There are significant developmental differences in physiology and pharmacology in neonates that make the conduct of a safe anesthetic much more challenging in a neonate.
ObjectivesComplete a focused review of the current knowledge of the physiological and pharmacologic differences seen in newborns that impact the safe administration of anesthesia.
MethodsA selective review of literature in developmental changes in physiology and pharmacology was completed.
ResultsThis knowledge acquired in the review was used to establish common principles for the safe administration of anesthesia to newborn patients.
ConclusionIn spite of the persistence of large gaps in our knowledge in this physiology and pharmacology, common modern anesthetic management principles for neonatal surgery have significantly improved clinical outcomes.
Existen diferencias significativas de desarrollo en la fisiología y la farmacología de los neonatos que hacen que sea mucho más difícil llevar a cabo una anestesia segura.
ObjetivosCompletar una revisión focalizada del conocimiento actual sobre las diferencias fisiológicas y farmacológicas observadas en recién nacidos que tienen un impacto en la administración segura de la anestesia.
MétodosSe llevó a cabo una revisión selectiva de la literatura sobre cambios en el desarrollo fisiológico y farmacológico.
ResultadosEl conocimiento adquirido en esta revisión fue usado para establecer principios comunes para la administración segura de la anestesia en pacientes recién nacidos.
ConclusiónA pesar de la persistencia de grandes lagunas en nuestro conocimiento en esta fisiología y la farmacología, los principios modernos y comunes del manejo de la anestesia en cirugía neonatal han mejorado significativamente los resultados clínicos.
One of the most challenging tasks an anesthesiologist will ever face is the provision of safe and effective anesthesia care of a newborn for surgery. Neonatal anesthesia demands a thorough understanding of the rapidly changing physiology and pathology of the neonate and both the pharmacokinetics and pharmacodynamics of the medications used to provide anesthesia. This knowledge is then incorporated into a well planned anesthetic plan of care. Furthermore, great manual skills and continuous experience with these unique challenges presented by neonates are essential for optimal clinical outcome for these vulnerable patients. This selective review aims to provide a brief summary of theoretical aspects of neonatal anesthesia and present some practical guidelines of care for this patient population.
Physiology of the neonateThe respiratory system has to undergo major physiologic changes in function in seconds when transitioning from the fetal to neonatal environment. To facilitate easier passage through the birth canal, the neonate's chest wall (rib cage) is flexible with little calcification of the bones.1 In contrast, the newborns lungs are populated with immature alveoli that contain little elastin; thereby making them stiff and difficult to inflate.2 This combination of flexible chest wall and stiff lung increases the closing volume of the lung and promotes lung collapse.3 Functional residual capacity (FRC) when normalized by body weight is relatively constant from birth through adult life.4 Spontaneously breathing neonates will dynamically compensate for their immature respiratory mechanics with rapid breathing without expiratory pauses and expiratory air breaking through the larynx.5 To overcome these developmental challenges under passive conditions, pediatric anesthesiologists will routinely recruit alveoli after each brief period of apnea and will use positive end-expiratory pressure (PEEP) when mechanically ventilating a neonate to maintain normal lung volumes.
