Quality of life is an important outcome in paediatric cancer patients. Its evaluation at different times during the clinical course of the disease is essential for clinical practice focused on the needs of the patients. There is not a specific assessment tool for this purpose in Colombia.
ObjectiveTo perform the cultural adaptation of the quality of life scale PedsQL (Paediatric Quality of Life) Cancer Module, version 3.0 for use in Colombia.
MethodsPermission for use of the scale was obtained and the algorithm steps of the Mapi Research Trust group were followed: Direct and independent translations of scale by two native Colombian Spanish speaking translators, obtaining a preliminary version from the translations. This was followed by a back translation by a native English speaking translator and a review of the process by the author of the scale, inclusion of suggestions, and implementation of the pilot study.
ResultsDirect translations were similar in the instructions and response options; a consensus meeting in 8 of the 27 items was required to choose the best translation. The author made no suggestions and gave his endorsement to the implementation of the pilot, in which, 2 items were modified in order to improve their understanding.
ConclusionsThere is a Colombian Spanish version of the PedsQL questionnaire 3.0 Cancer Module, to be submitted for a validation study prior to its use in the assessment of quality of life in paediatric cancer patients.
La calidad de vida es un objetivo importante para los pacientes pediátricos con cáncer. Su evaluación en distintos momentos del curso clínico de la enfermedad es indispensable para un ejercicio clínico centrado en las necesidades de los pacientes. En Colombia no se cuenta con un instrumento específico de evaluación para tal fin.
ObjetivoRealizar la adaptación transcultural del cuestionario de calidad de vida infantil Pediatric Quality of Life Cancer Module version 3.0 (PedsQL) para su uso en Colombia.
MétodosSe obtuvo el permiso para el uso de la escala y se siguieron las etapas del algoritmo del grupo Mapi Research Trust: traducciones directas e independientes de la escala por dos traductores hablantes nativos del español colombiano, obtención de una versión preliminar a partir de las traducciones, retrotraducción por un traductor hablante nativo del inglés, revisión del proceso por el autor de la escala e inclusión de sugerencias y realización de la prueba piloto.
ResultadosLas traducciones directas fueron similares en las instrucciones y opciones de respuesta; en 8 de los 27 ítems se requirió una reunión de consenso para escoger la mejor opción de traducción; el autor no hizo propuestas y dio su aval para la aplicación de la prueba piloto, en la cual 2 ítems sufrieron modificaciones con el fin de mejorar su comprensibilidad.
ConclusionesSe cuenta con la versión en español colombiano de la escala PedsQL 3.0 Cancer Module, para someterla a un estudio de validación previo a su uso en la evaluación de la calidad de vida en población pediátrica con cáncer.
Advances in medical treatments and health care have resulted in an increase in patients’ life expectancy, which means that a greater number of people suffer diseases over longer periods, thus making health-related quality of life (HRQoL) a fundamental aspect that must be taken into account in the health-disease process.1 The term “quality of life” has evolved to include aspects of health that were not previously considered and various interdisciplinary approaches.2 HRQoL is a multidimensional construct that generally encompasses areas such as physical function, psychological functioning, social function and general perception of health, among others2,3; these dimensions are useful in the creation of measurement instruments, since they allow an integrated and structured approach that facilitates data collection and analysis.
