Primary Sjögren's syndrome is an autoimmune disease where the salivary and exocrine glands do not function correctly. This is caused by lymphocytic infiltrates and neuroendocrine components. The diagnostic criteria in this syndrome have subjective and non-specific elements, but in the last few years the performing of IgA and IgG anti-alpha-fodrin antibodies has been considered as a diagnostic option. The main objective of this article is to evaluate the diagnostic performance of the anti-alpha-fodrin test in patients diagnosed with primary Sjögren's syndrome.
Materials and methodsThe study included patients with a diagnosis of primary Sjögren's syndrome according to the criteria of the American-European consensus or the consensus of the American College of Rheumatology. These were compared with healthy patients with other non-autoimmune diseases.
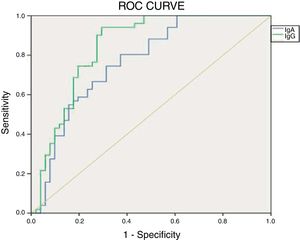
ResultsThe IgA type antibody was found to have a sensitivity of 0.18 and specificity of 0.92. The IgG type had a sensitivity of 0.05 and specificity of 0.96. The ROC curve for the IgG test was the closest to the point of greatest theoretical sensitivity and specificity.
DiscussionThe sensitivity of the test was found to be lower than that reported in other studies. This could be explained by factors such as: diagnostic criteria used, different immunoassay techniques, immunoglobulin evaluated, use of drugs, time of diagnosis, and different populations studied.
ConclusionIt is considered that the results regarding the specificity of the test in the population of the present study give it validity as a diagnostic test for primary Sjögren's syndrome, however, anti-alpha-fodrin antibodies have shown low sensitivity in our population, and it is considered that more studies are needed to define the role of these antibodies in this disease.
El síndrome de Sjögren primario es una patología autoinmune en la que se presenta disfunción causada por infiltrados linfocíticos y componentes neuroendocrinos en las glándulas salivales y exocrinas. En esta patología los criterios diagnósticos tienen elementos subjetivos y no específicos, por lo que en los últimos años se ha considerado la realización de anticuerpos anti alfa fodrina IgA e IgG como una opción diagnóstica. El objetivo principal es evaluar el rendimiento diagnóstico de la prueba anti alfa fodrina en pacientes diagnosticados con síndrome de Sjögren primario.
Materiales y métodosSe buscaron pacientes con diagnóstico de síndrome de Sjögren primario de acuerdo con los criterios del consenso americano-europeo o el consenso del Colegio Americano de Reumatología, los cuales se clasificaron como enfermos, estos se compararon con pacientes sanos, los cuales eran pacientes con otras enfermedades no autoinmunes.
ResultadosSe encontró que el anticuerpo de tipo IgA tuvo una sensibilidad de 0,18 y especificidad de 0,92. El de tipo IgG una sensibilidad de 0,05 y especificidad de 0,96. La curva ROC para el test IgG fue la más cercana al punto de mayor sensibilidad y especificidad teóricas.
DiscusiónSe encontró una menor sensibilidad de la prueba que la reportada en otros estudios, la cual puede ser explicada por factores como: criterios diagnósticos empleados, diferentes técnicas de inmunoensayo, inmunoglobulina evaluada, uso de medicamentos, tiempo de diagnóstico y diferentes poblaciones estudiadas.
ConclusiónSe considera que los resultados en cuanto a especificidad de la prueba en la población del presente estudio le otorgan validez como prueba diagnóstica para síndrome de Sjögren primario, sin embargo, los anticuerpos anti alfa fodrina presentan baja sensibilidad en nuestra población por lo que se necesitan más estudios para definir su papel en esta enfermedad.
