Attention deficit and hyperactive disorder (ADHD) is highly prevalent among children in Bogota City. Both genetic and environmental factors play a very important role in the etiology of ADHD. However, to date few studies have addressed the association of genetic variants and ADHD in the Colombian population.
ObjectivesTo test the genetic association between polymorphisms in the DAT1, HTTLPR, COMT and BDNF genes and ADHD in a sample from Bogota City.
MethodsWe genotyped the most common polymorphisms in DAT1, SERT, COMT and BDNF genes associated with ADHD using conventional PCR followed by restriction fragment length polymorphism (RFLP) in 97 trios recruited in a medical center in Bogota. The transmission disequilibrium test (TDT) was used to determine the association between such genetic variants and ADHD.
ResultsThe TDT analysis showed that no individual allele of any variant studied has a preferential transmission.
ConclusionsOur results suggest that the etiology of the ADHD may be complex and involves several genetic factors. Further studies in other candidate polymorphisms in a larger sample size will improve our knowledge of the ADHD in Colombian population.
El trastorno por déficit de atención e hiperactividad (TDAH) es una perturbación con elevada prevalencia en población infantil de Bogotá. Entre las causas de este trastorno se encuentran factores genéticos y ambientales, pero pocos estudios han tratado de abordar el componente genético en población colombiana.
ObjetivosRealizar un estudio de asociación genética entre diferentes polimorfismos y el TDAH en la población de Bogotá.
MétodosMúltiples polimorfismos de los genes DAT1, SERT, COMT y BDNF fueron genotipificados empleando las técnicas de PCR convencional y RFLP en 97 tríos de Bogotá. El test de desequilibrio de trasmisión (TDT) se empleó para determinar la asociación entre las diferentes variantes y el TDAH.
ResultadosEl análisis de TDT no identificó una transmisión preferencial de alelos de ninguna de las variantes estudiadas.
ConclusionesNuestros resultados indican que la etiología del TDAH es heterogénea e involucra diversos factores genéticos. Futuros estudios enfocados en otros polimorfismos candidatos en una muestra más grande ayudarán a comprender el TDAH en la población colombiana.
The Diagnostic and Statistical Manual of Mental Disorders (DSM) defines attention deficit hyperactivity disorder (ADHD) as high levels of inattention, hyperactivity and impulsive behaviors, among others.1 ADHD is one of the most common neurobehavioural disorders found in childhood, and may continue into adulthood. It is a complex, heterogeneous syndrome with a multifactorial aetiology.2
The worldwide prevalence of this disorder is 5.29% in school age children.3 In Colombia, prevalence of ADHD in Manizales and Medellín is higher than average, affecting 16% of the general population.4 The incidence of ADHD may increase due to risk factors such as extreme poverty, broken families, domestic violence and sparsity of health services, among others.4,5
Studies in monozygotic and dizygotic twins with ADHD in different countries have reported high rates of heritability (60–90%)6. Heritability estimates not only include genetic influences, but also gene–environment interaction,7 which appear to play a crucial role in the development of the disease. Among the environmental factors associated with the etiology of ADHD are prenatal, perinatal and postnatal risks, nutrition, stress, infections and exposure to toxins during gestation, which may affect the development of regions of the brain that are relevant to ADHD.8 Given the evidence of the high rate of heritability of ADHD, recent studies in molecular genetics have focused on identifying specific genes associated with the disorder. Initially, these studies focused mainly on genes involved in neurotransmission, specifically in the dopaminergic pathway.9,10 The dopamine active transporter gene (DAT1 or SLC6A3), which is associated with ADHD, was therefore an obvious target for many research studies.11–14DAT1 is located on chromosome 5 (5p15), and is an important regulator of dopaminergic neurotransmission, since it modulates the reuptake of dopamine into the presynaptic terminal. Although different polymorphisms across the DAT1 gene show an association with ADHD or similar disorders,15,16 the most widely studied polymorphism is a 40-nucleotide variable number tandem repeat (VNTR) variant located in the 3′ untranslated region (3′UTR) of the gene.17 The most common are 10-repeat (10R) and 9-repeat (9R) alleles, which occur at a frequency of 71.9% and 23.4%, respectively.18,19
