Ultrasonography performed by non-radiologist specialists is a tool that contributes to the diagnosis and monitoring of neurocritical patients. It is a non-invasive, low-cost, accurate and fast method that helps improve safety and timeliness in settings where prompt decision-making is imperative, such as in the operating room, critical care units or the emergency room.
The objective is to conduct a narrative review, presenting ultrasound applications focused on the central nervous system that may be useful in neuroanaesthesia and neurocritical care. A search was performed of related terms on databases in the medical literature. Relevant papers where selected and reviewed to perform a non-systematic review focusing on the visualization of the midline and lateral ventricles, the measurement of the optic nerve sheath as a surrogate marker of intracranial hypertension, and the use of colour Doppler for visualizing the middle cerebral artery.
It is expected that the use of ultrasound examination of the central nervous system will continue to evolve given its advantages, good correlation with studies considered as the gold standard, and the growing availability of the device. Advancements in this field are expected to improve timeliness and provide objective guidance for decision-making. We recognize the importance of developing skills in the use of this method of exploration in those services where it is required.
La ultrasonografía realizada por especialistas no radiólogos es una herramienta que contribuye al diagnóstico y monitoreo de los pacientes neurocríticos. Adicionalmente es económica, precisa, no invasiva y rápida, lo que mejora la seguridad y oportunidad en escenarios donde la toma inmediata de decisiones es imperativa tales como salas de cirugía, unidades de cuidado crítico o servicios de urgencias.
El objetivo es realizar una revisión narrativa presentando las aplicaciones ultrasonográficas enfocadas al sistema nervioso central (SNC) que pueden ser útiles en neuroanestesia y cuidado neurocrítico. Se realizó una búsqueda en bases de datos de los términos relacionados en la literatura médica. Se seleccionaron y revisaron artículos de relevancia para realizar una revisión no sistemática que se centró en la visualización de la línea media, ventrículos laterales, medición de la vaina del nervio óptico como subrogador de hipertensión endocraneana y en la visualización de la arteria cerebral media a través de doppler color.
Se prevé que continuarán los avances en la exploración ultrasonográfica del SNC debido a sus ventajas, la buena correlación con los estudios considerados como estándar de oro y la creciente disponibilidad de ecógrafo. Es de esperar que se mejore la oportunidad y ayude a dirigir la toma de decisiones objetivamente. Se reconoce la importancia del desarrollo de habilidades en el manejo de este método de exploración para aplicarlo en los servicios donde sea requerido.
Ultrasound performed by non-radiologist physicians has gradually positioned itself as a low-cost, non-invasive, safe, effective and fast tool to facilitate the work of paediatricians,1 emergency medicine and critical care specialists,2 and anaesthetists,3 among others. Routine use of ultrasound improves safety, and timeliness, and could even improve outcomes in different clinical situations.3
The most common applications include ultrasound-guided catheterization,4 abdominal assessment in trauma (Fast) and extended assessment (e-Fast),5 echocardiography,6 screening in aortic disease,7 and procedures such as drainage in ascitis and pleural effusion, and guidance of anaesthetic blockades.
However, different scenarios have been proposed for the use of ultrasound as a tool for solving specific clinical problems: what is the volemic status? Are inotropes required? Is decompressive craniotomy required? Some of the answers to these questions may be elicited by means of new applications such as inferior vena cava measurement for volemic status assessment,8 thoracic ultrasound, critical care protocols,9–11 ocular ultrasound,12 or CNS ultrasound.
This review focuses on relevant CNS applications in anaesthesia and critical care.
MethodologyA search of the terms echography, ultrasound, cerebral midline, CNS ultrasound, cerebral Doppler ultrasound, optic nerve ultrasound was conducted in the medical literature available in the Pubmed database until April 2014. Relevant articles were selected and reviewed with no date restriction and, finally, a narrative review on the applications of ultrasound in the CNS was performed.
Visualization of the midline and ventricles, and of parenchymal brain lesionsCerebral midline shift is one of the severity indicators in neurological disease and it determines surgical management in certain instances. Case reports have shown that it is possible to visualize the midline in the bone window though the temporal bone squama.13 Brain ultrasound requires a low-frequency transducer (1–5MHz) together with the transcranial Doppler software for image optimization.
The technique is essentially the same as in transcranial colour Doppler, except that the structures are visualized in the B mode (Fig. 1). Lateral ventricles and midline may be visualized, and there are reports of midline shifts in patients with stroke, dural haematomas and, occasionally, basal ganglia haemorrhage.