Primary Sjögren's syndrome is an autoimmune disease presenting with dysfunction from lymphocytic infiltrates and neuroendocrine abnormalities in the salivary and exocrine glands. The patients affected develop keratoconjunctivitis sicca, xeroderma, and xerostomia, but there may be extra-glandular manifestation with skeletal, pulmonary, neurological and kidney involvement, inter alia.1–3
The criteria from the American-European Consensus have used as a basis for the classification of Sjögren's syndrome visual and oral scales which are subjective and hinder the evaluation of treatment efficacy and do not allow us to make a prognosis. Schirmer's test does not correlate with the severity of the disease, although it has a high specificity for this condition. The minor salivary gland biopsy is an invasive procedure and fails to deliver adequate inter-observer reliability. The sialogram is an obsolete procedure, in addition to not being able to differentiate among the various causes of gland inflammation. As far as the criteria of the American College of Rheumatology is concerned, the major criticism is the fact that although these are just 3 objective criteria, their practical application is quite limited when administered by practitioners other than rheumatologists, ophthalmologists, or dental health practitioners. Moreover, there is an additional need to refer the patient to the ophthalmologist to undergo a keratoconjunctivitis test, which leads to delays and inaccuracies in the patient's assessment.4
Alpha fodrinAlpha fodrin was discovered in 1997 by the Japanese scientist Haneji et al.,5 who identified a fragment of 120kDa in the salivary glands, with lymphocytic infiltration of mice with Sjögren's syndrome. It is a 240kDa intracellular protein of the cytoskeleton that belongs to the spectrin family; its role is related to the organization of cell organelles, transmembrane ion exchange (channels and pumps) and the secretion of substances.6–8 The protein is cleaved during the disease process and some studies have found that the use of the 120 KDa fragment is able to regenerate a secondary in vitro and in vivo response in healthy laboratory mice.9
Different trials have validated the sensitivity and specificity of the anti alpha fodrin antibodies, as a diagnostic marker for Sjögren's syndrome; however, there are significant variations among the trials, since they use different diagnostic criteria and the study populations tend to be small (mainly due the epidemiology of the disease). The prevalence of the antibodies has been estimated at 64–72%, but it may reach 95% in populations with primary Sjögren's syndrome.5,6,10
It has been suggested that the use of the anti alpha fodrin antibody may be a non-invasive serological option for the diagnosis of primary Sjögren's syndrome, which also offers the possibility to contribute with serological findings in patients without positive results for the anti-Ro antibody. Keeping in mind that the sensitivity and specificity has not been studied in the Colombian population, this study determined the diagnostic performance of the test in our population.
Materials and methodsThe study was conducted in a Colombian population of patients diagnosed with primary Sjögren's syndrome, who came to the rheumatology clinic at Fundación Instituto de Reumatología Fernando Chalem in Bogotá, Colombia. The database of the Fundación was retrospectively reviewed until December 31st, 2017. The patients with a diagnosis of primary Sjögren's syndrome were identified, in accordance with the American-European consensus or the consensus of the American College of Rheumatology and were classified as ill individuals; the controls were patients with other non-autoimmune diseases presenting with visual or oral symptoms in the cohort of arthrosis and fibromyalgia.
The patients selected were contacted and they voluntarily agreed to participate in the trial.
Blood samples were drawn to establish the presence of the antibodies used routinely in the diagnosis of the disease (anti-Ro, anti-La, and rheumatoid factor; the samples were processed using indirect immunofluorescence in an automated HELIOSO machine. For the anti alpha fodrin antibodies, the ELISA kit from Aesku.Diagnostics was used and the samples were processed in the automated SQ2 system. All of the results were confirmed via a second specific test.
The software SPSS statistics version 22 was used for data processing. The bivariate analysis of the data and the ROC curve were developed with this software
The diagnostic performance of the anti alpha fodrin antibodies (IgG and IgA) was evaluated for primary Sjögren's syndrome, as compared against the standard of reference for the diagnosis of the disease in terms of sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio. The population was characterized based on some demographic variables (age, sex, race), time of diagnosis, availability of biopsy, presence of antibodies, and current management of the disease. The prevalence of IgA and IgG anti alpha fodrin was established in the population studied, and finally, the prevalence of the IgA and IgG anti alpha fodrin antibodies test was compared against the qualitative results of the various antibodies routinely used in the diagnosis of primary Sjögren's syndrome (anti-SSA [Ro], anti-SSB [La], rheumatoid factor and ENA), in order to show the proportion of these antibodies among our population; this same process was used between the IgA and IgG anti alpha fodrin antibodies and the results of the salivary gland biopsy.
