Supraclavicular block is usually performed using a lateral to medial approach, although a medial to lateral approach is also feasible. Block onset may be evaluated through the sympathetic effect associated with the sensitive and motor blockade.
ObjectiveTo describe the ultrasound-guided supraclavicular block using a medial approach, evaluating the sensitive, motor, and sympathetic block onset.
Materials and methodsAn ultrasound-guided supraclavicular block was performed in a fresh cadaver with 20ml volume (2ml of iodine and 1ml of methylene blue). A CT scan was performed and sagittal sections were obtained. The clinical phase included 10 patients undergoing a medial approach block; the onset of the block was evaluated based on a motor, sensory and sympathetic assessment (measuring flow changes in the humeral artery, the palmar temperature, and the perfusion index).
ResultsAdequate distribution of the contrast medium was observed in the cadaver, with complete spread through the brachial plexus, both in terms of the CT-reconstruction as in the anatomical cross sections. A significant change in all the sympathetic block parameters was observed 5min after the bock: temperature (32.5±1.8°C to 33.4±1.7°C; p=0.047), humeral arterial flow (105±70ml/min to 192±97ml/min; p=0.007), and thumb perfusion index (5±3 to 10±3%; p=0.002). The block was effective and uneventful in all patients.
ConclusionsThis supraclavicular approach achieves a homogeneous distribution throughout the brachial plexus, with high anesthetic efficacy. Regional changes secondary to the sympathetic block occur early after the block.
El bloqueo supraclavicular habitualmente se realiza mediante abordaje lateral a medial, si bien puede realizarse de medial a lateral y su instauración puede evaluarse por el efecto simpático asociado al bloqueo sensitivo y motor.
ObjetivoDescribir el bloqueo supraclavicular ecoguiado por abordaje medial evaluando la instauración del bloqueo sensitivo, motor y simpático.
Materiales y métodosSe realizó el bloqueo supraclavicular ecoguiado en cadáver fresco con 20ml de volumen (con 2ml de yodo y 1ml de azul de metileno). Se realizó una tomografía computarizada y posteriormente cortes anatómicos sagitales. En la fase clínica se incluyeron diez pacientes a quienes se les realizó el bloqueo y posteriormente se evaluó la instauración del bloqueo con valoración sensitiva, motora y simpática (cambios en flujo arterial humeral, temperatura palmar y el índice de perfusión).
ResultadosEn el cadáver se evidenció una adecuada distribución del medio de contraste bañando la totalidad del plexo braquial, tanto en la reconstrucción tomográfica como en los cortes seccionales anatómicos. A los 5 minutos del bloqueo se observó un cambio significativo de todos los parámetros de bloqueo simpático: temperatura (32,5±1,8°C a 33,4±1,7°C; p=0,047), flujo arterial humeral (105±70ml/min a 192±97ml/min; p=0,007) e índice de perfusión del pulgar (5±3 a 10±3%; p=0,002). El bloqueo fue efectivo en todos los pacientes y sin complicaciones.
ConclusionesEl abordaje supraclavicular propuesto logra una correcta distribución en el plexo braquial con elevada eficacia anestésica. Los cambios regionales secundarios al bloqueo simpático son precoces tras el bloqueo.
The supraclavicular access of the brachial plexus (BP) is an option for upper extremity surgical procedures.1–5 Initially, supraclavicular block (SCB) was widely popularized by Kulenkampff5 as a result of its high effectiveness since all the trunks of the plexus are closely bundled above the first rib and behind the pulse of the subclavian artery. Multiple supraclavicular (SC) approaches to the brachial plexus (BP) were described during the era of neurostimulation, based on the cutaneous point of entry and the direction of the needle.5–8 The perivascular technique described by Winnie relied on the idea of the neurovascular sheath to account for the clinical behavior and the efficacy of the block through a single point injection of the anesthetic agent, allowing for a distribution among the trunks and the divisions of the plexus.6 However, the high incidence of complications and side effects (arterial puncture, dysphonia secondary to recurrent laryngeal nerve block, hemi diaphragmatic paralysis due to ipsilateral phrenic nerve block, Horner's syndrome, pneumothorax as a result of accidental pleural puncture, and peripheral nerve injury neuropathy) led to the discontinuation of the technique.9
The SC approaches were reintroduced after the advent of ultrasound because of a significantly improved safety from visualizing the potentially vulnerable anatomical structures.9–15 The visualization through the short axis of the supraclavicular plexus is achieved using a lineal probe and using an in-plane approach the needle may be directed from lateral to medial (external to internal) until it reaches the lower trunk “corner pocket”. However, a medial to lateral (internal to external) approach is also feasible. Whilst this latter approach has been reported, the references about the description of the technique and final needle position are limited.9,12,14
Traditionally, the onset of regional blocks is determined through clinical parameters that evaluate the patient in response to a sensitive stimulus (cold or piercing) and the muscle contraction capacity (motor).4,9–11 When blocking the sympathetic nerve fibers, vasodilatation and increased blood flow of the blocked extremity follows. These regional hemodynamic alterations can be measured non-invasively via the skin temperature (T°), the perfusion index (PI), and the humeral arterial flow (HAF).16–19 These changes have not yet been reported with respect to the SCB.
