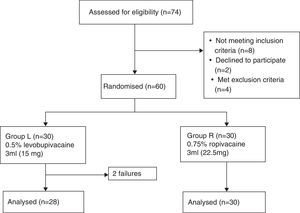
MethodologyFollowing approval by the Institutional Ethics Committee [(Ref. No. D1303/FM) and Clinical Trial Registry No. (NCT02201784)] and written informed consent, this prospective, randomised, double-blind, controlled, equivalence trial was conducted on sixty ASA grade I/II patients of either sex, aged between 18 and 60 years undergoing spinal anaesthesia for lower limb orthopaedic surgery. Patients with contraindication for spinal anaesthesia, known allergy to local anaesthetic drugs and patients having h/o diabetes, neurological or musculoskeletal diseases that could make our technique difficult were excluded. The patients were randomly divided into two groups of 30 each (group L and group R) by computer-generated randomisation (Fig. 1). Patients in group L received 3ml levobupivacaine 5mg/ml (15mg of LEVO-ANAWIN® 0.5% Neon Laboratories Ltd.) while in group R received 3ml ropivacaine 7.5mg/ml (22.5mg of ROPIN® 0.75% Neon Laboratories Ltd.). All drugs were loaded by an anaesthetist who did not have any involvement in further patient assessment while another anaesthetist administered anaesthesia and assessed all patients. Patients had standard monitoring including electrocardiography, pulse oximetry and non-invasive blood pressure monitoring (NIBP). Baseline heart rate (HR), NIBP and arterial oxygen saturation (SpO2) were measured. All patients received oxygen via Hudson mask at the rate of 6l/min until the surgery ends. Intravenous (IV) access was secured, patients were premedicated with i.v. ondansetron 0.1mg/kg body weight and preloading done with lactated Ringer's (LR) solution 15ml/kg body weight. Under strict aseptic precautions, skin was infiltrated with lidocaine 2% and lumbar puncture was performed in the sitting position with a 25-G Quincke spinal needle (Becton Dickinson, Madrid, Spain), using a midline approach at the L3–4 intervertebral space. Correct needle placement was identified by free flow of CSF and confirmed by aspiration and reinjection of CSF before and after the administration of the study drug solution. The study drug was injected over 20s. After the injection of the spinal medication, the patients were placed supine immediately, the time of which was recorded as ‘zero’. The level of sensory block was assessed every 5min till the loss of sensation to pinprick, using a 22-guage hypodermic needle with 2mm protrusion through the guard. Assessments continued at 30min intervals following the completion of surgery until normal sensation returned. After confirming the loss of sensation at T10 dermatome in comparison to C5–6 dermatome, patients were given i.v. midazolam 0.03mg/kg body weight and surgeons were allowed to proceed for the surgery. Inability to achieve T10 sensory level within 30min was considered as ‘Failure’. These patients were administered general anaesthesia. They were not included for analysis but only reported as total number of failures according to per protocol analysis. Motor block in the lower limbs was graded according to the modified Bromage scale14 (Grade 0=No motor block, Grade 1=Inability to raise extended leg, able to move knees and feet, Grade 2=Inability to raise extended leg and move knee, able to move feet, Grade 3=Complete motor block of the lower limbs). Thereafter, it was performed every 5min till the attainment of MB grade 3 followed by every 30min until complete recovery (MB grade 0). HR, NIBP and SpO2 was recorded before induction, every 3min till 15min, then, every 15min until discharge from the recovery room. Hypotension was defined as systolic BP<90mmHg and was treated with inj. mephenteramine of 6mg i.v bolus and fluids. Bradycardia was defined as HR<50beats/min and treated with i.v. atropine of 0.5mg, if symptomatic. For assessment of the onset of anaesthesia, the time for sensory block to develop to T10, maximum block height and time to achieve maximum height were noted. To assess the duration of the sensory block, time for regression to L1 and duration of analgesia (primary outcome) were compared. Time to achieve maximum motor block, duration of motor block along with any side effects were also noted.