Genes involved in the serotonergic pathway are also associated with ADHD. The serotonin transporter gene (SERT, SLC6A4), located on the long arm of chromosome 17, is one of the most widely studied. This gene encodes for an integral membrane protein that transports serotonin from the synaptic space to the presynaptic neurons,20,21 and is therefore an important regulator of serotonergic activity in the brain. The 5-HTTLPR (serotonin-transporter-linked polymorphic region) polymorphism has been studied to determine its association with depression and similar disorders,22 as well as with ADHD.23,24 The 5-HTTLPR is a 44-bp insertion/deletion polymorphism in the promoter region of the SERT gene, and is associated with changes in messenger RNA transcript levels and protein concentrations.25 The long allele (L) consists of 16 repeats, while the short allele (S) has 14.26 The homozygous variant of the long allele is associated with increased transcriptional activity of 5-HTTLPR, resulting in increased SERT expression and serotonin reuptake relative to that of S-containing variants.25
The brain-derived neurotrophic factor gene (BDNF), located on chromosome 11 (11p13),27 belongs to the family of proteins called neurotrophins, and is involved in different mechanisms, such as neuronal survival in the central nervous system and synaptic plasticity.28,29 Different studies have identified an amino acid substitution of valine for methionine (Val66Met) at codon 66 of the BDNF gene that may alter intracellular trafficking and BDNF secretion in the brain.30,31 With regard to ADHD, the G or Val allele is considered a risk factor.30,32
Finally, the catechol-o-methyltransferase (COMT) gene located on chromosome 22 (22q11)33 codes for an enzyme that catalyses the transfer of a methyl group from S-adenosylmethionine to catecholamines, including the neurotransmitters dopamine, epinephrine and norepinephrine.34 Most studies aimed at determining an association between COMT and ADHD have focused on the rs4680 polymorphism, which produces a valine-to-methionine substitution at codon 158 (Val158Met) located in exon 4 of COMT.35 This polymorphism alters COMT enzyme activity, where Met homozygote enzymatic activity is shown to be 3–4 times lower than that of Val homozygotes.35
Recently, studies have been carried out to determine the contribution of localized polymorphisms in different genes to the development of ADHD in the Colombian population.36–38 In our study, we analyzed the most common polymorphisms of the DAT1, SERT, COMT and BDNF genes in order to determine their possible association with familial ADHD in a sample of the population of Bogotá.
MethodsParticipantsBoys (83.01%) and girls (16.99%), with an average age of 10 years, treated at the Hospital Pediátrico de la Misericordia (Misericordia children's hospital), with a definitive diagnosis according to DSM-IV-TR criteria from a specialist in child and adolescent psychiatry were included in the study. Blood samples were drawn from patients and family members once their informed consent had been obtained and the study had been approved by the Ethics Committee of the Faculty of Medicine of the Universidad Nacional de Colombia. The children gave their assent for their data to be included in this study. The total sample size was 97 families, consisting of a father, mother and affected child (291 individuals).
GenotypingBlood samples from patients and controls were processed using the salting-out method.39 Polymerase chain reaction (PCR) was used to detect copy number variations (CNVs) in the 5-HTTLPR and DAT1 polymorphisms. Genotyping was done by means of 1.5% agarose gel stained with SYBR® Safe DNA Gel, and subsequently visualized under ultraviolet light. To identify single nucleotide polymorphisms (SNPs) of the BDNF (rs6265) and COMT (rs4680) genes, restriction fragment length polymorphism (RFLP) methodology using the Hin1ll (NlaIII) restriction enzyme was used in addition to conventional PCR. The results were analyzed in 2.5% agarose gel stained with SYBR® Safe DNA Gel, and visualized under ultraviolet light.