The purpose of this trial was to present an anatomical description of the ultrasound-guided supraclavicular block using a medial approach (MSCB) in a cadaver, evaluating its clinical evolution through a sensitive, motor, and sympathetic regional evaluation in an initial group of patients.
MethodologyPhase one: anatomicalUpon approval by the appropriate Ethics and Scientific Committee (ESC), ultrasound-guided MSCB was performed on a cadaver, using a high frequency lineal probe (6–13MHz) and a portable ultrasound machine (HFL 38X and M Turbo; Sonosite Inc., Bothell, WA, USA). A 50mm neurostimulation needle (Stimuplex D, Braun, Mengusen, Germany) was used. The ultrasound probe was initially placed parallel to the clavicle inside the supraclavicular fossa (obtaining an intermediate plane involving the three axes: axial, sagittal, and coronal) to identify the BP at the SC level with its three trunks (upper, middle and lower). With the pleura protected by the first rib and the subclavian artery above the rib, the needle was advanced from medial to lateral, passing between the subclavian artery (medial) and the BP (lateral). The objective was to reach the area proximal to the lower trunk of the BP under direct vision of the tip of the needle and, at this point, lateral to the subclavian artery and medial to the brachial plexus, 20ml of solution (17ml NaCl 0.9%+2ml of iodine contrast+1ml methylene blue) were administered (Fig. 1). A CT-scan of the SC area was obtained for future digital reconstruction and identification of the distribution of the contrast medium. Later on, the anatomical specimen was frozen (at −20°C over 48h) and sagittal sections were obtained (2–2.5cm) and photographed for evaluating the distribution of the contrast medium administered with respect to the BP.
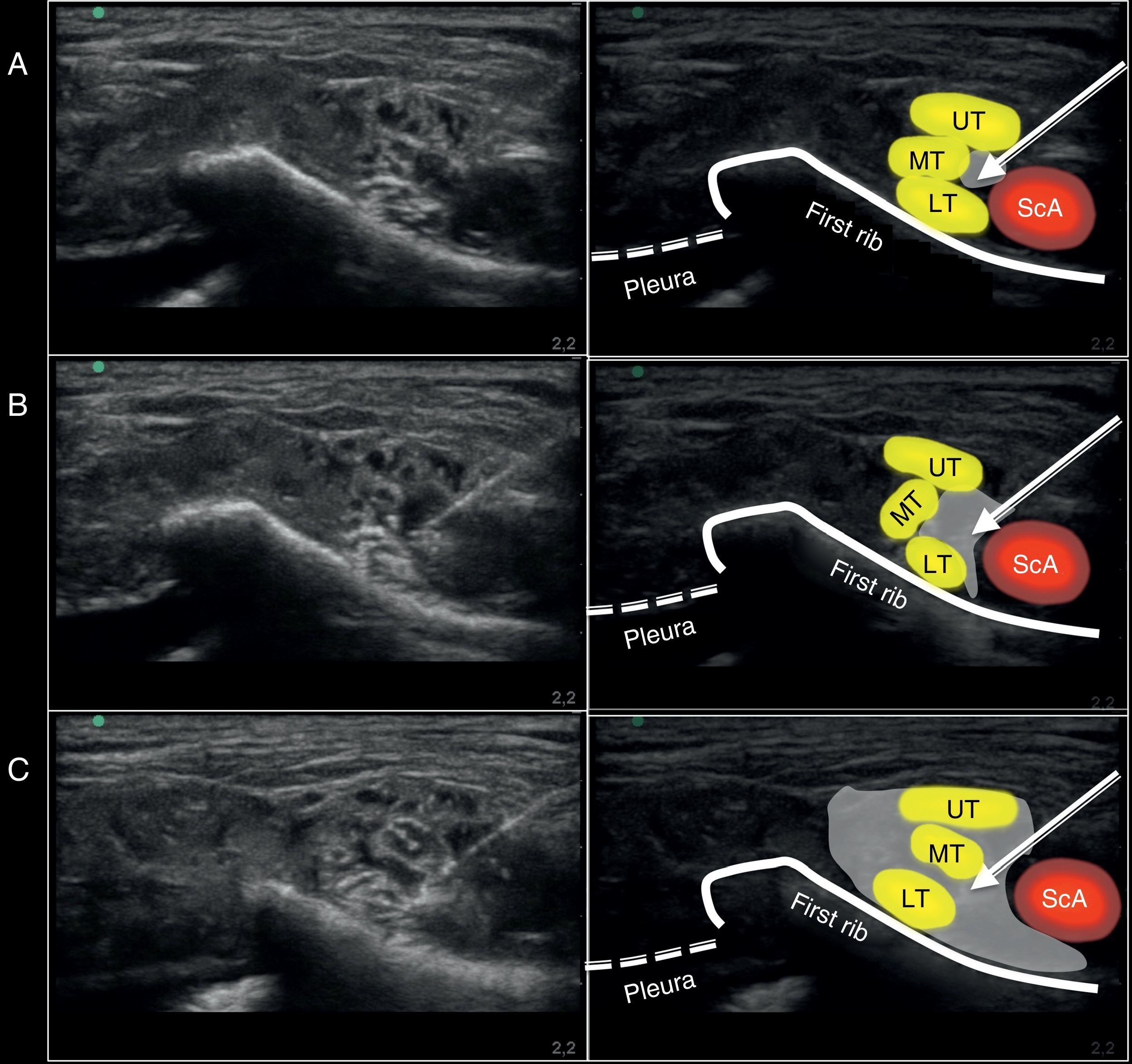
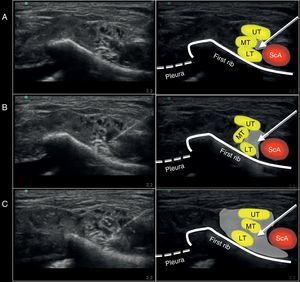
Ultrasound image captured during the administration of the supraclavicular block using a medial approach on the anatomic specimen. (A) Position of the needle at the injection site. (B) Ultrasound image during the 10ml injection. (C) Ultrasound image after completing the 20ml injection. ScA, subclavian artery; UT, upper trunk; MT, middle trunk; LT, lower trunk. The arrow points toward the position of the neurostimulation needle. The gray shade shows the distribution of the volume injected at the injection site evaluated with ultrasound.
An observational study was done of a series of cases with prior approval by the appropriate ESC and following acceptance and signature of the participant's informed consent. A MSCB was then performed in 10 adult ASA I–III patients that required upper extremity surgery. The exclusion criteria were as follows: patients with peripheral vascular disease, allergy to local anesthetic agents (LA), pregnancy, and coagulopathies.