Statistical analysisPower analysis estimated that a sample size of 30 patients per group would yield 95% power for testing the hypothesis at equivalence margin of 30-min difference in mean time to first analgesic requirement (PS Power and Sample Size Calculator-Version 3.0.43; Dupont WD, Plummer WD). The Type I error probability associated with this test, for the null hypothesis that levobupivacaine and ropivacaine in equipotent doses are similar in terms of duration of analgesia was α=0.05 (Fig. 2). Statistical analysis was performed using Excel 2013 (Microsoft, Redmond, VA), SPSS software (Version 19, SPSS Inc., USA) and Graph Pad Prism 5.00 (Graph Pad Software, San Diego, CA). Data are presented as mean (±SD), median (range), or frequencies (%) as appropriate. Group demographic data and adverse events were compared using unpaired t-test or chi-square (χ2) test, whichever applicable. Comparison of block characteristics, duration of analgesia and haemodynamics were made using unpaired t-test. To compare intragroup variations from baseline, one-way ANOVA with Dunnett's multiple comparisons tests was used. A p-value of <0.05 was considered statistically significant.
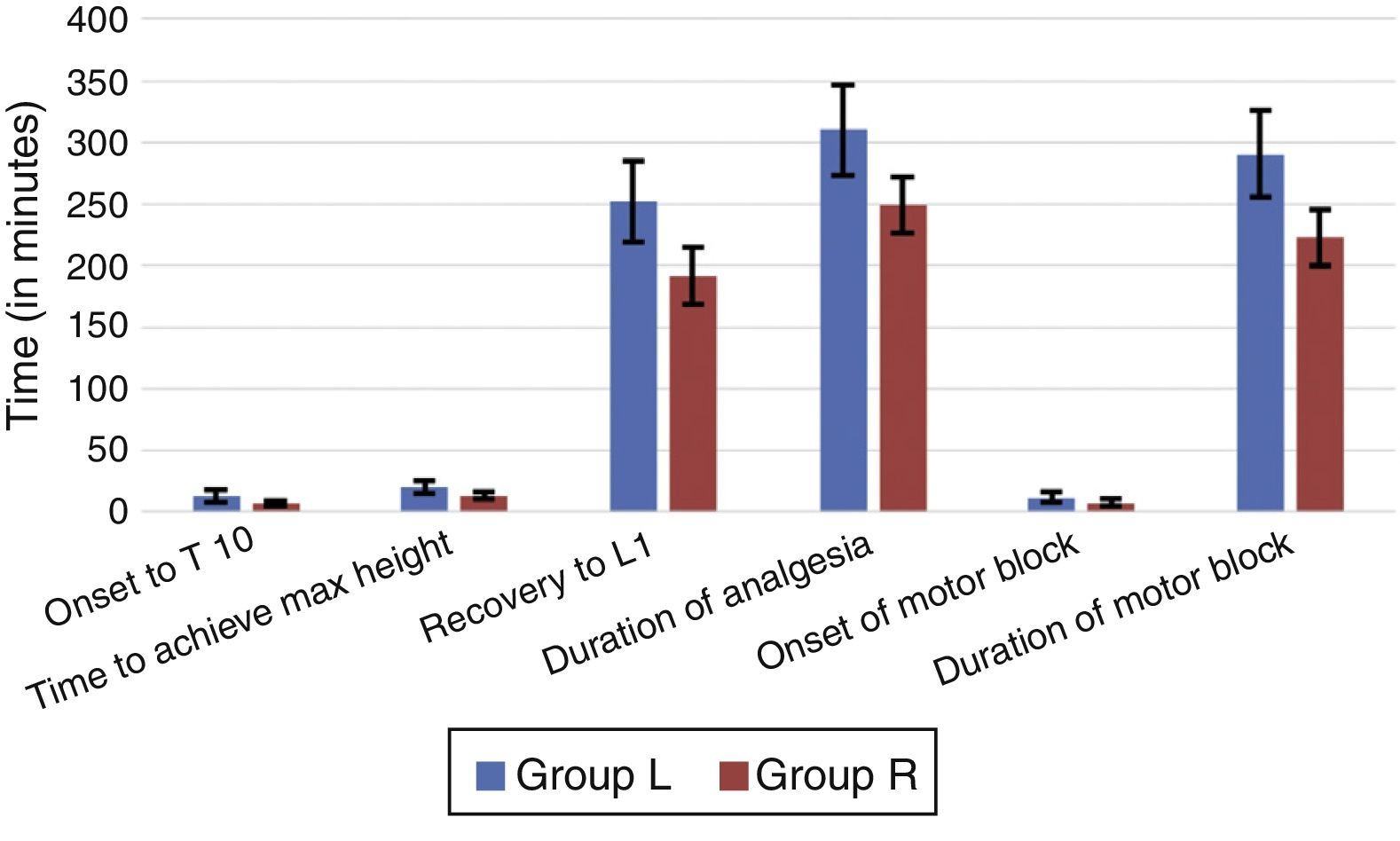
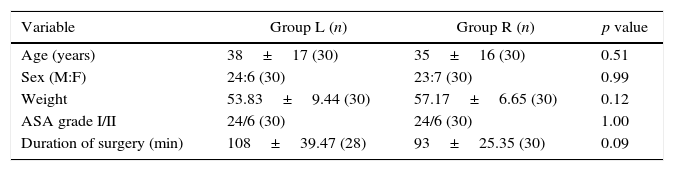
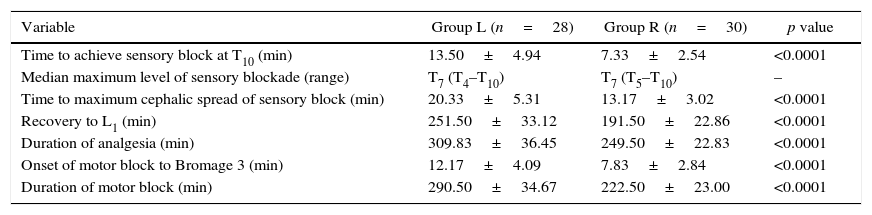
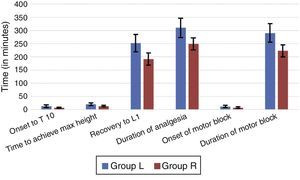
ResultsThere were no significant differences between the two groups with respect to age, sex, weight, ASA grade or duration of surgery (Table 1). Anaesthesia was successful in all patients except two failures in group L. Onset of anaesthesia to T10 was 