ResultsThe total number of patients recruited was 102, 51 ill and 51 controls, all of which met the inclusion criteria. The mean age was 54.6 years old, with minimum age of 25 and maximum age of 79 years. The age at which the disease was diagnosed was in average 49.4 years, with a minimum age at diagnosis of 23 years and a maximum of 78 years. The average time of evolution of the disease was 5.1 years, until the time they joined the trial, with a minimum of 3 months and a maximum of 22 years.
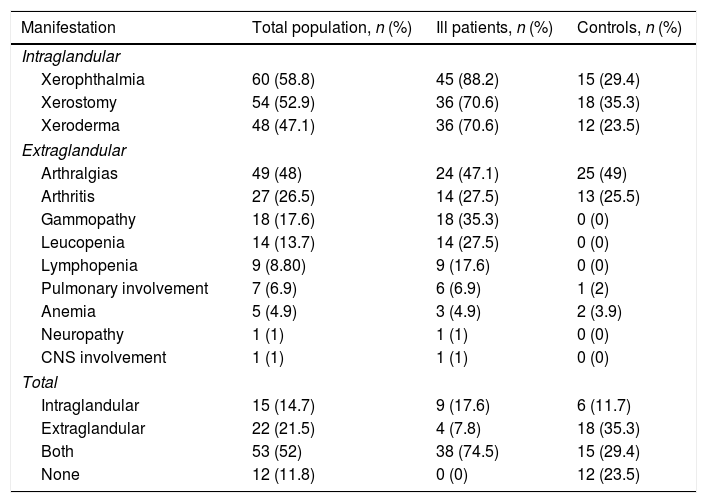
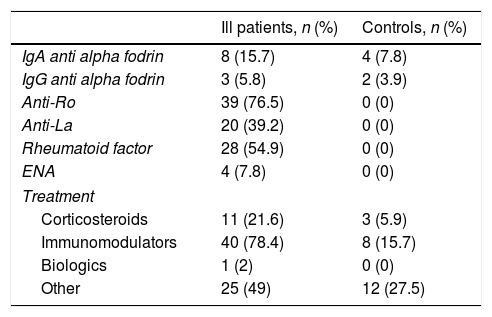
Table 1 depicts the clinical manifestations of the population studied, both the ill population and the controls. Table 2 illustrates the results obtained in terms of the presence of antibodies and treatment of both the ill and the control groups.
Clinical manifestations of the population studied.
| Manifestation | Total population, n (%) | Ill patients, n (%) | Controls, n (%) |
|---|---|---|---|
| Intraglandular | |||
| Xerophthalmia | 60 (58.8) | 45 (88.2) | 15 (29.4) |
| Xerostomy | 54 (52.9) | 36 (70.6) | 18 (35.3) |
| Xeroderma | 48 (47.1) | 36 (70.6) | 12 (23.5) |
| Extraglandular | |||
| Arthralgias | 49 (48) | 24 (47.1) | 25 (49) |
| Arthritis | 27 (26.5) | 14 (27.5) | 13 (25.5) |
| Gammopathy | 18 (17.6) | 18 (35.3) | 0 (0) |
| Leucopenia | 14 (13.7) | 14 (27.5) | 0 (0) |
| Lymphopenia | 9 (8.80) | 9 (17.6) | 0 (0) |
| Pulmonary involvement | 7 (6.9) | 6 (6.9) | 1 (2) |
| Anemia | 5 (4.9) | 3 (4.9) | 2 (3.9) |
| Neuropathy | 1 (1) | 1 (1) | 0 (0) |
| CNS involvement | 1 (1) | 1 (1) | 0 (0) |
| Total | |||
| Intraglandular | 15 (14.7) | 9 (17.6) | 6 (11.7) |
| Extraglandular | 22 (21.5) | 4 (7.8) | 18 (35.3) |
| Both | 53 (52) | 38 (74.5) | 15 (29.4) |
| None | 12 (11.8) | 0 (0) | 12 (23.5) |
Results of antibodies and treatment of underlying disease.