The study of HRQoL has focused mainly on adult patients, and until a few decades ago there was no marked interest in studying it among children, especially paediatric patients with chronic diseases.4 Although at first it was believed that the quality of life of children was comparable to that of adults, research revealed important aspects to be taken into account in the conceptualisation of the same in the paediatric area; for example, the stage of development of the child and the contexts specific to this stage of life, such as primary/secondary school and the home (the relationship with parents or caregivers).2–5
In order to understand and approach a construct as complex as HRQoL, standardised questionnaires have been created that comprise different dimensions, which in turn consist of questions whose answers are presented on scales (generally Likert-type). Instruments are also created in the paediatric area. The majority are seen to follow a psychometric model in their preparation,6 and are generic instruments from the English-speaking world3,6,7; however, the creation of specific instruments to be applied among this population is on the rise,3 along with their use in clinical studies on children with chronic health problems.6 Moreover, in the measurement of the paediatric population, the reports of the minors’ parents or caregivers have been considered important, since this information complements the reports of the children and, in some cases, is the only source of primary information that can be obtained.1,8,9
There are few instruments created in Latin America for the measurement of HRQoL, and the cultural adaptation of scales created in other languages for use in Spanish-speaking countries is more common7,10–12; however, said instruments are sometimes used without cross-cultural adaptation or the respective validation study having been performed.4 In Colombia, there are limited questionnaires in existence to measure quality of life in children; among those adapted to our population are the PedsQL™ 4.0 Generic Core Scale13 and KIDSCREEN-27,14,15 which are generic scales that can be used in children with or without disease. However, there are no specific instruments that measure the HRQoL of paediatric cancer patients, so a suitable instrument is required for this purpose. As a result of the foregoing and taking into account the criteria proposed in 2002 by the Scientific Advisory Board (SAC) of the Medical Outcome Trust,16 for choosing quality instruments, the Paediatric Quality of Life (PedsQL) Cancer Module version 3.0 was chosen to undergo cross-cultural adaptation for use in Colombia.
MethodsInstrumentThe PedsQL Cancer Module version 3.0 originally developed in English by Varni et al.,17 is a specific instrument (in cancer) for assessing paediatric quality of life; it is composed of an instructions section and 27 items grouped under 8 dimensions: a) pain and hurt (2 items); b) nausea (5 items); c) procedural anxiety (3 items); d) treatment anxiety (3 items); e) worry (3 items); f) cognitive problems (5 items); g) perceived physical appearance (3 items), and h) communication (3 items).