Shunts may be localized using this technique.14,15 It is important to remember that the ultrasound window is not good in up to 15% of cases, preventing the visualization of the brain structures.16
In patients taken to decompressive craniotomy (Fig. 2), the brain parenchyma may be visualized more readily given the absence of artefacts and bone acoustic shadowing. In these patients, the transducer is placed on sites with no bone tissue present, and no specific anatomic landmark is required. There are reports of the ability to follow-up on the size of intracranial haematomas, position shunt catheters,17 monitor midline shifts, or follow-up after treatment.18
Optic nerve measurement and correlation with intracranial hypertensionIntracranial hypertension is a life-threatening condition. It is measured with the help of intracranial devices, but this is associated with complications such as infection, bleeding or dysfunction.19
The search for diagnostic tools associated with less morbidity has focused on non-invasive methods such as nuclear magnetic resonance, computed axial tomography and transcranial Doppler ultrasound. However, there is limited correlation between these methods and specific intracranial pressure values.20,21
The use of ocular ultrasound was first reported in 1965.22 It has recently been proposed that measurement of the optic nerve sheath diameter through the ocular window may be a non-invasive method for the detection of intracranial hypertension.19,23 Moreover, good intra- and inter-observer reproducibility has been demonstrated.24
This measurement is based on the fact that the most distal portion of the nerve has a dural covering known as the optic nerve sheath,25 which becomes dilated as intracranial pressure rises and CSF is distributed through along the dura. These changes are greater in the anterior portion of the nerve sheath, just behind the eyeball, an area that is easily accessible by ultrasound.26–28
Given the late onset of clinical signs in cases of intracranial hypertension, early ultrasound detection allows for prompt therapeutic action, thus contributing to improved outcomes.29,30 Ultrasound is less time consuming when compared with other neuroimaging studies, and eliminates the need to transfer critically ill patients. Moreover, it offers the possibility of assessing response to treatment by means of serial measurements.31–33
A learning curve of 10 measurements with 3 abnormal scans is proposed for physicians with experience in ultrasound, and 25 scans may be adequate for a non-experienced ultrasonographer.32
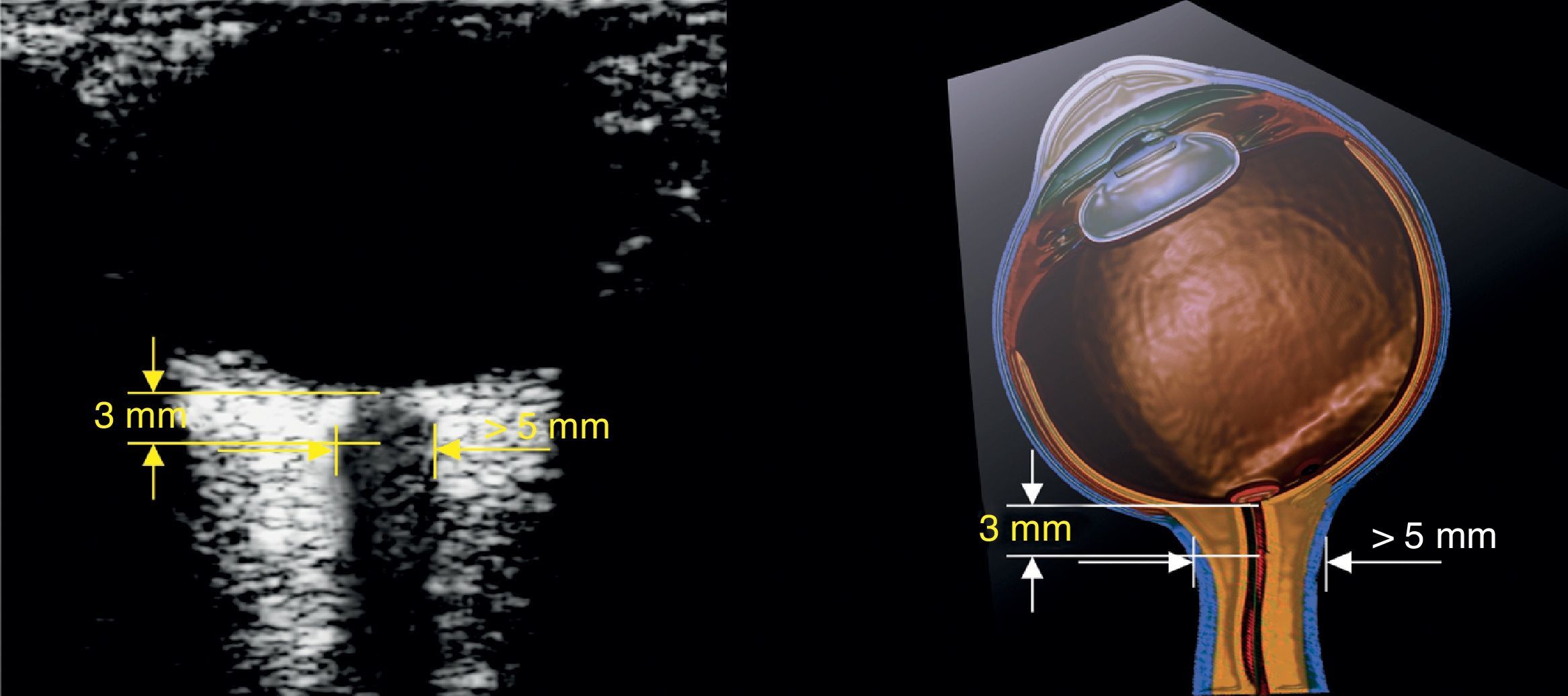
How to measure the optic nerve sheathA high-frequency (7–10MHz) linear transducer is required.22 The ultrasound machine is set to visualize structures up to 5–6cm deep. The transducer is placed over the closed eyelid after generous gel application.