| Ill patients, n (%) | Controls, n (%) | |
|---|---|---|
| IgA anti alpha fodrin | 8 (15.7) | 4 (7.8) |
| IgG anti alpha fodrin | 3 (5.8) | 2 (3.9) |
| Anti-Ro | 39 (76.5) | 0 (0) |
| Anti-La | 20 (39.2) | 0 (0) |
| Rheumatoid factor | 28 (54.9) | 0 (0) |
| ENA | 4 (7.8) | 0 (0) |
| Treatment | ||
| Corticosteroids | 11 (21.6) | 3 (5.9) |
| Immunomodulators | 40 (78.4) | 8 (15.7) |
| Biologics | 1 (2) | 0 (0) |
| Other | 25 (49) | 12 (27.5) |
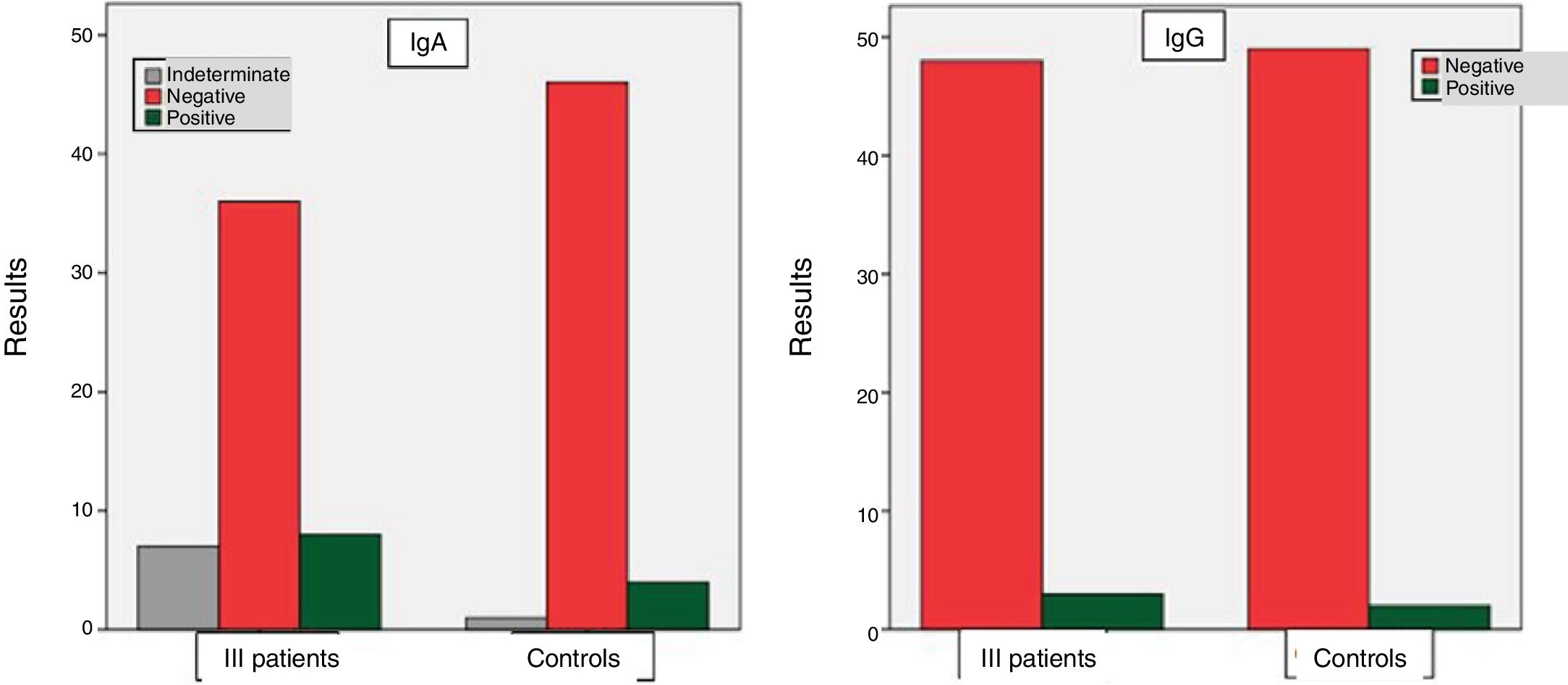
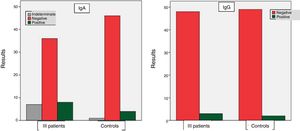
Among the group of ill patients, the results for the IgA anti alpha fodrin antibody were: positive in 8 cases (15.7%), indeterminate in 7 patients (13.7%), and negative in 36 patients (70.6%). With regard to the IgG antibody, the results were: positive in 3 patients (5.8%) and negative in 48 patients (94.2%). Among the controls, positive results of the IgA anti alpha fodrin were found in 4 patients (7.8%), indeterminate in one patient (2%), and negative in 46 patients (90.2%). With regard to the results of the IgG anti alpha fodrin antibodies, there were 2 patients with positive results (3.9%), and 49 patients with negative results (96.1%). These results are shown in Fig. 1.