For its application, it is divided into four age groups: 2–4 years (which is only applied to caregivers), 5–7, 8–12 and 13–18; the latter being applied to both children and caregivers. The Likert-type scale consists of five response options (never, almost never, sometimes, often, almost always), except for children aged 5–7 years who have three response options (never, sometimes and almost always) and a visual aid (happy face, neutral face and sad face).
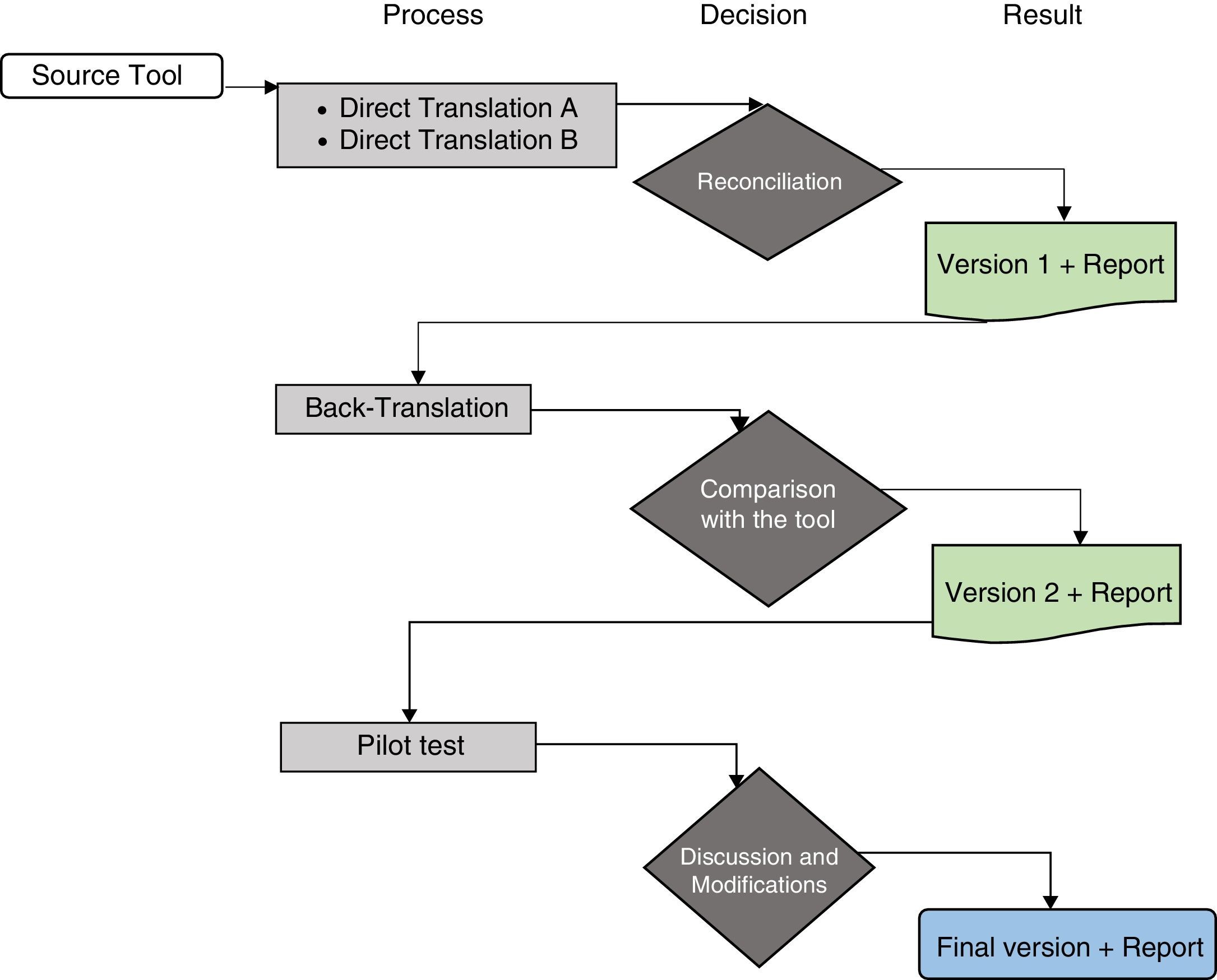
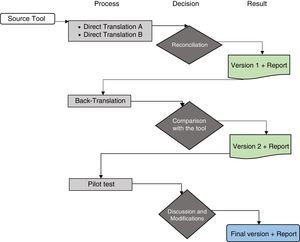
ProcedureThe study authors contacted the author, J.W. Varni, and the Mapi Research Trust group responsible for the scale, who authorised the use of the instrument and suggested following the translation process proposed by the group in order to undertake the cross-cultural adaptation process (Fig. 1).18 To follow this process, the following steps were performed:
- 1.
Direct translation: two Colombians with a good fluency in English conducted two direct translations from English into Colombian Spanish of the full scale, along with the instructions and response options. The translations were carried out independently.
- 2.
Preliminary version: by means of a consensus meeting between the study investigators, the two direct translations were compared in order to discuss and resolve discrepancies between the translations and to generate a preliminary version of the scale in Colombian Spanish. To resolve discrepancies, the following criteria were taken into account: if the translations were identical, no changes were made; if there was a difference, we opted for the translation whose meaning was more in line with the original, but adjusting it to the Colombian context.
- 3.
Back translation: following the guidelines of the MAPI group for the translation of the instrument, a native English-speaking translator with a good fluency of Colombian Spanish carried out a back translation from Spanish into English using the preliminary version.
- 4.
Author's review: the author of the scale, J.W. Varni, reviewed the back translation and compared it to the original English version. The proposed recommendations were included in the preliminary version.
- 5.
Pilot test: once the author's observations were included and in accordance with the guidelines of the MAPI group, the scale was presented to a group of 20 cancer patients aged 2–18 years who were undergoing treatment or attending a control visit at the Colombian National Cancerology Institute in Bogotá D.C. (Colombia) (5 in each age group), along with their caregivers, who gave their consent. Both the patients and their caregivers read the scale (or it was read to them, if they did not have good reading skills) and they assessed whether each item, along with the instructions and response options of the scale, was understandable, clear, offensive or irritating, or if they could be written with better paraphrasing. Once this process was complete, the Colombian Spanish version of the PedsQL Cancer Module version 3.0 was obtained.
Algorithm of the linguistic validation process. Taken and adapted from the guidelines “Linguistic validation of the PedsQL™ – a Quality of Life Questionnaire”.18
For the implementation of the pilot test, approval was obtained from the Institutional Ethics Committee.