The MSCB was administered in the blocks room (constant room temperature of 24°C). The patients underwent constant non-invasive monitoring, and intravenous access was placed on the opposite upper extremity, with 1.2mg of midazolam administered as needed, prior to the block. A pulse oximeter was placed (Oxy-100 pulse oximeter, GIMA S.p.A. Italy) on the thumb of the hand of the same side to be blocked and another one was placed in the contralateral thumb to measure the pulsatility index % (PI). The PI represents the relationship between the pulsatile and the non-pulsatile component of the pulse oximetry wave.16 Additionally, a palmar cutaneous thermometer was placed on the metacarpal of the third digit on both hands (Phillips Medical Systems, Eindhoven, The Netherlands; ±0.1°C reliability) for temperature recording (°C). A SonoSite M Turbo ultrasound machine and a lineal probe HFL 38X/13–16MHz (Sonosite Inc, Bothell, WA, USA) were used. Measurements were taken of the diameter (mm) and the HAF (ml/min) 2cm proximal to the elbow fold using pulsed-wave Doppler on the extremity to be blocked and the basal diameter and HFA values were determined, in addition to PI and T°. The patient was placed in supine decubitus with the head turned toward the opposite side of the block and the hand pointing toward the knee in order to pull the clavicle down. The SC area was aseptically cleaned followed by a cutaneous infiltration at the puncture site with 1ml of 2% lidocaine to proceed with the block as described in the anatomical phase. A neurostimulation needle (0.71×50mm and 22G×2in., Stimuplex D, Braun Inc., Melsungen, Germany) was used in every case; neurostimulation was used (neuroestimulator Stimuplex HNS 12; Braun Inc., Melsungen, Germany) for safety considerations to make sure that the local anesthetic was administered without any motor stimulus at an intensity equal to, or lower than, 0.3mA (with stimulation parameters of 2Hz and 100μs duration). 20ml of 1.5% mepivacaine were administered in every case.
Time was recorded immediately after injecting the LA and this was considered the onset of post-block time. PI and T° values were recorded after 5 and 15min, and the humeral diameter and humeral flow were measured 5min post-block.
The sensory function in response to a pin prick at C6 was evaluated (dorsal aspect of the inter-metacarpal area between the 1st and 2nd digit), C7 (tip of the 3rd digit), C8 (tip of the 5th digit), on a 0 to 3-points scale (0: no sensation at all, 1: painless sensation, 2: mild pain sensation, and 3 the patient had a normal sensation). The motor function was evaluated at the level of C6 (forearm flexion), C7 (forearm extension), and C8 (digit abduction), in a scale from 0 to 3 points (0: absence of strength, 1: decreased strength, and failed to overcome gravity, 2: decreased strength and able to overcome gravity, and 3: normal motor strength). Recordings were made after 5, 15, 25, and 35min post-block. For clinical purposes, surgical block was achieved when the sensitive and motor block values were 0 or 1 in all areas. The occurrence of post-block neurological deficit was evaluated after 24h via a telephone call, and at 7 and 30 days in person.