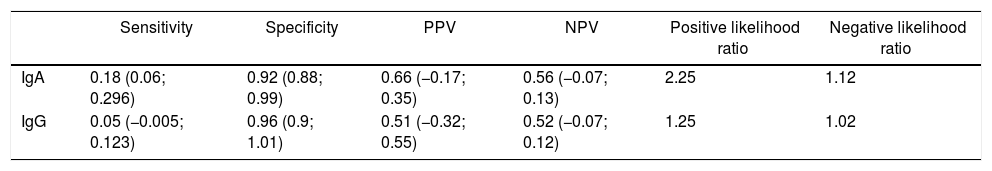
The results of the diagnostic performance of the anti alpha fodrin test as compared against the gold standard are shown in Table 3.
Diagnostic performance of the test versus the gold standard (American-European Consensus criteria or American College of Rheumatology).
| Sensitivity | Specificity | PPV | NPV | Positive likelihood ratio | Negative likelihood ratio | |
|---|---|---|---|---|---|---|
| IgA | 0.18 (0.06; 0.296) | 0.92 (0.88; 0.99) | 0.66 (−0.17; 0.35) | 0.56 (−0.07; 0.13) | 2.25 | 1.12 |
| IgG | 0.05 (−0.005; 0.123) | 0.96 (0.9; 1.01) | 0.51 (−0.32; 0.55) | 0.52 (−0.07; 0.12) | 1.25 | 1.02 |
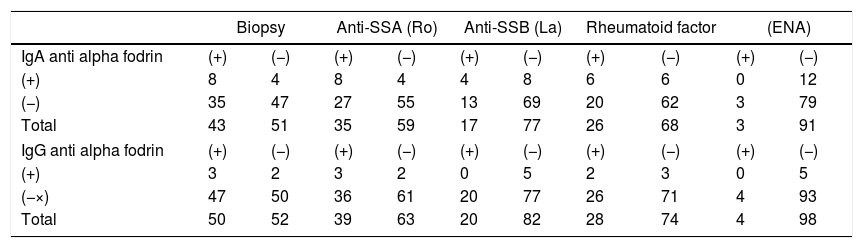
As depicted in Table 4, for the group of patients with a positive biopsy, the IgA alpha fodrin test was positive in 18.6%, whilst the anti-IgG antibody was only positive in 6%. With regard to the ill patients with anti-SSA (Ro) positive antibodies, 22.8% has a positive result for the IgA anti alpha fodrin antibody, and 7.69% for the IgG anti alpha fodrin antibody. Of the patients with anti-SSB (La) positive antibodies, the IgA type antibody was positive in 20% and the IgG type had no positive results. In terms of the patients with a positive rheumatoid factor, the IgA type antibody was positive in 30%, and the IgG type was positive in 7.14%. With respect to the patients that were ENA positive, none had positive results for the IgA or IgG.