ResultsTranslation processThe results obtained from the linguistic translation process of the PedsQL Cancer Module version 3.0 from English to Colombian Spanish are presented below.
Direct translationTwo Colombians with an excellent command and fluency of English, an internal medicine doctor and a psychologist with a M.Sc. in Neuropsychology who is a candidate for a PhD in Psychology, performed two direct translations (from the original English scale into Colombian Spanish) independently.
Preliminary versionBy means of a consensus meeting between the team of investigators from the NCI responsible for the project, the two direct translations were compared in order to discuss and resolve discrepancies between the translations and to generate a preliminary version of the scale. The discussion took place according to the criteria presented in the methodology.
- •
Scale instructions and response options. These were taken from the Paediatric Quality of Life (PedsQL™) Inventory version 4.0 – Spanish (Colombia), since they are equivalent and this instrument has already been adapted and validated for the Colombian population.
- •
Scale items: adolescent (13–18 years) and child (8–12 years) report. The direct translations of items 2 and 3 in the Nausea domain, 2 and 3 of Procedural Anxiety, 2 and 3 of Treatment Anxiety, 1 and 2 of Worry, 2 of Perceived Physical Appearance and 1–3 of Communication had identical wording. The direct translations of items 1, 4 and 5 of the Nausea domain, 1 of Treatment Anxiety, 3 of Worry, 1–5 of Cognitive Problems and 1 and 3 of Perceived Physical Appearance used the same words and, although they showed variations in composition, were adapted to the Colombian context, and therefore the one that would prove easier to understand for the patients was chosen. Different words were observed in the translations of items 1 and 2 of Pain and Hurt and 1 of Procedural Anxiety; in the first case, the first translator translated the word “hurt” as ardour and the second as molestia; at the consensus meeting it was decided to use molestia, as it was considered more appropriate conceptually and better suited to the context.
- •
Scale items: young children (5–7 years) report. The direct translations of items 2 of the Nausea domain, 1 and 3 of Procedural Anxiety, 2 and 3 of Worry, 1 of Cognitive Problems, 3 of Perceived Physical Appearance, and 1–3 of Communication had identical wording. The direct translations of items 1 of the Pain and Hurt domain, 1 of Nausea, 2 and 3 of Procedural Anxiety, 2 of Treatment Anxiety, 1 of Worry, 2–4 of Cognitive Problems and 1 and 2 of Perceived Physical Appearance used the same words and, although they showed variations in composition, were adapted to the Colombian context, and therefore the one that was considered easier to understand for the patients was chosen. Different words were observed in the translations of items 2 of the Pain and Hurt domain, 1 of Procedural Anxiety and 3–5 of Nausea, as follows:
- –
Items 2 of Pain and Hurt and 1 of Procedural Anxiety: the first translator translated the word “hurt” as ardour and the second as molestia; at the consensus meeting it was decided to use molestia, as it was considered more appropriate conceptually and better suited to the context.
- –
Items 3–5 of Nausea: the first translator translated the expression “sick to your stomach” as enfermar del estómago and the second as náuseas; at the consensus meeting it was decided to use enfermar del estómago as it was considered more appropriate conceptually and linguistically.
- –
- •
Parent report for adolescents (13–18 years), children (8–12 years), young children (5–7 years) and toddlers (2–4 years). The direct translations of items 1 and 2 of the Pain and Hurt domain, 2 and 3 of Nausea, 1 and 3 of Procedural Anxiety, 1 and 2 of Worry and 5 of Cognitive Problems had identical wording. The direct translations of items 1, 4 and 5 of the Nausea domain, 2 of Procedural Anxiety, 1–3 of Treatment Anxiety, 3 of Worry, 1–4 of Cognitive Problems, 1–3 of Perceived Physical Appearance and 1–3 of Communication used the same words and, although they showed variations in composition, were adapted to the Colombian context, and therefore the one that was considered easier to understand for the patients was chosen.
After obtaining the reconciled version of the scale in Spanish from the direct translations, this version was sent to a bilingual English-speaking certified translator, who carried out the back translation.
Author's reviewThe author of the scale reviewed the back translation and compared said version to the original in English. The proposed adjustments were included in the preliminary version once they had been reviewed and considered by the research team.