In spite of equivalent FRCs, neonates will rapidly desaturate with apnea even with effective pre-oxygenation with 100% inspired oxygen.6 This principally is due to a doubling of the rate of oxygen consumption when normalized by body weight in comparison to an adult.7 To compensate for this high oxygen consumption, neonates also have twice the alveolar ventilation of an adult. Pediatric anesthesiologists routinely take advantage of this during inhalational induction of anesthesia where the rapid wash in of anesthetics leads to a more rapid induction of anesthesia in the young compared to an adult.8
Anesthesiologists also must understand that neonates are obligate nose breathers and more easily experience airway obstruction under anesthesia due to the relatively larger tongue and more compliant (collapsible) airway soft tissue.9 Similarly, practitioners in neonates realize the importance of the artificial airway diameter because airway resistance increases by the fourth power as radius decreases.10
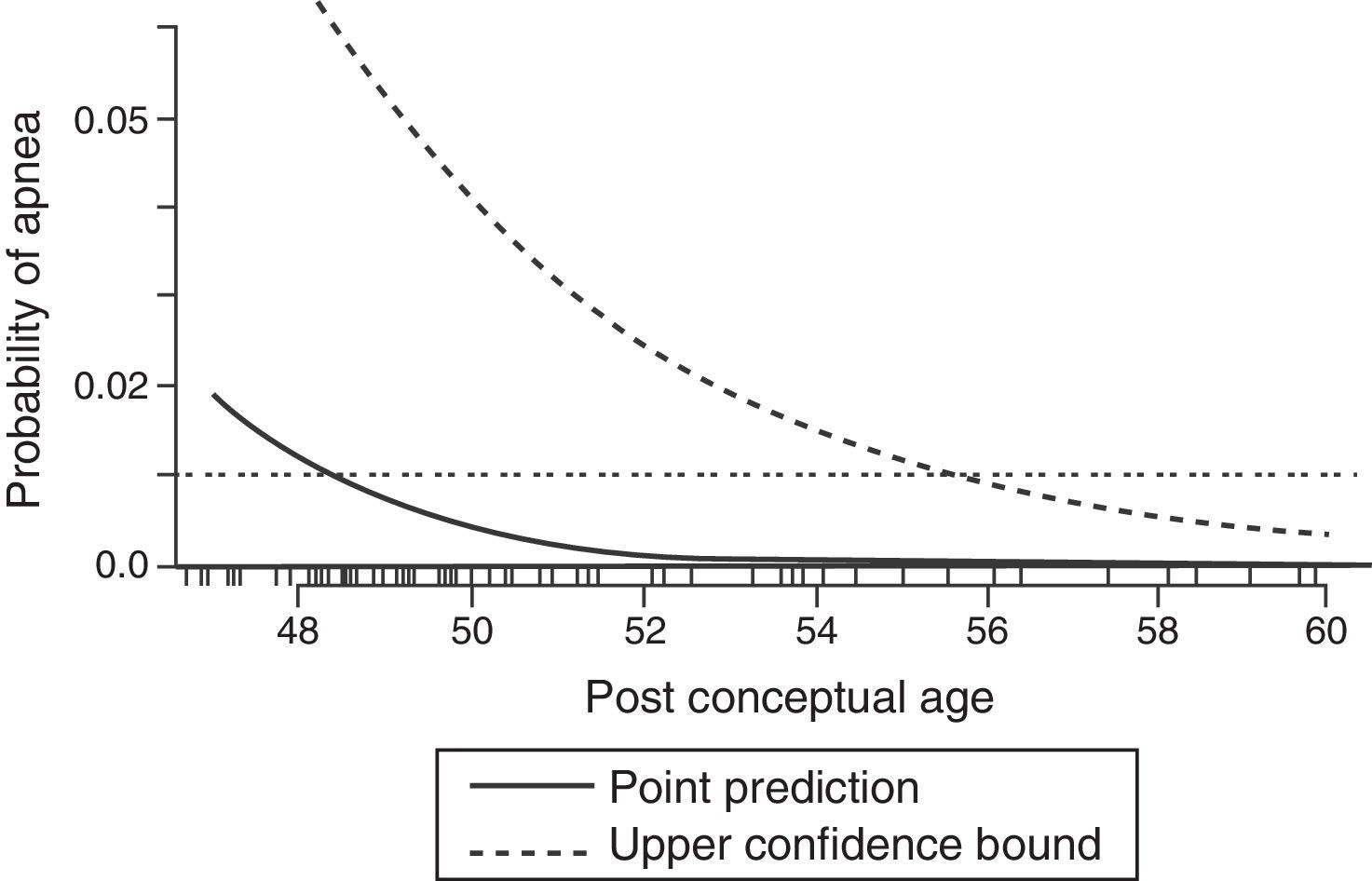
During the neonatal period the control of breathing varies significantly from that seen in older children and adults. The increase in ventilation to hypercapnia is less in a neonate compared to an adult; however, it is the response to hypoxemia that differs dramatically. While an adult will have a sustained increase in ventilation when exposed to hypoxia, neonates will demonstrate a brief increase follow by a sustained depression of ventilator drive when exposed to hypoxemia.11 The immaturity of the respiratory centers in the central nervous system in neonates are likely responsible in part of the pronounced affect anesthetics have in this population. In the only published systematic review of studies evaluating perioperative apnea in this population, general anesthesia increased the risk for postoperative apnea in premature infants less than 60 weeks postconceptional age.12 This risk is further increased by anemia (Hct<30%). Even at 56 postconceptional weeks the risk for postoperative apnea remains approximately 1% (see Fig. 1); therefore, most textbooks and leaders in the field of pediatric anesthesia recommend that patients under this threshold should be admitted to the hospital for monitoring for 12–24h.13
Predicted probability of apnea by postconceptional weeks (solid line) with 95% upper confidence limit (broken line). The 1% risk for postoperative apnea reaches 95% confidence at approximately 56 weeks postconceptional age.
Following birth the circulatory system undergoes dramatic changes from a fetal parallel system characterized by both ventricles pumping the majority of their output into the system circulation to an extra-uterine series system with the right and left ventricles assuming responsibility for the pulmonary and the systemic circulations respectively.14 In addition, the three embryological shunts (foramen ovale, ductus venosus, and ductus arteriosus) will functionally close in the early postnatal period.