IgA and IgG anti alpha fodrin antibodies and biopsy, anti-SSA (Ro), anti-SSA (Ro), rheumatoid factor and ENA.
| Biopsy | Anti-SSA (Ro) | Anti-SSB (La) | Rheumatoid factor | (ENA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgA anti alpha fodrin | (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) |
| (+) | 8 | 4 | 8 | 4 | 4 | 8 | 6 | 6 | 0 | 12 |
| (−) | 35 | 47 | 27 | 55 | 13 | 69 | 20 | 62 | 3 | 79 |
| Total | 43 | 51 | 35 | 59 | 17 | 77 | 26 | 68 | 3 | 91 |
| IgG anti alpha fodrin | (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) | (+) | (−) |
| (+) | 3 | 2 | 3 | 2 | 0 | 5 | 2 | 3 | 0 | 5 |
| (−×) | 47 | 50 | 36 | 61 | 20 | 77 | 26 | 71 | 4 | 93 |
| Total | 50 | 52 | 39 | 63 | 20 | 82 | 28 | 74 | 4 | 98 |
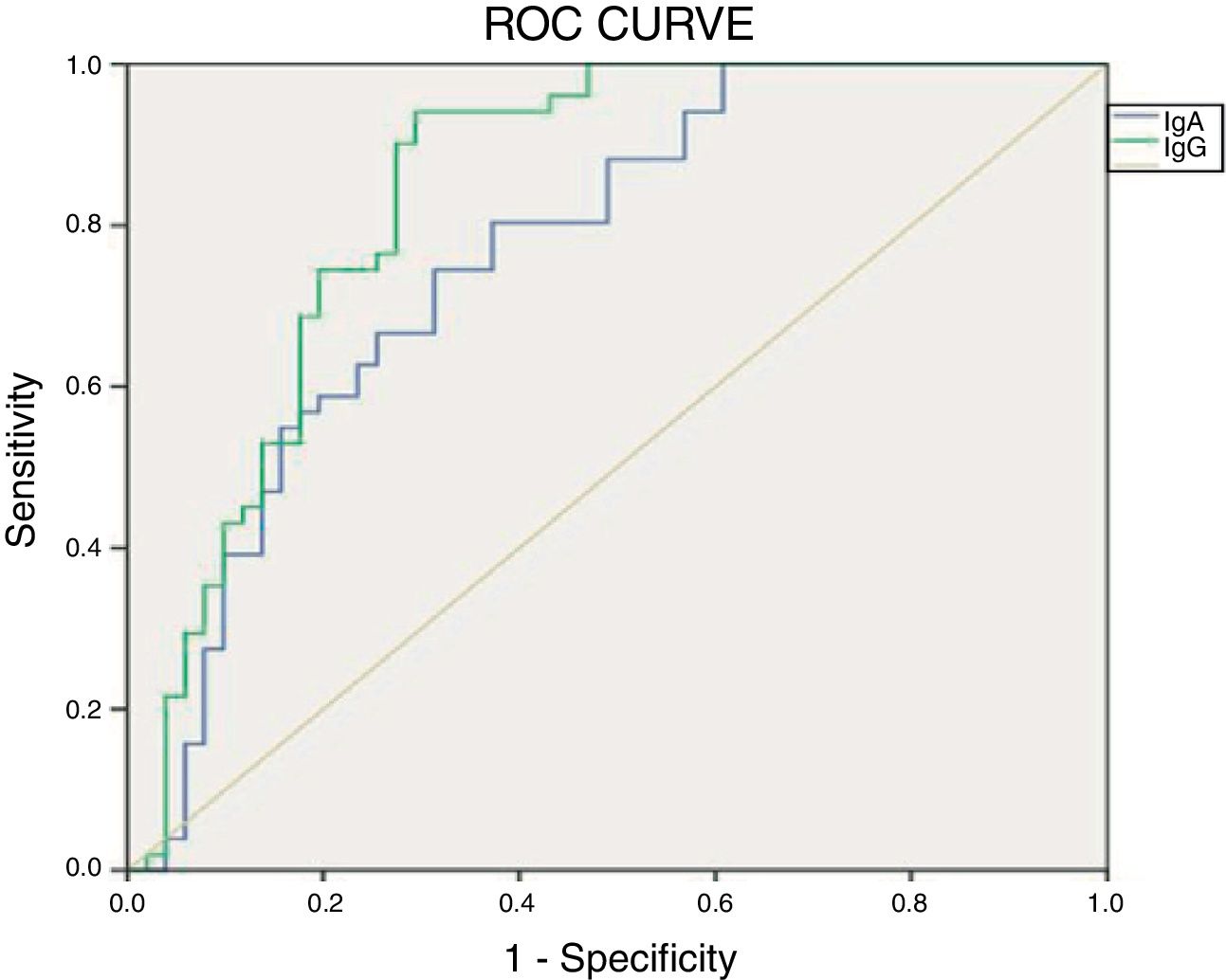
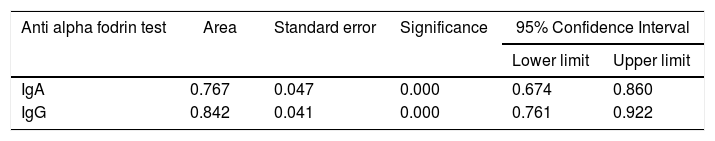
Fig. 2 shows the results of the ROC curve for the anti alpha fodrin antibodies.