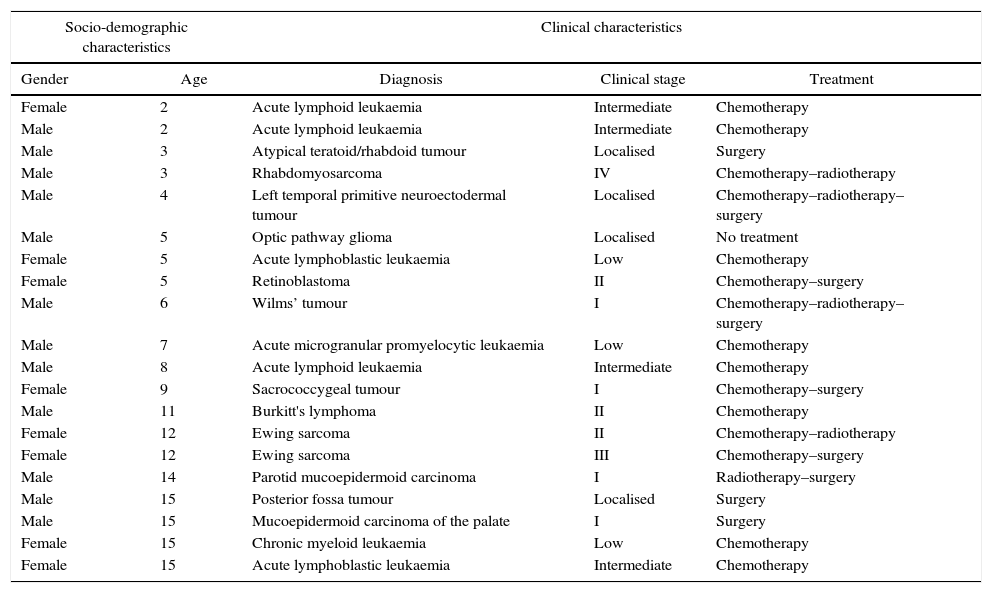
Pilot testThe pilot test was performed between 3 and 13 November 2015. Table 1 shows the individual socio-demographic and clinical characteristics of the patients who participated in the test. The scale was applied to 15 children and 20 caregivers, who in turn were interviewed in order to evaluate each of the items along with the scale instructions. The following was found:
- •
In item 2 (original: “food does not taste very good to me”; translated into Spanish as: “la comida no me sabe muy bien”) of the Nausea domain, 3 children and 2 caregivers said they were confused and had difficulty understanding and answering the item, since it is asked negatively and caused confusion in relation to the response options; they proposed asking it in an affirmative manner or removing the word “no”. As such, the research team rephrased the item: “la comida me sabe mal”.
- •
In item 1 (original: “I feel I am not good looking”; translated into Spanish as: “siento que no tengo buena apariencia”) of the Perceived Physical Appearance domain, 3 children and 1 caregiver reported difficulty understanding the question and confusion, on the one hand, due to it being asked negatively, which caused confusion in relation to the response options and, on the other, because 1 child and 1 caregiver did not know the meaning of the word “apariencia”. As such, the research team rephrased it: “siento que tengo mal aspecto”.
Socio-demographic and clinical characteristics of the patients included in the pilot test.