Certain neonatal conditions (i.e., birth asphyxia, meconium aspiration, septicemia, and congenital diaphragmatic hernia) may prevent this normal transition in circulation from occurring due to persistent elevation in pulmonary vascular resistance and blood pressure. This persistent fetal circulation (PFC), also known as pulmonary hypertension of the newborn, is characterized by profound hypoxia due to right-to-left shunting through the fetal extra-pulmonary shunts combined with right ventricular strain and circulatory compromise.15 Conventional treatment for this condition includes tracheal intubation and mechanical ventilation to restore normal lung volume without excessive distention, correction of metabolic acidosis, induction of mild alkalosis via hyperventilation, restoration of circulatory volume and inotropic support. Over the last decade the selective pulmonary vasodilatation by means of inhalation of nitric oxide (iNO) has become one the principle treatments for PFC.16 The effective dose range appears to be between 1 and 30 parts per million (ppm). Abrupt withdrawal of iNO can result in sudden severe rebound of pulmonary hypertension so this therapy needs to weaned slowly once initiated. For the few patients that failed to respond to iNO, the short-term use of extracorporeal life support for several days has been shown to achieve 90% survival in patients with a predicted mortality of 50%.17
The neonatal cardiac myocyte contains more non-contractile elements, has a disorganized intracellular arrangement of contractile proteins, and is less elongated than in the adult.18 This leads to a reduction in the force generation capability of the neonatal myocardium. In addition, the sarcoplasmatic reticulum and the T-tubular system are also immature, leading to an increased dependence on extracellular calcium for contraction.19 Developmental changes in the cytoskeleton and the extracellular matrix make the neonatal myocardium less compliant with reduced early and late diastolic filling compared to adults.20 The parasympathetic innervation of the neonatal heart is considered to be more mature compared to the sympathetic system, leading to reduction in heart rate with stimulation. While slow heart rates can reduce cardiac output, the rapid heart rate of the neonate can also limit diastolic filling and stroke volume. After optimizing filling pressures via administration of crystalloid fluid, heart rated between 120 and 180 beats per minute should be the target for neonates.
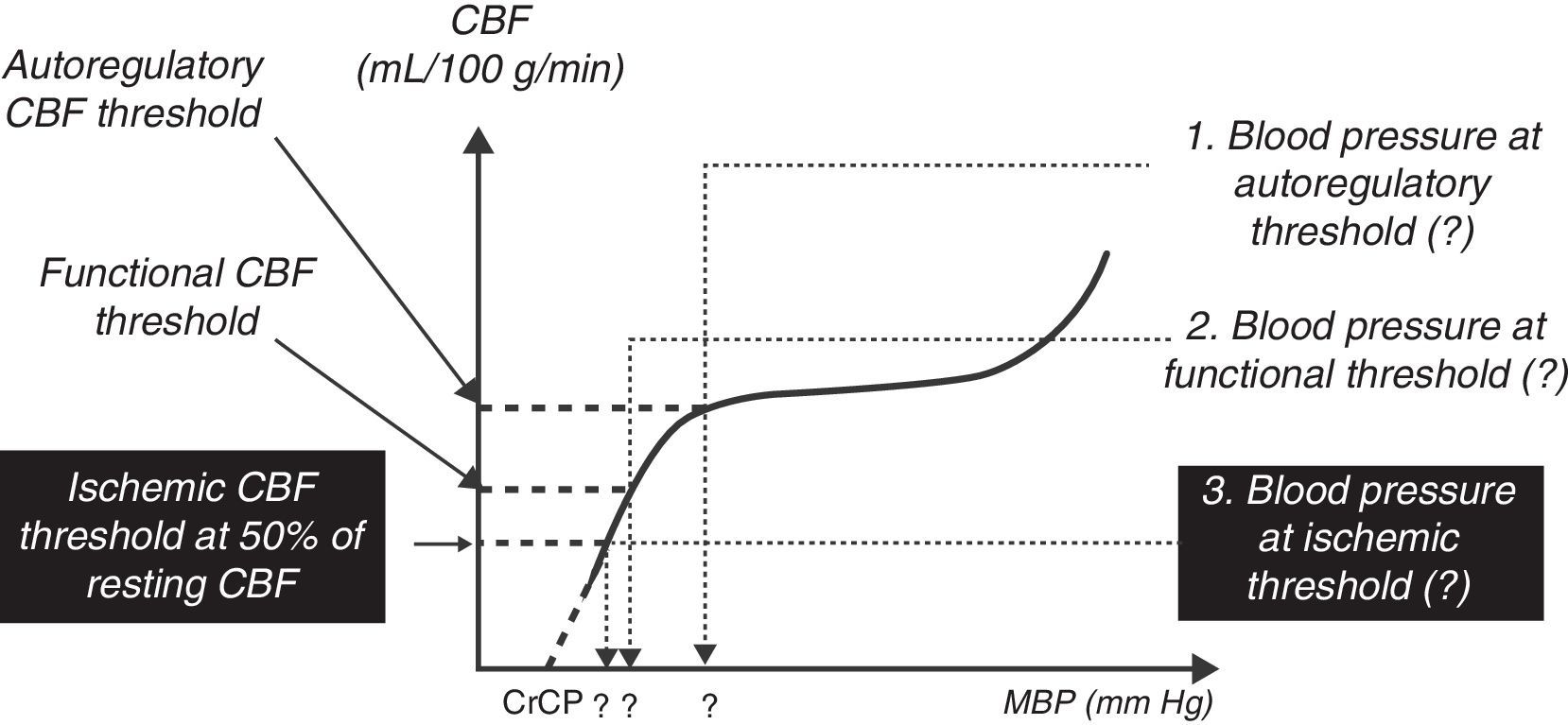
The central nervous system is incompletely developed at birth. However, pain pathways are integrated with somatic, neuroendocrine, and autonomic changes early in gestation. The hormonal responses to pain and stress may be exaggerated in newborns,21 although the clinical importance of this has not yet been defined. Recent studies suggest the cerebral blood flow responses to oxygen and carbon dioxide as well as the autoregulation of blood flow are present in the newborn22,23; however, the range of autoregulation is narrow and very close to the limits of normal blood pressure (see Fig. 2). The lower threshold of autoregulation remains somewhat uncertain. Although an association between the incidence of intraventricular hemorrhage and fluctuations in blood pressure has been noted,24 there has been no confirmation of a causal relationship.