The aim of the study was to describe a wide series of cases. This observational study was intended to evaluate the feasibility of the technique described, considering an initial sample of 10 patients as appropriate. The data were analyzed using the SPSS statistical tool (IBM: Statistical Package for the Social Sciences) and presented in terms of number of patients, mean and interquartile, or means and standard deviation, according to the cases. Comparative intra-group studies were done (basal and post-block) using non-parametric testing of repeated measures (Wilcoxon, Chi square). p<0.05 was considered significant.
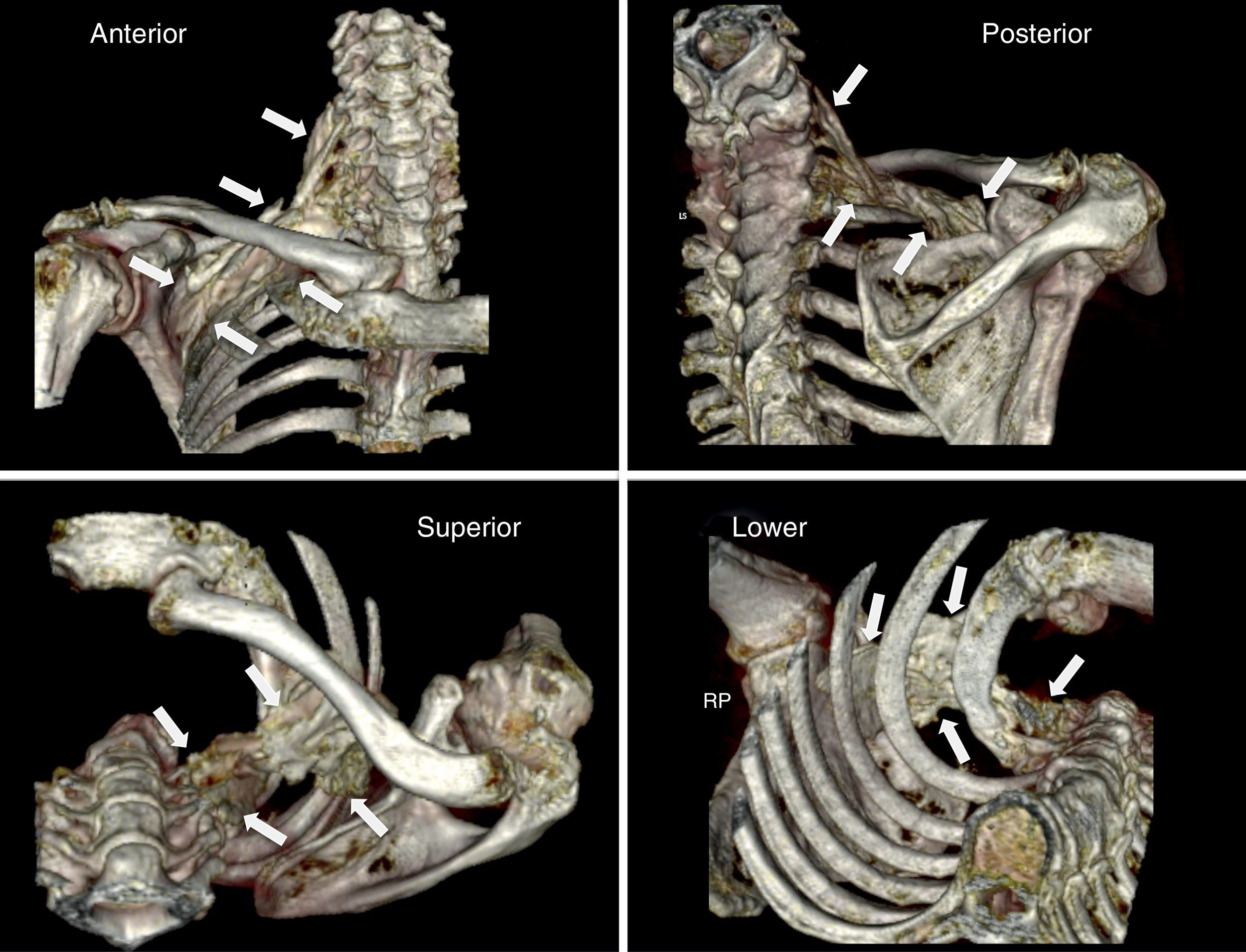
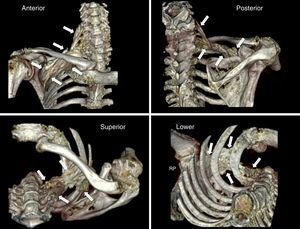
ResultsPhase one: anatomicalThe technical procedure developed uneventfully. The CT-scan 3-D reconstruction showed the periclavicular–cranial spread of the contrast medium (toward the roots of the plexus: C6, C7, C8, and T1) and caudal (at the infraclavicular level to a point next to the choracoid process) (Fig. 2).