7.33±2.49min in group R and 13.50±4.86min in group L (p<0.0001). The median (range) maximum height achieved in terms of dermatomes in group R was T7 (T5–T10) while in group L was T7 (T4–T10). The time to reach maximum height was shorter in group R (13.17±3.02min) as compared to group L (20.33±5.31min) with a p<0.0001 (Table 2, Fig. 3).
Patient characteristics.
| Variable | Group L (n) | Group R (n) | p value |
|---|---|---|---|
| Age (years) | 38±17 (30) | 35±16 (30) | 0.51 |
| Sex (M:F) | 24:6 (30) | 23:7 (30) | 0.99 |
| Weight | 53.83±9.44 (30) | 57.17±6.65 (30) | 0.12 |
| ASA grade I/II | 24/6 (30) | 24/6 (30) | 1.00 |
| Duration of surgery (min) | 108±39.47 (28) | 93±25.35 (30) | 0.09 |
n, number of patients; M:F, male:female; min, minutes; p≤0.05 is considered significant.
Source: Authors.
Block characteristics.
| Variable | Group L (n=28) | Group R (n=30) | p value |
|---|---|---|---|
| Time to achieve sensory block at T10 (min) | 13.50±4.94 | 7.33±2.54 | <0.0001 |
| Median maximum level of sensory blockade (range) | T7 (T4–T10) | T7 (T5–T10) | – |
| Time to maximum cephalic spread of sensory block (min) | 20.33±5.31 | 13.17±3.02 | <0.0001 |
| Recovery to L1 (min) | 251.50±33.12 | 191.50±22.86 | <0.0001 |
| Duration of analgesia (min) | 309.83±36.45 | 249.50±22.83 | <0.0001 |
| Onset of motor block to Bromage 3 (min) | 12.17±4.09 | 7.83±2.84 | <0.0001 |
| Duration of motor block (min) | 290.50±34.67 | 222.50±23.00 | <0.0001 |
n, number of patients; “T” is dermatomal level; min, minutes; data are expressed as mean±SD or median (range); p-value <0.05 is considered significant.