A schematic drawing of the blood flow/mean arterial pressure relation of the normal cerebral circulation in the preterm newborn baby. The flat portion represents the autoregulatory plateau. The lower blood pressure autoregulation threshold is 30mm Hg or less.
Immaturity of the central nervous system also contributes to the development of retinopathy of the premature (ROP). This condition begins as retinal vascular narrowing and obliteration followed by neovascularization, hemorrhage and in the most severe cases retinal detachment and blindness. Infants suffering from ROP frequently require anesthetics for ocular examination and potential laser treatment for hemorrhage and retinal detachment. While the etiology of ROP is likely multifactorial25; oxygen toxicity (perhaps from short-term exposure during brief surgical procedures) contributes to this common complication of prematurity.
Thermoregulation in the neonate differs markedly from the older child and adult. Heat loss is favored by the comparatively larger body surface-to-body weight relationship, the poorly developed insulating subcutaneous tissues, and the inability to use shivering thermogenesis. These limitations are partially compensated for by the unique thermal capacity for non-shivering thermogenesis via brown fat. Both volatile and intravenous anesthetics have been shown to inhibit non-shivering thermogenesis,26,27 potentially contributing to heat loss in the perioperative period. Hypothermia can reduce metabolism of medications, slow emergence from anesthesia and subject neonate to cardiovascular stress. Maintaining a neutral thermal environment (36–37°C) via warming of the operative suite, use of radiant warmers, force air heating units, and warmed intravenous and irrigation fluids are beneficial.28
The total body water is significantly higher in a preterm infant (>80%), term neonate (75%) compared to an adult (60%).29 Extracellular water represents in excess of 50% of a premature infant's body weight. Similarly the blood volume is higher in premature infants (90–100mL/kg) compared to term neonates (85mL/kg) compared to adults.30 Fluid requirements increase the first several days of life (60, 80, 100, 120mL/kg/day at day 1, 2, 3, and 4 respectively), and then remain stable the remainder of the neonatal period (approximately 150mL/kg/day).31 Glomerular filtration rate is low in the term infant, then doubles within the first 2 weeks of life, but does not reach adult levels until 2 years of age.32 Glycogen stores develop in the last several weeks of gestation. Therefore, premature infants are susceptible to hypoglycemia if they are deprived from a source of continuous glucose administration.33
Developmental pharmacologyThe major routes of elimination by which drugs and their metabolites leave the body are the hepatobiliary, renal and respiratory systems; all of which undergo significant maturation after birth.34 Hepatobiliary clearance via the P450 iso-enzymes is present at term and reaches approximately 85% of adult levels by 44 weeks postmenstrual age.35 Protein binding to albumen and α1-acid glycoprotein is reduced in neonates due to lower concentrations of these proteins in the serum.36 This lower protein binding leads to larger amounts of free (unbound) drug which potentiates the risk for adverse side-effects in neonates (particularly local anesthetics).37 These proteins reach approximate adult concentrations at 6 months of age.
Renal elimination of drugs and their metabolites is determined by glomerular filtration, tubular secretion and tubular reabsorption. Glomerular filtration rate (GFR) is only 10% of the mature value at 25 weeks, 35% at term and 90% of the adult value at 1 year of age.32 This reduction has significant implications on dosing intervals of drugs principally eliminated via renal clearance (i.e. aminoglycosides). The same factors that determine anesthetic absorption through the lung (i.e., alveolar ventilation, functional residual capacity, cardiac output, and blood/gas solubility) also contribute to their elimination kinetics.