| Socio-demographic characteristics | Clinical characteristics | |||
|---|---|---|---|---|
| Gender | Age | Diagnosis | Clinical stage | Treatment |
| Female | 2 | Acute lymphoid leukaemia | Intermediate | Chemotherapy |
| Male | 2 | Acute lymphoid leukaemia | Intermediate | Chemotherapy |
| Male | 3 | Atypical teratoid/rhabdoid tumour | Localised | Surgery |
| Male | 3 | Rhabdomyosarcoma | IV | Chemotherapy–radiotherapy |
| Male | 4 | Left temporal primitive neuroectodermal tumour | Localised | Chemotherapy–radiotherapy–surgery |
| Male | 5 | Optic pathway glioma | Localised | No treatment |
| Female | 5 | Acute lymphoblastic leukaemia | Low | Chemotherapy |
| Female | 5 | Retinoblastoma | II | Chemotherapy–surgery |
| Male | 6 | Wilms’ tumour | I | Chemotherapy–radiotherapy–surgery |
| Male | 7 | Acute microgranular promyelocytic leukaemia | Low | Chemotherapy |
| Male | 8 | Acute lymphoid leukaemia | Intermediate | Chemotherapy |
| Female | 9 | Sacrococcygeal tumour | I | Chemotherapy–surgery |
| Male | 11 | Burkitt's lymphoma | II | Chemotherapy |
| Female | 12 | Ewing sarcoma | II | Chemotherapy–radiotherapy |
| Female | 12 | Ewing sarcoma | III | Chemotherapy–surgery |
| Male | 14 | Parotid mucoepidermoid carcinoma | I | Radiotherapy–surgery |
| Male | 15 | Posterior fossa tumour | Localised | Surgery |
| Male | 15 | Mucoepidermoid carcinoma of the palate | I | Surgery |
| Female | 15 | Chronic myeloid leukaemia | Low | Chemotherapy |
| Female | 15 | Acute lymphoblastic leukaemia | Intermediate | Chemotherapy |
Health-related quality of life is an important objective for paediatric cancer patients. Its evaluation at different times during the clinical course of the disease is essential for clinical practice focused on the needs of the patients. For these reasons, it is important to have quality instruments that allow a reliable approach and which reflect the patient's perception of their health status and other areas of their life linked to the health-disease process.
Since these measurements are of a subjective nature, it is essential that the patient be entrusted with providing the information first-hand; however, previously in the paediatric setting, only the parents’ or caregivers’ reports were routinely taken, and it was only after years of research and improving the questionnaires created that the importance of the child or adolescent giving the report on their state of health was highlighted, given that the most suitable person to describe the effects of any intervention on their health is the very person to whom it is applied.
Moreover, it is necessary to take into account the differences between children and adults when evaluating and taking their report on quality of life, as their views of the world and manner of processing information differ substantially; in toddlers it is extremely important to consider their present stage of development and, furthermore, for paediatric patients with chronic diseases, the degree to which their health has deteriorated should be taken into account. As reported by Varni et al., when it comes to very young children, those with cognitive deficits or who are too sick or fatigued to complete the instruments, parent reports are quite useful, although they should not replace those of the children in the event they are able to provide one.19 In such cases, the parent or caregiver report plays a vital role, as it constitutes the primary information source; however, caution should be exercised in the management of such information, since studies consistently show there to be differences in the degree of agreement between parent and child reports.20–22
In spite of the variety of instruments created to measure children's HRQoL, the majority comprise age brackets of over 8 years, even though this information is also essential in younger children. The main barrier is the child's comprehension, given their stage of development, but it has been proven that 5-year-olds are able to give a report when the right tools are used.19
The PedsQL 3.0 Cancer Module has proven to be a suitable and quality instrument that also includes the 2–4 (parent report) and 5–7 (parent and child report) age brackets, using visual aids for young children, thereby facilitating the toddler's understanding. Moreover, several studies have evaluated its psychometric properties in different languages and cultures,23–26 finding it to be a useful and quality instrument that may be used in countries where it has been adapted and validated.
ConclusionsAs a result of the cross-cultural adaptation process used here, there is a version of the scale that guarantees linguistic and conceptual equivalence to the original. Before its use in studies on the Colombian population, it is necessary to evaluate its psychometric properties.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank J.W. Varni, the author of the scale, who authorised the use of the instrument for the study of the cross-cultural adaptation and validation, and performed the back translation review process. The MAPI group, which through Marie Sidonie Edieux provided access to the instrument and the guidelines for cross-cultural adaptation. Jorge Enrique Martínez and Alejandra Rodríguez, who carried out the direct translations. Steven William Bayless, who undertook the back translation. And the patients of the Colombian National Cancerology Institute, as well as their family members who participated in the pilot test for the scale.
Please cite this article as: Fontibón LF, Ardila SL, Sánchez R. Adaptación transcultural del cuestionario PedsQL Cancer Module version 3.0 para su uso en Colombia. Rev Colomb Psiquiat. 2017;46:161–167.