Statistical analysisThe different alleles and genotypes obtained from each study subject were arranged in standard PED and MAP formats for subsequent analysis with PLINK software.40 The Hardy–Weinberg equilibrium (HWE) was determined using a chi-square test. The odds ratio (OR) of the different variants was calculated using the transmission disequilibrium test (TDT), with a 95% confidence interval (95% CI) and statistical significance if p<0.05.
ResultsClinical characteristicsA total of 97 trios were evaluated, mostly from Bogotá (92.45%). The mean age of the children was 10.17 years. In total, 83.01% were boys. Over half (62%) of the children studied were in primary school, 39% in secondary and 4% in preschool. In terms of the Colombian socio-economic stratification system, most of the children studied came from stratum 3 (48%) and 2 (36%) (low income) areas, followed by stratum 4 (12%) (middle income) and stratum 1 (8%) (very low income).
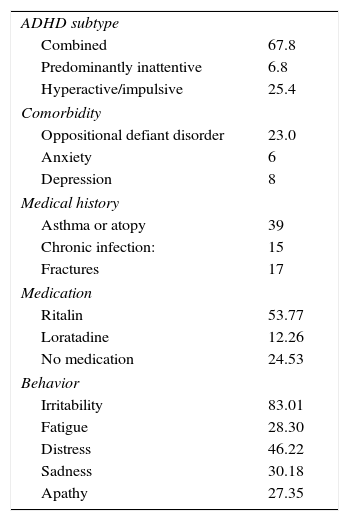
Table 1 shows the clinical characteristics of the study population. All percentages were obtained from the medical history and observations during the visits. In terms of ADHD subtypes observed in our sample, the combined presentation was the most common (67.8%), followed by hyperactive/impulsive (25.4%) and inattentive (6.8%). The most common psychological comorbidity found in the sample was oppositional defiant disorder (23%). The most prevalent concomitant diseases or conditions found were asthma or atopy (39%), chronic infection (15%) and fractures (17%). Most study subjects were taking methylphenidate (Ritalin®) (53.77%) for their ADHD, or loratadine (12.26%) for allergies.
Clinical characteristics of the study population.
| ADHD subtype | |
| Combined | 67.8 |
| Predominantly inattentive | 6.8 |
| Hyperactive/impulsive | 25.4 |
| Comorbidity | |
| Oppositional defiant disorder | 23.0 |
| Anxiety | 6 |
| Depression | 8 |
| Medical history | |
| Asthma or atopy | 39 |
| Chronic infection: | 15 |
| Fractures | 17 |
| Medication | |
| Ritalin | 53.77 |
| Loratadine | 12.26 |
| No medication | 24.53 |
| Behavior | |
| Irritability | 83.01 |
| Fatigue | 28.30 |
| Distress | 46.22 |
| Sadness | 30.18 |
| Apathy | 27.35 |
ADHD: attention deficit hyperactivity disorder.
Values are expressed as percentages.
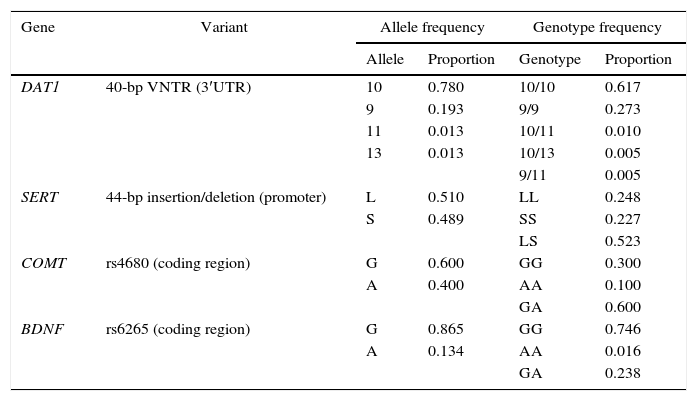
Table 2 shows the frequency of the different alleles and genotypes of the variants analyzed. Four VNTR alleles of the DAT1 gene were identified, but 10-repeat and 9-repeat alleles were the most frequent. In the case of the 44-bp insertion/deletion polymorphism in the SERT promoter region, only the L and S alleles were observed at similar frequencies. Of the two alleles identified in the COMT and BDNF genes, G is more frequent in both cases. All the polymorphisms studied are in Hardy–Weinberg equilibrium.