The results obtained when comparing the validity of the IgA and IgG anti alpha fodrin test are shown in Table 5. Based on the Youden Index calculation, the cut-off point that optimizes the sensitivity and the specificity for the IgA test, discriminating appropriately between healthy and ill patients, was 4.33U/ml, with sensitivity and specificity values of 80.4% and 61%, respectively. For the IgG test, the cut-off point that optimizes the sensitivity and specificity is 1.43U/ml, with sensitivity and specificity values of 94.1% and 69%, respectively.
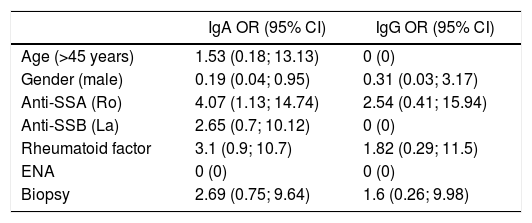
Bivariate analysisTable 6 illustrates the results of the bivariate analysis conducted between the IgA and IgG anti alpha fodrin antibodies, with the following variables: age, sex, and other antibodies. The chi square test was used for this analysis.
Odds ratio between IgA and IgG anti alpha fodrin antibody and the variables: age, gender, and other antibodies.
| IgA OR (95% CI) | IgG OR (95% CI) | |
|---|---|---|
| Age (>45 years) | 1.53 (0.18; 13.13) | 0 (0) |
| Gender (male) | 0.19 (0.04; 0.95) | 0.31 (0.03; 3.17) |
| Anti-SSA (Ro) | 4.07 (1.13; 14.74) | 2.54 (0.41; 15.94) |
| Anti-SSB (La) | 2.65 (0.7; 10.12) | 0 (0) |
| Rheumatoid factor | 3.1 (0.9; 10.7) | 1.82 (0.29; 11.5) |
| ENA | 0 (0) | 0 (0) |
| Biopsy | 2.69 (0.75; 9.64) | 1.6 (0.26; 9.98) |
This study found a lower sensitivity of the test than the sensitivity reported in other trials that assessed the diagnostic performance of the anti alpha fodrin antibodies, such as Zandbelt,11 Qin,12 Chen,13 Hu14 and Witte.15 They found that the sensitivity of the test ranges between 35% and 48%. It should be stressed however, that the above-mentioned authors found that the IgA values perform better than the IgG values in terms of the sensitivity of the test; this result is similar to our population. The variation in sensitivity can be explained through multiple factors, including the implementation of different diagnostic criteria for primary Sjögren's syndrome, and inclusion criteria, the use of different immunoassay techniques, the type of immunoglobulin evaluated (IgA, IgG), and the different populations studied.
The lack of standardization of the immunoassay techniques (ELISA, LIA, ALBIA) used for the detection of antibodies in the various studies, contributes to differential findings in terms of prevalence of antibodies in the various populations studied.7 According to Turkcapar,16 in order to determine the presence of anti alpha fodrin antibodies, the immunoblot technique may be more specific than ELISA, but it is worth noting that in this study, the technique used was ELISA.