3-D reconstruction (scanner) of the distribution of the contrast medium following a medial supraclavicular block on the anatomical specimen. The white arrows indicate the limits of the diffusion. Please note the craniocaudal distribution from the supraclavicular area to the infraclavicular region of the brachial plexus, in proximity to the choracoid process.
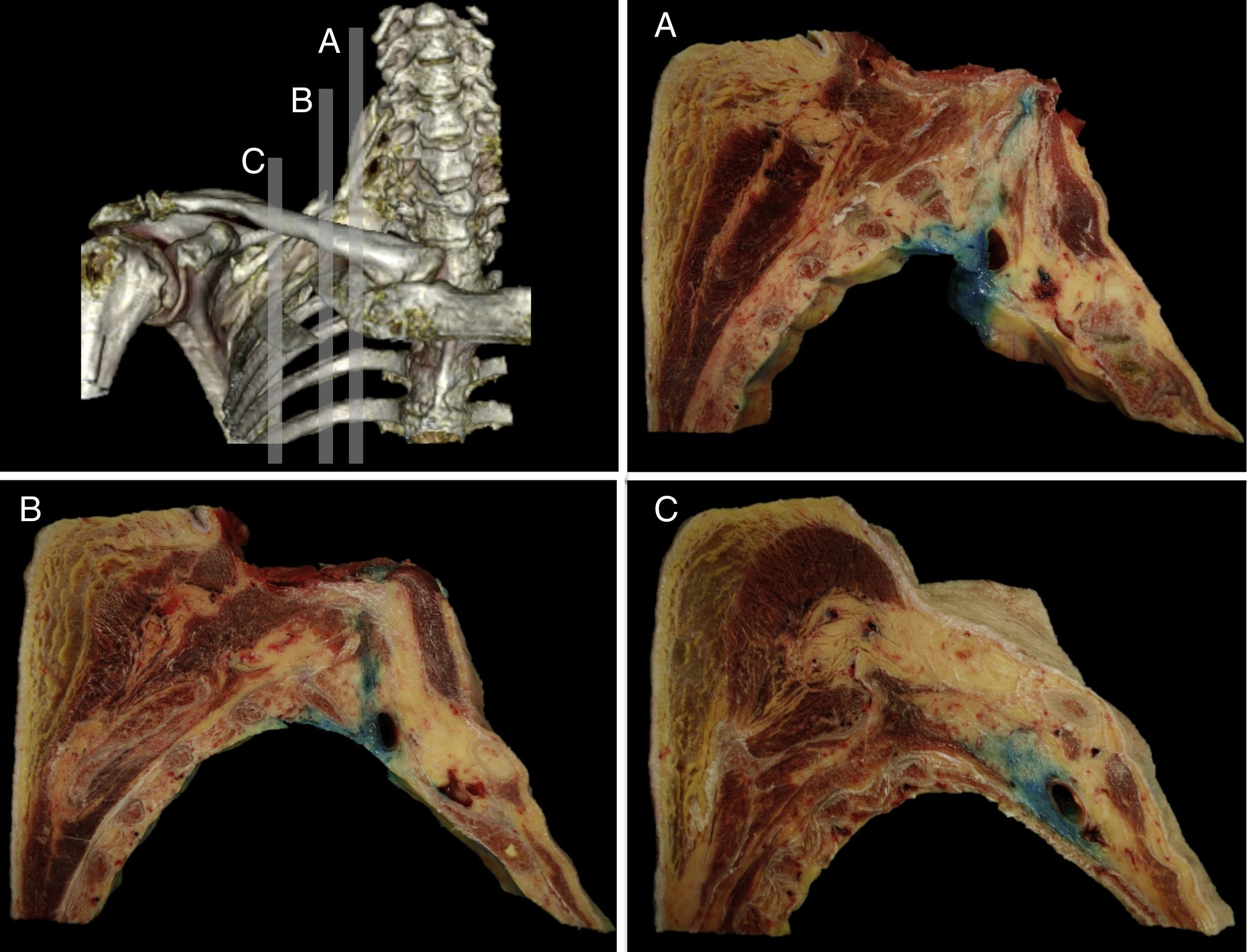
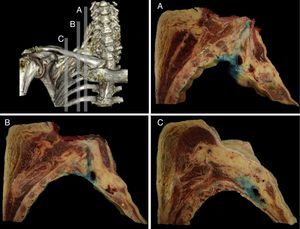
The distribution was further verified with the sagittal sections performed, identifying the neurovascular complex and the anatomical relationship of the BP at the periclavicular level. The contrast medium spread throughout the SC region surrounding the upper, middle, and lower trunks (Fig. 3) and longitudinally spread from the roots of the BP (interscalene), to the secondary trunks (infraclavicular).