The optic nerve is identified as the hypoechoic structure traversing along a regular course behind the eyeball. For measurement, a vertical line is drawn from the junction between the optic nerve and the eyeball. This line serves just as a reference and must be 3mm long. Once the 3mm length is established, a horizontal line is drawn across the optic nerve. This second line provides the measurement of the optic nerve in mm (Figs. 3 and 4).32,33
For most of the authors included in the review, 5mm is the cut-off point for determining that the scan is positive for intracranial hypertension; other authors propose different values (Table 1).
Adapted from Hasan.23
| Study | Optic nerve diameter (mm) | % Sensitivity | % Specificity |
|---|---|---|---|
| Blaivas et al.19 | 5.0 | 100 | 95 |
| Goel et al. | 5.0 | 98.6 | 92.8 |
| Tayal et al.19 | 5.0 | 100 | 63 |
| Kimberly et al. | 5.0 | 88 | 93 |
| Moretti et al. | 5.2 | 93.1 | 73.8 |
| Moretti et al. | 5.2 | 94 | 76 |
| Geeraerts et al.27 | 5.9 | 95 | 79 |
| Geeraerts et al.27 | 5.9 | 87 | 94 |
| Soldatos et al. | 5.9 | 74.1 | 100 |
| Major et al. | 5.0 | 100 | 86 |
Worth mentioning is a systematic review published by Dubourg et al.33 in 2011 in which the authors assess the diagnostic accuracy of optic nerve measurement compared with the invasive measurement of the intra-parenchymal pressure as the gold standard; 6 prospective cohort studies totalling 231 patients were included, but no significant heterogeneity was found for the sensitivity or the specificity of the ultrasound measurement.33 The sensitivity and specificity of the optic nerve sheath measurement are 0.90 (95% CI, 0.80–0.95) and 0.85 (95% CI, 0.73–0.93), respectively. This review also showed a reliability of 0.2–0.3mm among reviewers. The problem was the lack of an accurate cut-off point for defining optic nerve sheath dilatation in all the studies. The measurement of the optic nerve sheath diameter has been found to have good diagnostic accuracy for detecting intracranial hypertension and influences the decision of patient referral to specialized centres.33
There are authors who are against the use of optic nerve measurement for determining intracranial hypertension.34 However, the absence of correlation so far does not seem to be associated with the technique or the pathophysiological process but rather with the lack of a standard cut-off point suggesting the boundary between normalcy and hypertension.35 There is also a need to standardize the scanning technique because, as a sphere, the eye may be scanned longitudinally or cross-sectionally and there is no way of knowing how this may affect the results.24,35–37
To solve this issue, the PROSPERO initiative was created in 2013 with the aim of assessing the diagnostic accuracy of ultrasound measurement of the optic nerve sheath for the detection of intracranial hypertension and the establishment of an accurate cut-off point for the creation of an individualized patient database.38
Transcranial colour DopplerThe sonographic approach to the intracranial arteries poses greater difficulties when compared with optic nerve or midline assessment. The learning curve is steep and a good sonograhic window will not be achieved in 15% of patients, making access to that form of imaging difficult.16
This may be overcome in part with the use of transcranial colour Doppler, which requires a machine equipped with a 1–5MHz microconvex transducer and colour Doppler functionality. The sonographic window is obtained through the squama of the temporal bone (Fig. 5). A second window may be performed through the eye in order to find the ophthalmic artery, with a third window through the post-auricular area in order to access the posterior cerebral artery. The use of the colour function allows visualization of the intracranial arteries, in particular the middle cerebral artery38 (Fig. 5).
The two most widely accepted applications for transcranial Doppler ultrasound are vasospasm control in patients with subarachnoid haemorrhage (Class IIa evidence),16,38–47 and brain death confirmation (Class IIa evidence).39–48 Other applications, although with limited evidence, include the use of flow velocities in the main cerebral arteries as a means to monitor brain haemodynamics.42–45 The use of the relationship between the middle cerebral artery flow and velocities as measured by Doppler and the mean arterial pressure has been proposed for assessing the integrity of cerebral autoregulation.46
Of interest in neurocritical care and neuroanaesthesia has been the possibility of having a way to monitor critically ill patients in the intensive care room or in the operating room. Transcranial colour Doppler has the invaluable potential of providing non-invasive, real time and dynamic information about modifiable brain haemodynamic variables that may have an impact on patient outcomes.49,50
ConclusionsCentral nervous system ultrasound performed by non-radiologist specialists is an increasingly available low-cost, versatile and accurate approach that helps improve timeliness and objective decision-making. These applications are expected to expand greatly in the near future and to offer improved correlation with specific pathophysiological processes that influence decision-making in neurocritical patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThe authors did not receive sponsorship to undertake this article.
Please cite this article as: Ochoa-Pérez L, Cardozo-Ocampo A. Aplicaciones de la ultrasonografía en el sistema nervioso central para Neuroanestesia y cuidado neurocrítico. Rev Colomb Anestesiol. 2015;43:314–320.