Source: Authors.
The time to modified Bromage 3 (MB-3) grade was 7.83±2.84min in group R and 12.17±4.09min in group L with p<0.0001. Levobupivacaine produced significantly longer duration of motor block (290.50±34.67min) compared to ropivacaine (222.50±23.00min), p<0.0001. Time for regression of sensory block to L1 was longer in the group L than group R (251.50±33.12min versus 191.50±22.86min; p<0.0001). Duration of analgesia was also significantly longer in group L (309.83±36.45) than group R (249.50±22.83), p<0.0001.
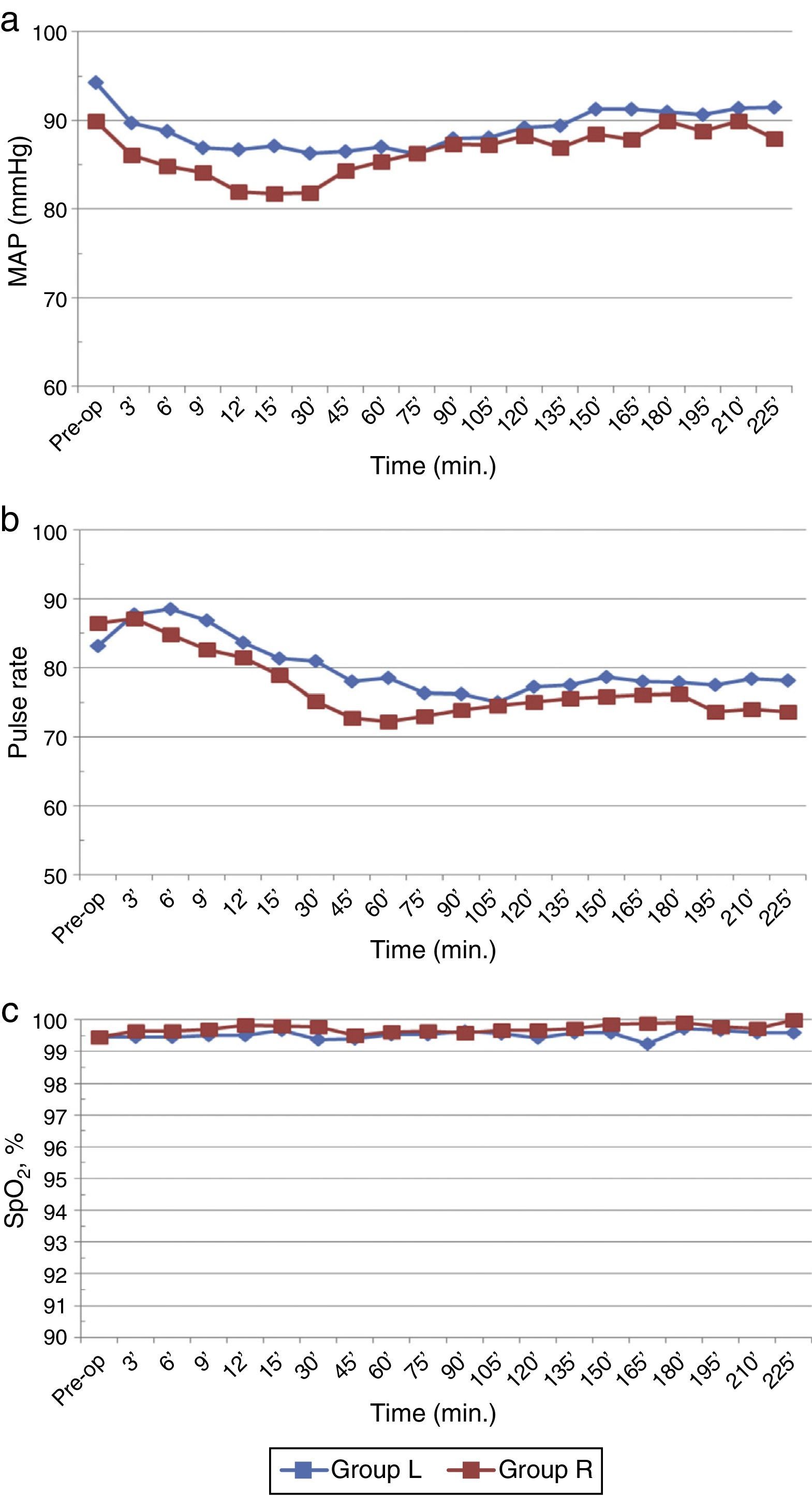
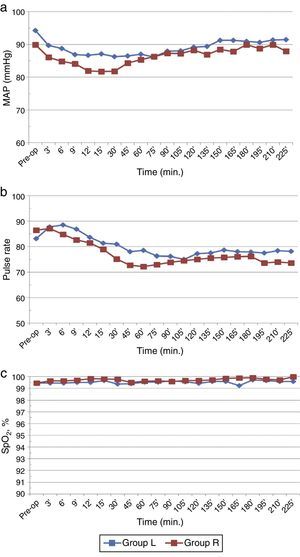
Baseline haemodynamic parameters were comparable in both the groups. The mean MAP decreased significantly in both the groups compared to baseline/preoperative values (p<0.05) but overall incidence of hypotension was not significantly different (Fig. 4a). Furthermore, it was transient (30min) in ropivacaine group compared to levobupivacaine which was sustained (100min). There were no significant differences between the two groups with respect to PR and SpO2 (p>0.05) (Fig. 4b and c).
No incidence of Post Dural Puncture Headache (PDPH) or any other significant adverse effect was observed in either group (Table 3). Hypotension was the most common side effect seen in both the groups, however, total amount of mephentermine used was significantly not different (p>0.05). Bradycardia occurred during intra-op period in 2 patients of each group.
DiscussionIn our study isobaric levobupivacaine showed significantly slower onset of sensory and motor block but with prolonged duration of analgesia compared to ropivacaine.
No significant differences in patient characteristics and baseline haemodynamic parameters were observed between the two groups.