Sagittal anatomical sections from the anatomical specimen showing the methylene blue diffusion from the supraclavicular interscalene region (A), at the puncture point (B), and the medial infraclavicular aspect of the choracoid process (C). In every case, the methylene blue distributed around the neurovascular components of the brachial plexus.
10 ASA I–III patients were included for upper limb surgery (4 cases of shoulder and proximal humerus surgery, 1 case of elbow surgery, and 5 hand surgeries). Seven of the 10 patients were females and three males, with mean age of 56±15 years, 68±10kg of body weight, 163±11cm, with a BMI of 26±3.
The technique was performed without any associated secondary complications (vessel puncture, pneumothorax, hemi-diaphragmatic paralysis) in the 10 patients, with a 9±4-min duration. The affected extremity was the right side in 4 patients and the left in 6 patients. During the needle manipulation one of the patients reported paresthesia that was immediately resolved after removal of the needle. The motor response to neurostimulation exhibited an intensity of >0.3mA in 8 patients (80%). None of the patients developed post-block neurological deficit.
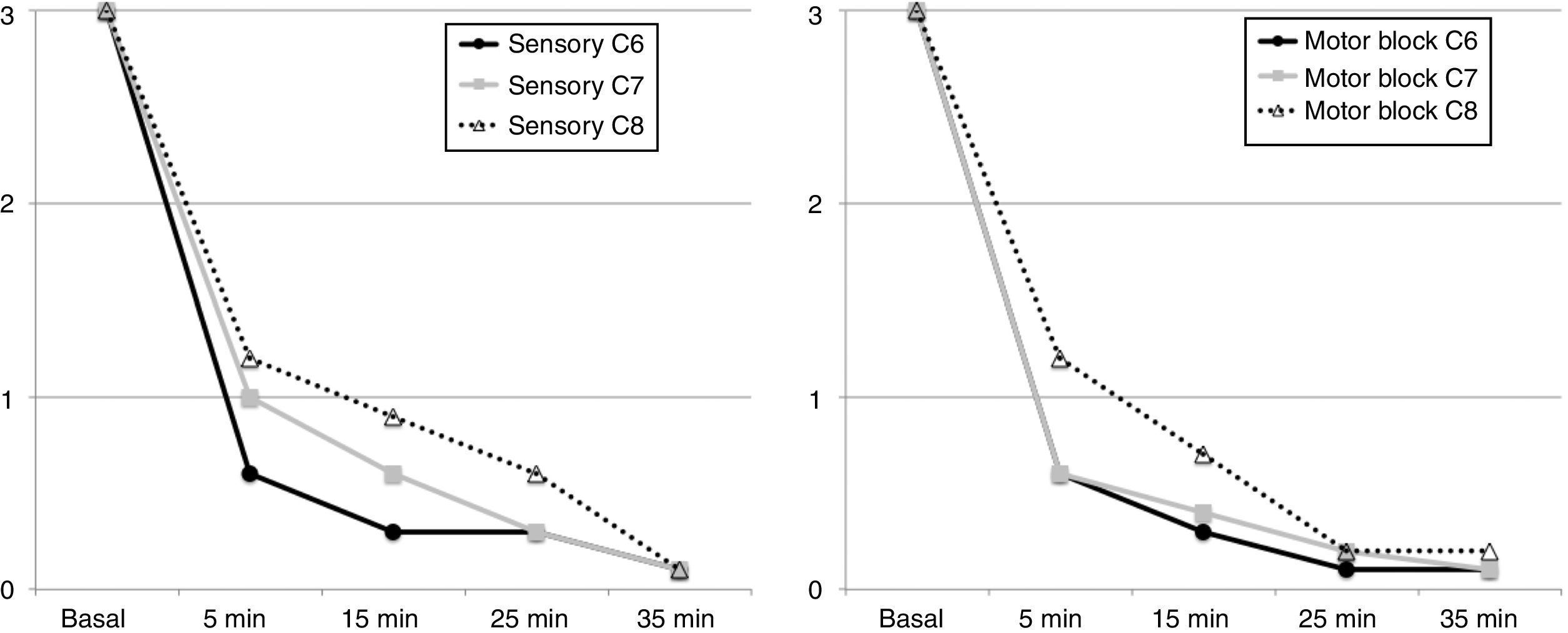
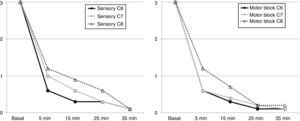
The block was effective in all patients. 7 of them (70%) experienced a surgical sensory block after 5min, 8 patients after 15min, in 9 patients after 25min and the 10 of them after 35min, in the area between C6 and C8. Complete motor block was achieved in 6 patients after 5min, in 8 patients at 15min, in 9 patients at 25min, and in the 10 of them at 35min (Fig. 4).