The pharmacokinetic variables important to anesthesiologists are known. Clearance (CL) is the most important, as it determines maintenance dosing or drug infusion rates. Volume of distribution (V) has relatively minor importance except in relation to loading doses. These parameters determine the shape of the time-concentration curve. The three major sources of pharmacokinetic variability in neonates have been identified (size, age, and organ function).38 Size is the most common co-variable used to determine dose in children even though normal variation in weight with age is large. Size alone is insufficient to predict CL in neonates and infants, the addition of a maturation effect (age) is required. The cause for the slower drug clearance seen in newborns is not limited to just the normal developmental changes in hepatic and renal organ function; compromised organ function due to critical illness in the newborn may also play a significant role in slowing drug CL in neonates.39
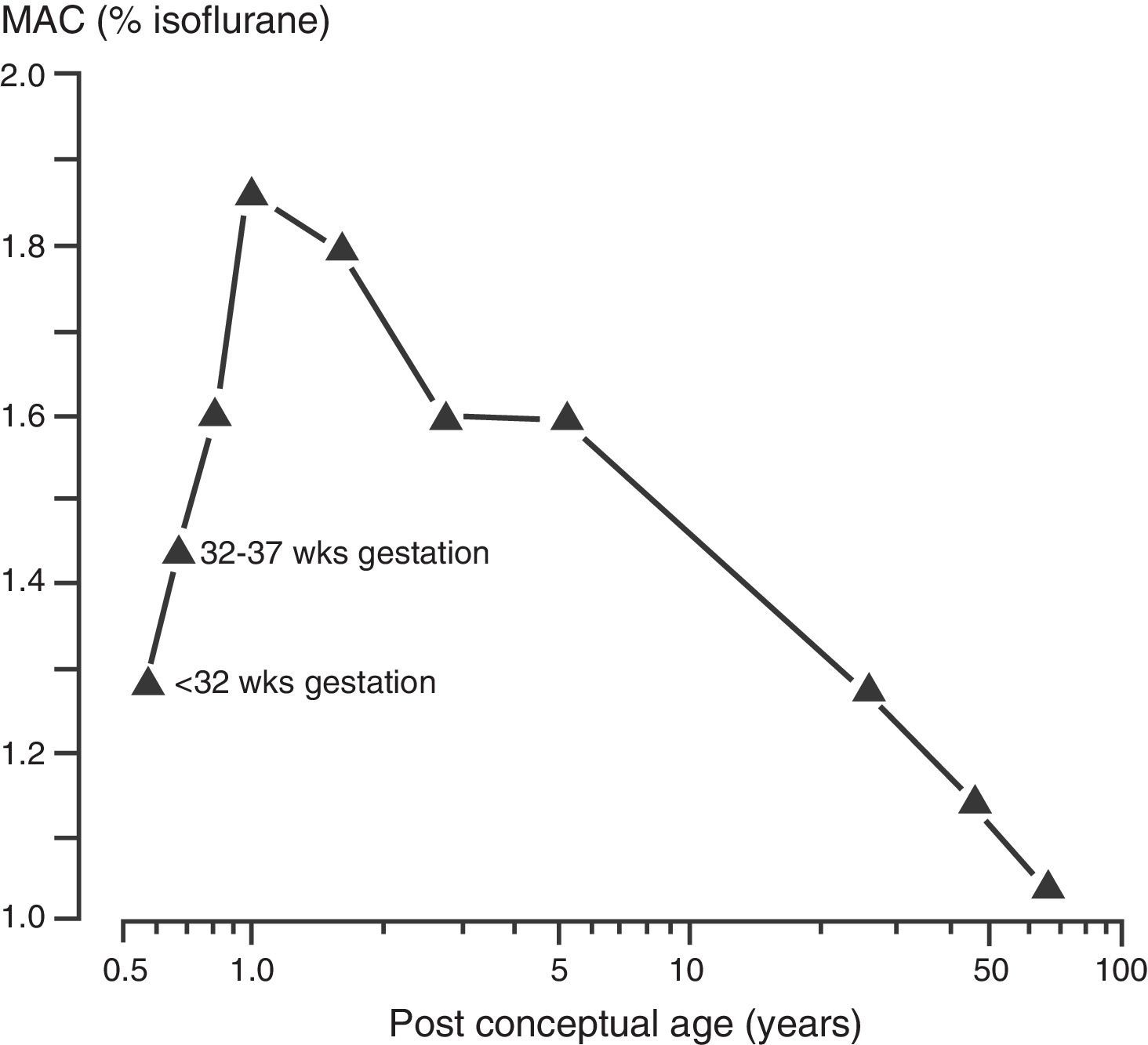
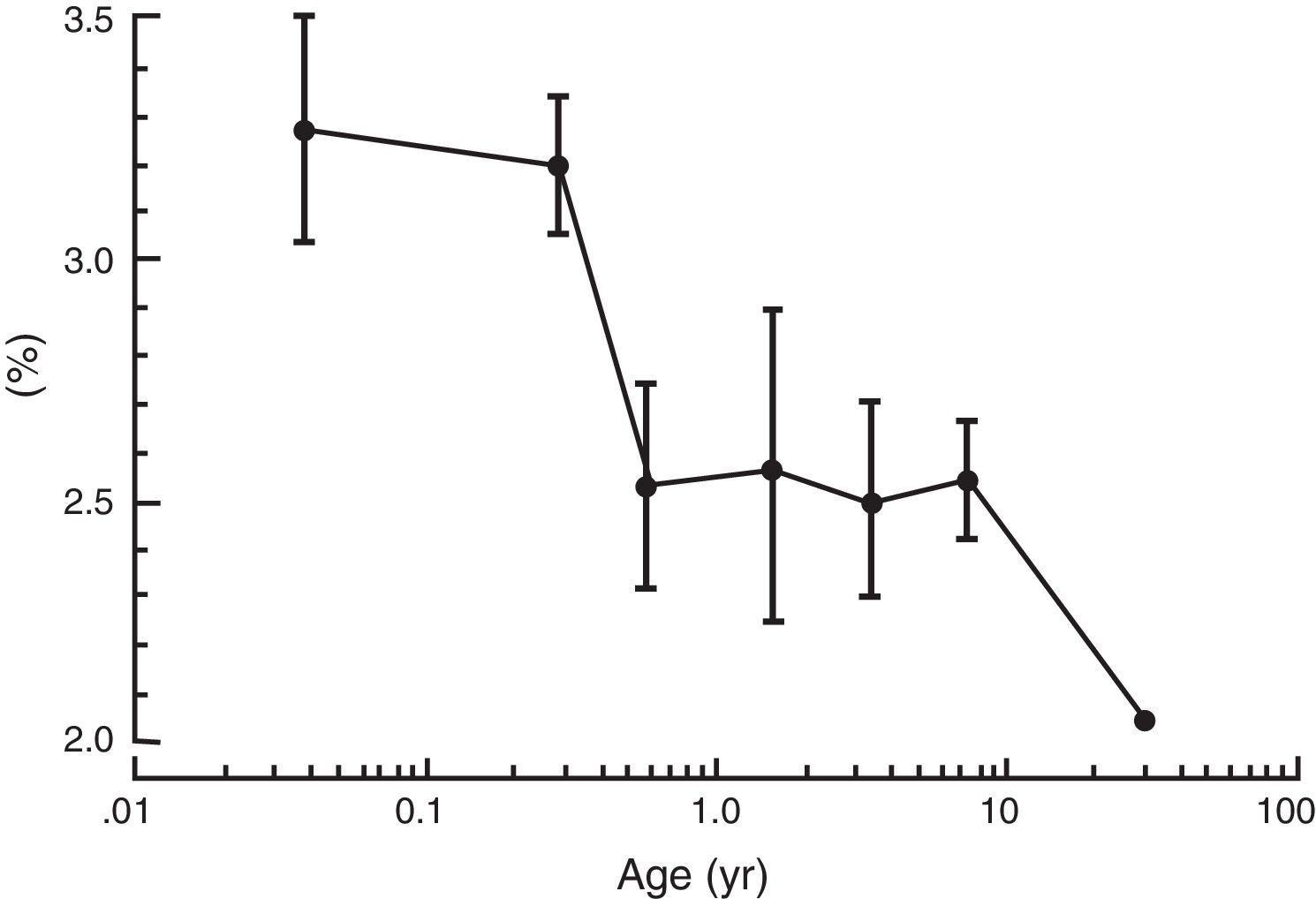
As described earlier, the increase in metabolic rate (i.e. oxygen consumption) leads to an increase in alveolar ventilation and volatile anesthetic uptake in neonates when compared to adults. In addition, an increase percentage of the cardiac output is delivered to vessel rich groups (brain and other vital organs) and the solubility of volatile anesthetics is reduced, both factors contributing to an increase in volatile anesthetic uptake.40 Minimum alveolar concentration (MAC) is commonly used to express anesthetic vapor potency. The MAC typically is reduced in pre-terms and peak at 1–6 months of age before decreasing to adult values in adolescence (see Fig. 3).41 These agents tend to have more myocardial depression in the newborn compared to adults. Contrary to other volatile anesthetic agents, the MAC of sevoflurane is not reduced in pre-term and newborn infants compared to older children (see Fig. 4).42 Thus, the MAC for sevoflurane in the neonate is approximately 3.2%, which is similar to the MAC values for the 1–6 month old infants. The safety of these agents is potentially compromised by the ability to deliver multiple MAC concentrations from the vaporizer and to further increase uptake via excessive minute ventilation with mechanical ventilator support. This problem has been significantly reduced by the elimination of halothane production in developed countries.
The minimum alveolar concentration (MAC) of isoflurane and post-conceptual age.