Levobupivacaine and ropivacaine have been produced in order to address the issues of bupivacaine toxicity.1,15 Several studies have been undertaken in the past to evaluate the clinical efficacy and toxicology of these local anaesthetics in different dosage and baricity. Most of these clinical studies suggested that levobupivacaine was slightly less potent than bupivacaine but more potent than ropivacaine.16 Higher potency of levobupivacaine than ropivacaine could partly be explained by its greater lipid solubility and formulation17 which underestimates the active molecules by 12.6% than its racemate.18 However, many recent studies19,20 have found greater than 30% difference in potency which implies that levobupivacaine is actually more potent than ropivacaine. Its potency compared to ropivacaine remained inconsistent and varied from 1 to 1.67.17 So, based on the above facts and various previous studies,7,19,21,22 we assumed levobupivacaine to be 1.5 times more potent than ropivacaine.
Previous authors have used different doses (5–17.5mg) of levobupivacaine.11,12,23,24 Taking into consideration the previous studies,11–13 MLAC and potency ratio we have used levobupivacaine 15mg (5mgml−1) to compare ropivacaine 22.5mg (7.5mgml−1), so as to achieve adequate sensory and motor block for most of the orthopaedic procedures.
There is gross variation in the findings of various authors regarding sensory block onset time. According to some authors, there is no significant difference in onset time.11–13,24 Contrary to this, some are of the opinion that there is significant difference in the onset time of two drugs.23,25,26 However, in the present study, ropivacaine achieved sensory level of T10 significantly faster than levobupivacaine consistent with the past researches.23,25,26 The variations in the finding of these studies could be due to sample size, demographic profile, methodology, drug dose and baricity. Cuvas et al.27 has only taken elderly (>60 yrs) males while Sananslip et al.28 recruited females posted for gynaecological surgery.