With regard to the specificity of the test, the value identified for IgA anti alpha fodrin antibodies was 92% and 96% for IgG anti alpha fodrin antibodies. It is important to stress the relevance of the value for specificity of the test in the population in this study, because it highlights its validity as a diagnostic test for primary Sjögren's syndrome. A negative value in our test indicates that there is a 0.92 likelihood for IgA anti alpha fodrin antibodies and a 0.96% likelihood for IgG anti alpha fodrin antibodies, to detect with the test the absence of disease in healthy individuals; these results are similar to those described in the meta-analysis by Hu.14 Bearing in mind that the primary Sjögren's syndrome has a prevalence of 3 a 4% in the general population, the results obtained in terms of specificity for IgA and IgG anti alpha fodrin antibodies are considered to be valid in the population studied.
We found that this is a specific test for the diagnosis of a low prevalence disease such as primary Sjögren's syndrome, with a significant impact on the positive predictive value. Its cost, when compared against the cost of the different antibodies used for the routine diagnosis of the disease (non-specific antibodies) may be profitable for a healthcare system like the Colombian system; nevertheless, it is important to highlight that economic evaluation studies are required to establish the actual implications of its implementation in a healthcare system.
The presence of the following factors was associated to a higher risk of obtaining a positive result for the IgA antibody: a positive salivary gland biopsy, positive anti-Ro antibodies and rheumatoid factor, in addition to age over 45 years old. However, only a statistically significant association was found for the positive anti-Ro antibodies and gender.
With regard to the IgG antibody, the factors associated with a higher risk of having a positive result of the antibody were a positive biopsy, positive anti-Ro antibodies and positive rheumatoid factor. It was impossible to establish the association between age and the presence of IgG antibody. None of the factors associated with a higher risk of having a positive IgG antibody had statistical significance.
Additionally, a statistically significant association was found between the presence of IgA anti alpha fodrin antibodies and the expression of extraglandular manifestations such as anemia, lymphocytopenia, gammopathy and pulmonary involvement.
The results obtained from the bivariate analysis may have been due to the fact that the IgA and IgG anti alpha fodrin antibodies tend to express in early stages of the disease, sometimes long before there are any alterations in antibodies such as anti-SSA (Ro)17; in our population, the average time of diagnosis until they joined the trial was 5 years, so one may assume that the time of evolution of the disease influences the resulting sensitivity, specificity and prevalence.
With regard to treatment, no statistically significant association was found between the presence of IgA and IgG antibodies and the type of drug therapy of the disease. Nonetheless, such result in not consistent with the revised literature; for instance, Witte described a statistically significant association between the presence of anti alpha fodrin antibodies and the extent of lymphocyte infiltration of the salivary gland.5,18,19 In addition to the association between the use of immunomodulation medications with less involvement of the salivary gland and hence a decreased expression of anti alpha fodrin antibodies. For the purpose of this study, the result obtained in terms of therapy could be explained because most patients used corticosteroids and immunomodulators to treat their condition, and the mechanism of action of these medications could have been involved in the results of the IgA and IgG anti alpha fodrin test.
ConclusionsIn accordance with the results obtained and the discussion in this study, the conclusion is that the IgA anti alpha fodrin antibody represents a potential alternative test for the diagnosis of the disease; however, the limitations of the trial, the target population, the prevalence and the natural history of the disease, shall all be considered in order to continue researching in this area.
The results for the IgG anti alpha fodrin test as a diagnostic test for primary Sjögren's syndrome in our population are not considered favorable; therefore, we may conclude that in terms of decision-making for implementation as a valid diagnostic tool, further studies are required, both in the Colombian population and in the rest of the world.
With regard to the sensitivity of the test, the results obtained in the population studied are poor and other immunological parameters need to be considered to define their role in the classification and diagnosis of patients with primary Sjogren's syndrome.
Conflict of interestThe authors have no conflict of interests to declare.
The authors acknowledge the collaboration and sponsorship of Aesku.Diagnostics and Fundación Instituto de Reumatología Fernando Chalem to conduct this study.
Please cite this article as: Arteaga C, Barrera C, Morales G, Barrera N, Prieto N. Alfa fodrina y diagnóstico de síndrome de Sjögren primario, experiencia de un centro de reumatología de Bogotá, Colombia. Rev Colomb Reumatol. 2019;26:4–10.