The mean (± standard deviation) end-tidal concentration of sevoflurane in oxygen for each of the six age groups from neonates to older children up to 12 years of age.
Intravenous agents play a significant part in the delivery of modern anesthesia care to a neonate. It goes without saying the delivery of any intravenous medications requires patient stable access to the circulatory system. Practitioners of anesthesia for neonates must be able to safely establish peripheral and occasionally central venous access for medication delivery.
Unfortunately data regarding the dose-response relationship of most medications is well known in adults and frequently not well understood in neonates. Thus, it is common practice to extrapolate dose guidelines for neonates from adult data. As described previously, neonates generally have reduced serum protein levels which lead to higher concentrations of free (unbound) drug. In addition, reductions in hepatic metabolism and renal elimination generally increase the interval between doses necessary for therapeutic effect.
Because of their more frequent use, the pharmacology and side-effects of narcotics in neonates are more thoroughly understood.43 Morphine has been associated with more hypotension and the development of a prolonged elimination half-life due to its dependence on renal elimination. It was the release of potent, synthetic narcotic fentanyl in the 1980s with its minimum affect on the cardiovascular system that allowed the first safe and effective treatment of surgical pain in the neonate. Larger doses were able to partially suppress the neonatal surgical stress response and improve outcomes (survival) following neonatal surgery.44 The wide spread use of propofol for the induction of anesthesia and facilitation in placement of an endotracheal tube for adults has filtered into neonatal practice as well. Propofol has been shown to more commonly cause adverse hemodynamic effects (hypotension) when administered for placement of endotracheal tubes in critically ill neonates.45 In addition, propofol has delayed redistribution and clearance which lead to prolonged action in neonates. Lastly the long-term continuous infusion of propofol (hours to days) has been associated with the development of metabolic acidosis, organ failure and death in neonates and young children46; therefore, the dose and duration of infusions for sedation should be limited or prohibited to avoid this serious complication.
It is very common to use neuromuscular blocking agents to facilitate patient relaxation during neonatal surgery. The use of a depolarizing neuromuscular agent (succinylcholine) is now typically reserved for emergency placement of an endotracheal tube. Because of its very large volume of distribution, the recommended dose for intubation in a neonate (2mg/kg) is twice that recommended in an adult patient (1mg/kg). In spite of this large dose, the duration of action in not prolonged due to rapid clearance by plasma esterase's. The similarity of structure between succinylcholine and acetylcholine along with the fully mature parasympathetic nervous system of the neonate commonly results in the development of a relative bradycardia with succinylcholine administration. For this reason, many authorities recommend pre-treatment with an anticholinergic agent (atropine or glycopyrrolate).
The response to non-depolarizing neuromuscular agents in neonates is quite variable and more difficult to predict. All of these agents have an increase in their volume of distribution and decrease in their clearance. In addition, the neonatal myoneural junction appears to demonstrate an increased sensitivity to this class of agents. Pancuronium historically has been popular for this patient population due to its vagal lytic (increase in heart rate) properties. However, this use is significantly limited by the long duration of action in neonates. Vecuronium and rocuronium have fewer vagal lytic properties and are reported to by shorter in duration; however, their duration of action remains longer than 60min in most infants.
Perioperative care of the neonateThe anesthesiologist scheduled to care for a newborn must have a reliable comprehensive system to assess the stability of the patient and use this information in the development of their anesthetic plan of care. Important historical information that can be gathered from the medical record includes gestational age, weight, and birth events (including Apgar scores at 1, 5, and 10min). The anesthesiologist should consult with their neonatal and surgical colleagues to ascertain the presence of congenital anomalies. Ultrasound examinations are frequently used to non-invasively identify these anomalies. Finally the anesthesiologist must closely inspect the care requirements of the neonate of the last 24h to ascertain the likely starting point for support in the operative suite.
Physical examination of the infants is required to assess the stability and patency of the neonate's airway and gas exchange. If mechanical ventilator support is needed, close inspections of the placement of the airway on chest X-ray, current ventilator settings and the result of the most recent blood gas are needed. The anesthesia provider must perform a careful assessment for signs or symptoms of cardiovascular abnormalities and stability. Hydration (volume) status should be assessed via vital signs (heart rate, blood pressure), capillary refill and urine output. Any evidence of dehydration should be fully corrected before the induction of anesthesia except in true life-threatening emergent situations. Lastly the anesthesiologist must assess the safety of the current vascular access and associated infusions and determine if the access will be sufficient for the perioperative care.
The last task in the pre-operative assessment is the review of available laboratory studies. Typically complete blood counts are available to assess the risk for anemia associated with significant blood loss and platelet counts to look for evidence of disseminated intravascular coagulopathy. Low platelets counts (less than 100,000) likely indicate intravascular consumption and should prompt further work-up for coagulopathy. Serum electrolytes will assist with acid-base assessment and possible risk for hyperkalemia with rapid blood transfusion. In addition, the serum glucose level during current therapy will help the anesthesia provider lessen the risk for intraoperative hypoglycemia.