In the present study, both groups achieved the median dermatomal height of T7 but levobupivacaine took longer time to achieve maximum block level than ropivacaine. Furthermore, levobupivacaine (T4–T10) showed slightly greater variability compared to ropivacaine (T5–T10). According to most of the authors,12,21,23,26–29 median height attained was in the range of T8–T9 with similar dosing and technique. Nevertheless, few authors11,12 obtained varied results which may be attributed to different doses and baricity of the ropivacaine used in their study.
Similar to the sensory blockade, ropivacaine also showed faster onset of motor blockade compared to levobupivacaine. However, Khaw et al.30 used a measured isobaric preparation of ropivacaine for spinal anaesthesia in right lateral position administered over 60s did not find any significant difference. As we have not measured the specific gravity of the drug in our study, taking into consideration the fact that bupivacaine and ropivacaine are hypobaric at 37°C,31 it can be assumed that the hypobaric nature of our drug, sitting position32,33 and comparatively faster rate of injection29 has resulted in quicker onset of motor blockade.
In our study, sensory (L1) and motor regression of ropivacaine was comparatively faster than levobupivacaine. Various authors in the past obtained similar results with ropivacaine showing faster sensory11,24,25 and motor recovery.13,23,27
Ropivacaine and levobupivacaine, apart from being slightly different in potencies, are assumed to be almost similar in clinical hands.9 But the present study showed that even at equipotent doses of 1.5:1 (Ropi:Levo), ropivacaine offer significantly shorter duration of analgesia compared to levobupivacaine. This was similar to the findings of previous authors who showed early regression of ropivacaine as compared to levobupivacaine.12,23,34 However, Gautier et al.35 documented no difference while comparing 12mg ropivacaine with 8mg levobupivacaine in Caesarean section. Different pharmacodynamic response due to lower dose and different study population seems to be the most reasonable explanation for this discrepancy.
Decrease in MAP and PR are two most frequently encountered complications of neuraxial blocks. In our study, it was observed that fall in BP was transient in ropivacaine group but sustained in levobupivacaine group. In spite of this, there was no significant difference in the overall incidence of hypotension and these were promptly treated without any serious consequences. Further, the total mephentermine dose required in both the groups were comparable (p>0.05). However, the higher incidence of transient hypotension seen with ropivacaine could arise due to quicker attainment of maximum height of block in comparison to levobupivacaine resulting in fall blood pressure. This was in accordance with the opinion of Carpenter et al.36
Extreme care and vigilance were taken to avoid biases by making the study randomised and double blind. However, biases and limitations often creep during research and this study is not an exception. Study design might have led certain degree of biases to sneak in as we used per protocol analysis. An important limitation of our study was that we did not measure the specific gravity of either of the drug. Maintenance of temperature could be a problem in tropical countries which could have influenced the overall results.30 Besides, higher sodium concentration and osmolality of levobupivacaine further increases its density. The quality of anaesthesia was also not measured in the study.
ConclusionWe, therefore, conclude that isobaric levobupivacaine and ropivacaine doses used in the study produce adequate anaesthesia and analgesia for lower limb orthopaedic surgery without any serious side effects. Levobupivacaine produces significantly longer duration of analgesia than ropivacaine when used in a ratio of 0.6:1. Hence, drugs should be used taking into consideration the condition of patient, nature and duration of surgery. Efficacy, toxicity and haemodynamic profile make ropivacaine suitable agent for day care and other surgeries with low threshold for hypotension, while levobupivacaine can be a suitable agent for prolonged surgeries.
FundingNone.
Conflicts of interestNone declared.
Please cite this article as: Athar M, Ahmed SM, Ali S, Doley K, Varshney A, Siddiqi MMH. Levobupivacaína o ropivacaína: un ensayo aleatorio doble ciego controlado con dosis equipotentes en la anestesia espinal. Rev Colomb Anestesiol. 2016;44:97–104.
Clinical Trial Registry Number: NCT02201784.