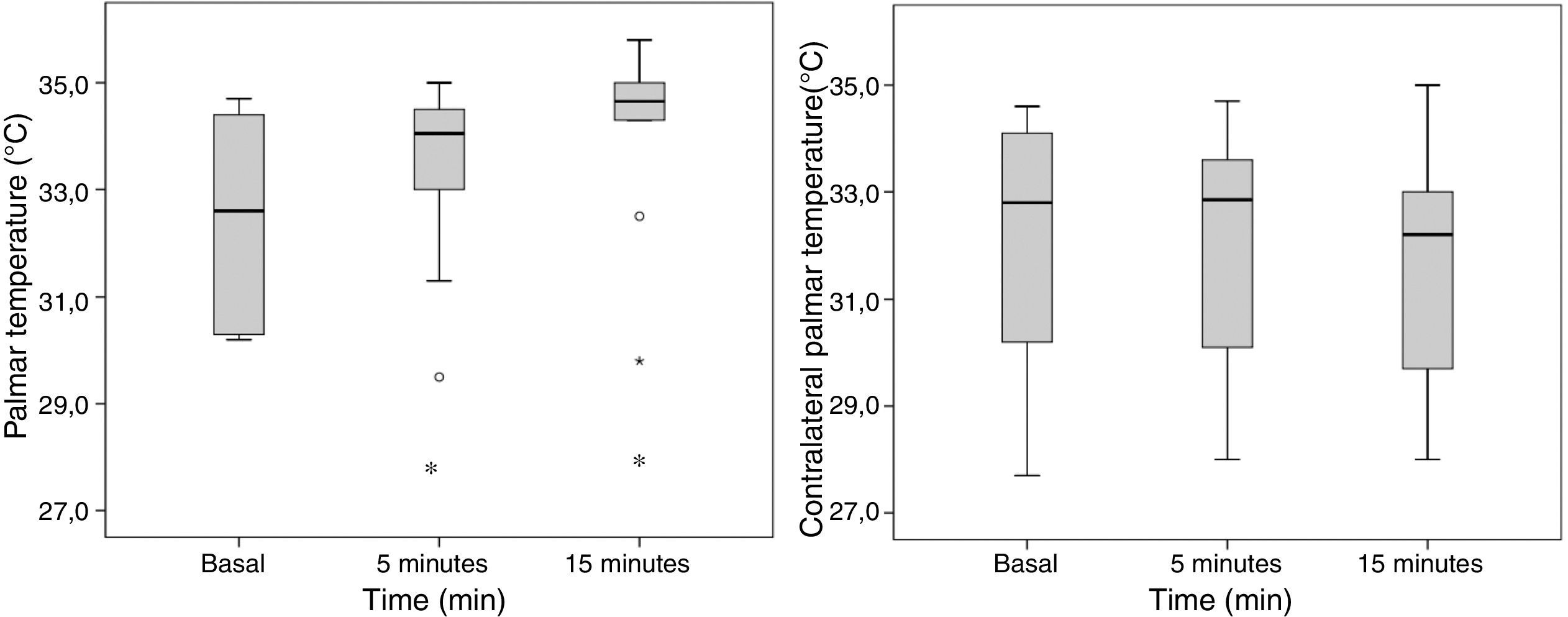
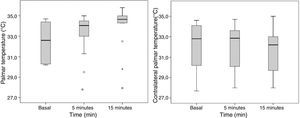
The palmar T° of the blocked extremity increased significantly 5min post-block (32.5±1.8°C to 33.4±1.7°C; p=0.047), without any T° changes in the contralateral palm (32.1±2.4°C to 32.1±2.2°C; p=1) (Fig. 5).
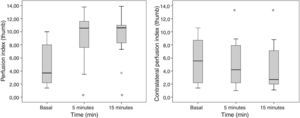
The diameter of the humeral artery did not show any significant changes after the block (36±5mm to 39±4mm; p=0.064), while the HAF increased significantly 5min after the block (105±70ml/min to 192±97ml/min; p=0.007). The PI in the thumb of the blocked extremity increased significantly from 5±3 to 10±3 (p=0.002) 5min after the block and from 5±3 to 10±3 (p=0.002) 15min after the block. A remarkable decrease in the PI of the contralateral extremity was observed, 5min after the end of the block, from 6±4 a 5±3; p=0.041 and at 15min a drop from 6±4 to 4±3; p=0.009 (Fig. 6).