Frequency of alleles and genotypes.
| Gene | Variant | Allele frequency | Genotype frequency | ||
|---|---|---|---|---|---|
| Allele | Proportion | Genotype | Proportion | ||
| DAT1 | 40-bp VNTR (3′UTR) | 10 | 0.780 | 10/10 | 0.617 |
| 9 | 0.193 | 9/9 | 0.273 | ||
| 11 | 0.013 | 10/11 | 0.010 | ||
| 13 | 0.013 | 10/13 | 0.005 | ||
| 9/11 | 0.005 | ||||
| SERT | 44-bp insertion/deletion (promoter) | L | 0.510 | LL | 0.248 |
| S | 0.489 | SS | 0.227 | ||
| LS | 0.523 | ||||
| COMT | rs4680 (coding region) | G | 0.600 | GG | 0.300 |
| A | 0.400 | AA | 0.100 | ||
| GA | 0.600 | ||||
| BDNF | rs6265 (coding region) | G | 0.865 | GG | 0.746 |
| A | 0.134 | AA | 0.016 | ||
| GA | 0.238 | ||||
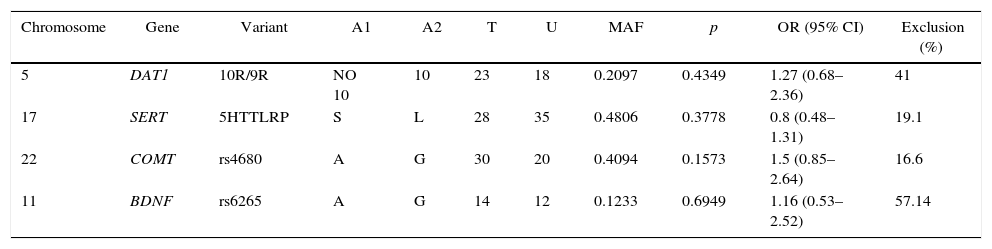
The TDT function in the PLINK software40 was used to determine the possible preferential transmission of alleles in the SERT, DAT1, BDNF (rs6265) and COMT (rs4680) gene variants in Bogotá trios analyzed (Table 3). The two most frequent alleles of each variant were used to perform the analyses. No clear evidence of preferential transmission of any of the alleles of the heterozygous parents to their respective offspring was found. This result may be due to the high percentage of exclusion of both homozygous trios and those in which one parent was not genotyped, which reduced the sample size for TDT analysis (Table 3). However, we observed an OR of >1 for DAT1, COMT and BDNF polymorphisms, although this was not statistically significant.
Transmission disequilibrium test (TDT).
| Chromosome | Gene | Variant | A1 | A2 | T | U | MAF | p | OR (95% CI) | Exclusion (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | DAT1 | 10R/9R | NO 10 | 10 | 23 | 18 | 0.2097 | 0.4349 | 1.27 (0.68–2.36) | 41 |
| 17 | SERT | 5HTTLRP | S | L | 28 | 35 | 0.4806 | 0.3778 | 0.8 (0.48–1.31) | 19.1 |
| 22 | COMT | rs4680 | A | G | 30 | 20 | 0.4094 | 0.1573 | 1.5 (0.85–2.64) | 16.6 |
| 11 | BDNF | rs6265 | A | G | 14 | 12 | 0.1233 | 0.6949 | 1.16 (0.53–2.52) | 57.14 |
A1: low-frequency allele; A2: high-frequency allele; 95% CI: 95% confidence interval; MAF: minor allele frequency; OR: odds ratio; T: low-frequency allele transmission; U: no low-frequency allele transmission.