Common anesthesia practices in neonates include the routine use of physiologic monitors (ECG, non-invasive blood pressure, temperature, pulse oximetry and end-tidal carbon dioxide). Care must be taken to preserve normal temperature during transport to and from the operative suites and well has heating the operative environment. Continuous glucose delivery throughout the duration of the procedure is required with potential to monitor intermittent serum glucose levels. General anesthesia with placement of endotracheal tubes is generally required for the safe care of the neonate. Anesthesia typically consists of balanced combination of volatile agent supplemented with narcotic (typically fentanyl) for hemodynamic stability. This fentanyl-based anesthetic frequently leads to delayed awakening and the need for post-operative mechanical ventilation. Lung recruitment maneuvers are applied after each period of apnea at ambient pressure to restore normal lung volumes. Mechanical ventilator support routinely includes delivery of PEEP to maintain normal lung volumes with sufficient intermittent positive pressure to maintain normal alveolar ventilation and carbon dioxide clearance.
With these modern advances in anesthesia practice multiple studies over the last 3 decades have shown that the risk of anesthesia-related mortality has decreased significantly over time for both adult and pediatric patients47–50; however, neonates continue to have the highest risk for perioperative cardiac arrest of any age group.51,52 In addition, the risk of mortality following cardiac arrest is much higher in the vulnerable group of patients. It is primarily this evidence of increased anesthesia-related risk that led first to formal (certified) advanced pediatric anesthesia fellowship training programs in the United States and more recently the development of board certification in pediatric anesthesiology by the American Board of Anesthesiology.
Controversies in neonatal anesthesiaIn spite of the dramatic improvements in care for the neonate in the perioperative environment of the last decade there remains many issues without clear evidence for best practice. What is clear is that there remain many opportunities for clinical investigators to closely evaluate current practice options in efforts to further reduce the risk of anesthesia for the vulnerable neonate.
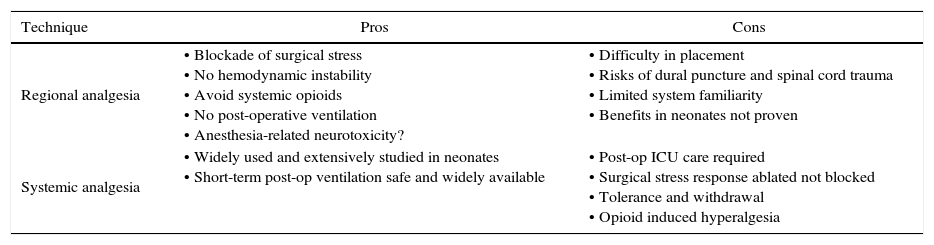
Over the last 15 years many investigators have produced evidence in the laboratory setting that exposure to many different anesthetic agents can potentially induce injury to the developing nervous system with long lasting effects.53 These theoretical concerns regarding anesthesia-related neurotoxicity in infants along with dramatic technical improvements in portable ultrasound has led to a resurgence in the interest and application of regional anesthesia in the neonate. The potential advantages and disadvantages of regional anesthesia as compared to systemic analgesia in a neonate have been debated (see Table 1).54 While there is no clear consensus on the preferred option, it is clear that many anesthesiologists caring for neonates increasingly value the benefits of regional anesthesia in neonates based on the increasing use of this care option.55
A comparison of the pros and cons of regional anesthesia and systemic analgesia in neonates.
| Technique | Pros | Cons |
|---|---|---|
| Regional analgesia | • Blockade of surgical stress • No hemodynamic instability • Avoid systemic opioids • No post-operative ventilation • Anesthesia-related neurotoxicity? | • Difficulty in placement • Risks of dural puncture and spinal cord trauma • Limited system familiarity • Benefits in neonates not proven |
| Systemic analgesia | • Widely used and extensively studied in neonates • Short-term post-op ventilation safe and widely available | • Post-op ICU care required • Surgical stress response ablated not blocked • Tolerance and withdrawal • Opioid induced hyperalgesia |
Source: author.
A comprehensive review summarizes the growing body of evidence that the use of high concentrations of oxygen during neonatal resuscitation is associated with oxidative stress, adverse effects on breathing physiology and cerebral circulation, and potential tissue damage from oxygen free radicals.56 This same evidence has been used to suggestion that short exposures to excessive oxygen in the perioperative period could also lead to adverse outcomes.57 Indeed, some investigators now recommend avoidance of excess oxygen for even brief periods in the perioperative setting with an upper limit of oxygen saturation of 95%.58
FundingThe author did not receive sponsorship to undertake this article.
Conflicts of interestThe author has no conflicts of interest to declare.
Please cite this article as: Martin LD. Principios básicos de la anestesia neonatal. Rev Colomb Anestesiol. 2017;45:54–61.