DiscussionThe supraclavicular BPB has the advantage of a bundled anatomy of the superior trunk (with its anterior, posterior, and suprascapular divisions), the middle trunk and the lower trunk.14 The proposed technique combines the two basic concepts of regional anesthesia: the nervous sheath, and the perivascular approaches (to access the sheath of the neurovascular bundle), developed by Winnie in the 60s–70s.1,7,20 Hence, the ultrasound-guided approach herein described focuses on the subclavian vein and suggests that this vein should be an integral part of the neurovascular complex. The close relationship of the three nerve components of the BP (upper, middle, and lower trunks within their nerve sheath), together with the fourth structure – the subclavian vein – localized medially to these trunks, comprise the neuromuscular bundle. The target is precisely the center of this neuromuscular bundle (lateral to the artery, medial to the middle trunk, deep in the upper trunk, and superficial in the inferior trunk). The association between a central position and the fascia enables a broad distribution around and throughout the nerve structure of the plexus with a single point injection.
While the medial to lateral direction of the needle in the approach suggested puts the needle away from the pleura, the technique herein discussed could be considered in the future as a means to reduce the incidence of the most feared complication of supraclavicular block: i.e., pneumothorax. However, given the low incidence of this complication, it may be difficult to show proof beyond a common sense argument. Another potential advantage is the lateral distribution of the anesthetic rather than a medial spread. However, we cannot proof that this approach may impact the incidence of hemi-diaphragmatic paralysis, paralysis of the recurrent laryngeal nerve and vascular puncture, as compared to the classical lateral to medial approach, though other authors have suggested likewise.7–9,14,15
The history of this ultrasound-guided approach dates back to the anteroposterior or plumb-line approaches suggested by Brown7 to avoid pneumothorax as a complication, until the description by Pham-Dang that evaluated the advantages of this lateral direction of the needle for inserting catheters and a more distal and homogenous distribution of the LA to achieve a metameric block on the more proximal areas of the extremity (C5, C6 and C7) and of the more distal areas of C8 and T1 that require longer times to be affected.12 The rate of onset of the sensory block during the exploration of C6 and C7 was longer as compared to C8 which was just 5min post-block (see Fig. 5), similar to the onset of the motor block. This is consistent with the pattern of sensory and motor block onset described in other trials, where the most distal metameric area takes longer to block.11
The associated sympathetic block causes vasodilatation and increased blood flow. This effect was overtly manifested in our trial with early changes occurring when measuring the PI, the T° and the HAF rate in the blocked extremity. These reflect a decrease of the vasomotor tone.17,21–23 Most of the hemodynamic changes occurred 5min after the block, probably because the diameter of the sympathetic nervous fiber is smaller as compared against the diameter of a motor or a sensitive fiber.22,23 The increased HAF rate is due to changes in the peripheral vascular resistances.16,17 However, it is impossible to ascertain that these changes associated with the sympathetic block are predictors of the block efficacy, including among other reasons that there were no failed blocks in our study. Neither is it possible to assess whether any of the parameters measured (IP, T° or flow) has more or less predictive value with regards to the onset of the block, since the assessment after 5min showed significant changes in the three parameters and the sample size was small. Despite these limitations, we believe that the simplicity of the measure, and its non-invasive nature, makes PI a potential monitoring tool for the future.
Just doing an ultrasound-guided SCB involves less complications because of the ability to visualize structures that are prone to injury and to evaluate the distribution of the local anesthetic.24 A report on 510 patients by Perlas et al.9 did not show any cases of pneumothorax when performing a SCB with a medial to lateral needle approach, or with a lateral to medial approach. There were no pneumothorax events in our trial and this is consistent with the literature. The only adverse effect a patient presented was Horner's syndrome. We are not aware of any cases of asymptomatic ipsilateral hemidiaphragmatic paralysis since no specific studies were done on the diaphragmatic function in any of the patients.24 The paresthesia reported by 1 patient (10%) – described as painful but transient – was observed during the insertion of the neurostimulation needle; however, no post-bloc neurological deficit was ever reported. The objective and the characteristics of the observational study herein discussed hinder any considerations with regards to improved safety versus other SC approaches, though the Brown and Pham-Dang techniques similar to our proposal, were introduced on the basis or improved safety.
ConclusionThe ultrasound-guided MSCB as presented, with a 20ml volume, achieves adequate diffusion of the LA into the periclavicular space, from the roots of the BP (inerscalene) to the secondary trunks (infraclavicular), resulting in a highly significant efficacy of the block. A second characteristic is that the rises in IP, the distal T°, and the HAF rate are seen early after the block. Since PI is an objective, easy to quantify measurement that causes no discomfort and does not need patient cooperation, IP could be a potential future monitoring tool for post-block clinical evaluation. Any future large clinical trials shall take into consideration the role of this ultrasound-guided SC approach versus other BP approaches, and establish the potential advantages in terms of safety.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Herrera AE, Mojica V, Nieuwveld D, Prats-Galino A, López AM, Sala-Blanch X. Bloqueo supraclavicular ecoguiado por abordaje perivascular medial. Descripción anatómica, técnica de bloqueo y cambios de perfusión regionales. Rev Colomb Anestesiol. 2017;45:272–279.