This study was based on 97 trios, in which the age of the affected child varied from 6 to 17 years. According to an earlier study in the Colombian population, ADHD is most prevalent among children aged from 12 to 17 years (36.9%), followed by 4–5 year olds (33.1%) and 6–11 year olds (30%).5 The male:female ratio, however, is consistent with that of studies in other populations, with ratios of between 6:1 and 12:1 in clinical samples and 3:1 in population samples.41 In addition, our results are similar to those obtained in previous studies in the Colombian population, in which male sex was a risk factor for diagnosis of ADHD.4 The greatest prevalence of this disorder was observed in primary school children from low socioeconomic strata, a finding consistent with other studies.5,42 Socioeconomic and cultural factors are likely to have a negative effect on the proper development of a child's skills, such as the ability to concentrate.42
Regarding genetic factors, several studies carried out in recent years have shown the importance of different gene variants in the development of ADHD.43,44 These studies have identified polymorphisms (SNPs and VNTRs) located in the coding and regulatory region of candidate genes, located mainly in the dopaminergic and serotonergic pathways, which are associated with this disorder.9,12,17,23,24 In our study, we analyzed the most common polymorphisms of the DAT1, SERT, COMT and BDNF genes in order to determine their possible association with familial ADHD in the population of Bogotá. TDT showed that none of the genetic variants analyzed showed a preferential transmission from parents to offspring of any of the alleles. Similar results have been reported in other populations, where the association between these variants and ADHD is not significant. In the case of the COMT gene, studies in the val/met polymorphism (rs4680) in exon 445,46 and other variants15,47 have reported a negative association with ADHD. However, children with the Val158Met variant have shown good response to methylphenidate (MPH) to control hyperactivity and impulsiveness.48 Methylphenidate has also been shown to reduce symptoms in up to 62.5% of children with the val/val genotype compared to other genotypes after 8 weeks of treatment.49 Although plasma levels of BDNF have been associated with the severity of inattention symptoms,50 other studies, including our own, have been unable to replicate the previously observed association between the BDNF rs6265 polymorphism and ADHD.44 For the DAT1 gene, studies on the VNTR located in the 3′UTR region and other variants suggest a heterogeneous pattern.11,13,14 In addition, the low odds ratio (OR=1.10) reported in a meta-analysis of the VNTR in the 3′UTR variant suggest that this VNTR has a relatively minor effect.44 Despite the non-significant association observed in our study, the OR=1.27 is very similar to that obtained in a previous study by Gizer et al. in 2009,44 which contributes to the reported heterogeneity between this variant and ADHD. Similarly, there is evidence of a significant association between the 5-HTTLPR polymorphism of the SERT gene and ADHD,23,51 although other authors have been unable to reproduce this association.24,52 The lack of association with the 5-HTTLPR variant observed in this study is further evidence of this discrepancy.
Our findings, and those obtained in previous studies associating gene variants of DAT1, SERT, COMT and BDNF with ADHD, show that the etiology of this disorder is complex, and does not depend on a single genetic or environmental factor. Further research into other candidate genetic variants of these and other genes involved in neurotransmission and/or similar functions – such as DRD4, DRD5, HTR1B, SNAP-25, LPHN3 and NOS1,53 together with more robust clinical analyses and a larger population sample, may give better insight into ADHD in the Colombian population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work received funding from the Fundación para la Promoción de la Investigación y la Tecnología [Foundation for the Promotion of Research and Technology] (FPIT) of the Bank of the Republic of Colombia, the Colombian Administrative Department of Science, Technology and Innovation (COLCIENCIAS) code 110165745043, and the Faculty of Medicine of the Universidad Nacional de Colombia, by means of a grant to C.E. Orboleda-Bustos for a short postdoctoral stay (Reg. No. 30041).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ortega-Rojas J., Arboleda-Bustos C.E., Morales L., Benítez B.A., Beltrán D., Izquierdo Á., et al. Estudio de variantes de los genes BDNF, COMT, DAT1 y SERT en niños colombianos con déficit de atención. Rev Colomb Psiquiat. 2017;46:222–228.